Abstract
Background and Purpose
Amyloid-β (Aβ), a peptide that accumulates in the brain and circulates in the blood of patients with Alzheimer’s disease (AD), alters the regulation of cerebral blood flow (CBF) and may contribute to the brain dysfunction underlying the dementia. However, the contributions of brain and circulating Aβ1–40 to the vascular dysfunction have not been elucidated.
Methods
we used transgenic mice overexpressing mutated forms of the amyloid precursor protein in which Aβ1–40 is elevated in blood and brain (Tg-2576) or only in brain (Tg-SwDI). Mice were equipped with a cranial widow and the increase in CBF induced by neural activity (whisker stimulation) or by topical application of endothelium-dependent vasodilators was assessed by laser-Doppler flowmetry.
Results
The cerebrovascular dysfunction was observed also in Tg-SwDI mice, but, despite ≈40% higher levels of brain Aβ1–40, the effect was less marked than in Tg-2576 mice. Intravascular administration of Aβ1–40 elevated plasma Aβ1–40 and enhanced the dysfunction in Tg-SwDI mice, but not in Tg-2576 mice.
Conclusions
The results provide evidence that Aβ1–40 acts on distinct luminal and abluminal vascular targets, the deleterious cerebrovascular effects of which are additive. Furthermore, the findings highlight the importance of circulating Aβ1–40 in the cerebrovascular dysfunction and may provide insight into the cerebrovascular alterations in conditions in which elevations in plasma Aβ1–40 occur.
Keywords: β-Amyloid, cerebral blood flow, Tg-2576, Tg-SwDI, somatosensory cortex
Introduction
There is increasing evidence that the regulation of the cerebral circulation is disrupted in Alzheimer’s disease (AD) 1, 2. While resting cerebral blood flow (CBF) is reduced in selected brain regions of AD patients, the increases in CBF induced by neural activity are attenuated early in the course of the disease 3, 4. Studies in mice overexpressing mutated forms of the amyloid precursor protein (APP) have indicated that the Aβ peptide, Aβ1–40 in particular, alters key factors regulating CBF 5–7. Thus, the increases in CBF induced by neural activity or by endothelium-dependent vasodilators are attenuated in these mice 5, 6. Furthermore, the ability to keep CBF independent of changes in arterial pressure (cerebrovascular autoregulation) is profoundly disrupted 8, 9. These findings have raised the possibility that Aβ leads to brain dysfunction, not only by its deleterious effects on neurons and glia, but also by reducing cerebrovascular reserves and increasing the susceptibility of the brain to injury 2, 10.
In AD patients, Aβ is elevated both in brain and plasma 11. Furthermore, plasma Aβ is also elevated in cerebral amyloid angiopathy, small vessel disease and Down syndrome 12–14. However, the relative contribution of plasma and brain Aβ to the cerebrovascular dysfunction has not been defined. In particular, it is unclear whether increases in brain Aβ1–40 are necessary and sufficient to alter cerebrovascular regulation or whether elevations both in brain and circulating Aβ are needed. Therefore, it would be of interest to determine the cerebrovascular effects of elevations in plasma Aβ in the context of elevated brain Aβ 15.
Mice expressing the Swedish, Dutch and Iowa APP mutations under the control of the Thy1.2 neuronal promoter (Tg-SwDI) have Aβ increases in brain but not in plasma 16. In contrast, Tg-2576 mice, which express APP with the Swedish mutation driven by a prion protein promoter, have elevations in brain and plasma Aβ 17, 18. We used Tg-SwDI and Tg-2576 mice to investigate the relative contribution of brain and circulating Aβ1–40 to the cerebrovascular dysfunction. We found that elevations in brain Aβ1–40 are sufficient to alter cerebrovascular regulations. However, circulating Aβ1–40 enhances the cerebrovascular dysfunction induced by brain Aβ1–40. The findings provide the first evidence for distinct luminal and abluminal targets mediating the deleterious cerebrovascular effects of circulating and brain Aβ, and provide insight into the cerebrovascular alterations in conditions associated with chronic elevations of circulating Aβ.
Materials and Methods
1. Mice
All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College. Studies were performed in 3–4 month-old Tg-2576 17, Tg-SwDI transgenics 16, and their littermates. Because Tg-2576 mice are on a congenic 129S6 background and Tg-SwDI mice are on a congenic C57BL6 background 16, 17, we first tested cerebrovascular responses in transgene-negative age-matched wild type (WT) littermates of both transgenic backgrounds. No differences between transgene-negative 129S6 and C57BL/6J in CBF responses were observed and the results from the WT mice were pooled.
2. General surgical procedures
As described in detail elsewhere 19, 20, mice were anesthetized with isoflurane (1–2%, vol/vol). A femoral artery was cannulated for recording of arterial pressure and collection of blood samples. In some studies, the external carotid artery ipsilateral to the cranial window was catheterized for intracarotid (i.c.) infusion of human Aβ1–40 (see below) 19. Mice were intubated and artificially ventilated with an O2/N2 mixture adjusted to provide an arterial PO2 (PaO2) of 120–140 mmHg (Supplemental Table 1). Rectal temperature was maintained at 37 °C using a thermostatically controlled rectal probe connected to a heating pad. After surgery, isoflurane was discontinued and anesthesia was maintained with urethane (750 mg/kg; i.p.) and α-chloralose (50 mg/kg; i.p.). The level of anesthesia was monitored by testing corneal reflexes and motor responses to tail pinch.
3. Monitoring of cerebral blood flow
The somatosensory cortex was exposed by drilling a small opening through the parietal bone (2x2 mm), the dura was removed, and the site was superfused with modified Ringer’s solution (37 °C, pH 7.3–7.4) 5, 21, 22. CBF was continuously monitored at the site of superfusion with a laser-Doppler flow probe (Vasamedic) positioned stereotaxically above the cortical surface and connected to a computerized data acquisition system. CBF values were expressed as percent increase relative to baseline 5, 22.
4. Measurement of brain and plasma Aβ
Brain and plasma Aβ levels were determined using ELISA-based assays, as described previously 16, 19. Briefly, cerebral hemispheres were sonicated and centrifuged, and Aβ1–40 concentration (pmol/mg) was determined using the 2G3/3D6 and m21F12/3D6 sandwich ELISA assay (antibody reagents were generously provided by Lilly Research Laboratories). For determination of plasma concentrations, plasma samples were treated with 0.5×v/v of 5 M guanidine HCl for 30 min at room temperature, and Aβ1–40 concentration (pmol/ml) was determined as described above for brain Aβ1–40.
5. Immunohistochemistry
Anesthetized mice were perfused transcardially with heparinized saline, followed by 4% (wt/vol) paraformaldehyde 19, 20. Brains were removed, postfixed and sectioned (thickness of 40 µm). Free-floating sections were randomly selected and processed for labeling endothelial cells with glucose transporter-1 (Glut-1) (rabbit anti-glut-1,1:500, EMD Chemicals). The specificity of the labeling was established by omitting the primary antibody or by preabsorption with the antigen. Images were acquired using a confocal laser scanning microscope (Leica) in somatosensory cortex underlying the cranial window (0.38 to −1.94 mm from Bregma). Brain sections from Tg-2576, Tg-SwDI and WT littermates were processed under identical conditions and imaged using identical settings. The number of Glut-1-positive vascular profiles and the % area occupied by the profiles were quantified using ImageJ (National Institutes of Health).
6. Experimental protocol for CBF experiments
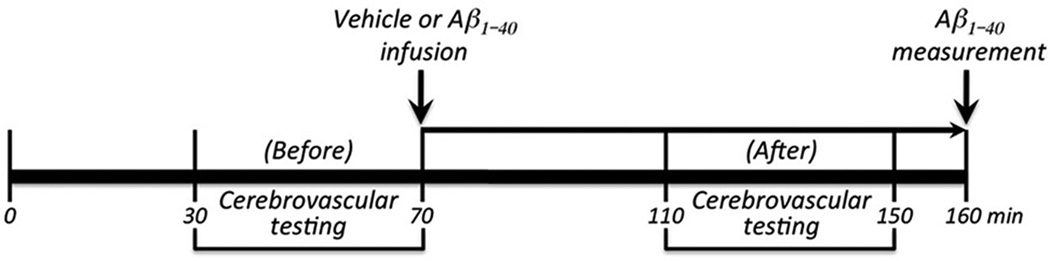
CBF recordings were started after arterial pressure and blood gases were in a steady state (Supplemental Table 1). All pharmacological agents studied were dissolved in Ringer’s solution, unless otherwise indicated. To study the increase in CBF produced by somatosensory activation, the whiskers were activated by side-to-side deflection for 60 sec. The endothelium-dependent vasodilators acetylcholine (10 µM; Sigma), A23187 (3 µM), and bradykinin (50 µM) were topically superfused for 3–5 min and the resulting changes in CBF monitored 5, 22. CBF responses to the smooth muscle relaxant adenosine (400 µM; Sigma) were also examined 19, 20. In experiments with i.c. infusion of human Aβ1–40 (rPeptides; in DMSO, final DMSO conc. <0.05%), CBF responses were first tested without infusion. Then, vehicle or Aβ1–40 (1 µM, 150µl/hr) was infused for 30–40 min into the internal carotid artery and responses were tested again (fig. 1).
Figure 1.
Experimental protocol of studies involving i.c. infusion of Aβ1–40. Cerebrovascular responses were tested before and after i.c. infusion of Aβ1–40. Aβ1–40 was measured in plasma and brain at the end of the experiment.
8. Data analysis
Data are expressed as means ± SEM. Two-group comparisons were analyzed by the two-tailed t-test. Multiple comparisons were evaluated by the analysis of variance and Tukey’s test. Differences were considered statistically significant for probability values less than 0.05.
Results
Brain Aβ1–40 is sufficient to induce cerebrovascular dysfunction
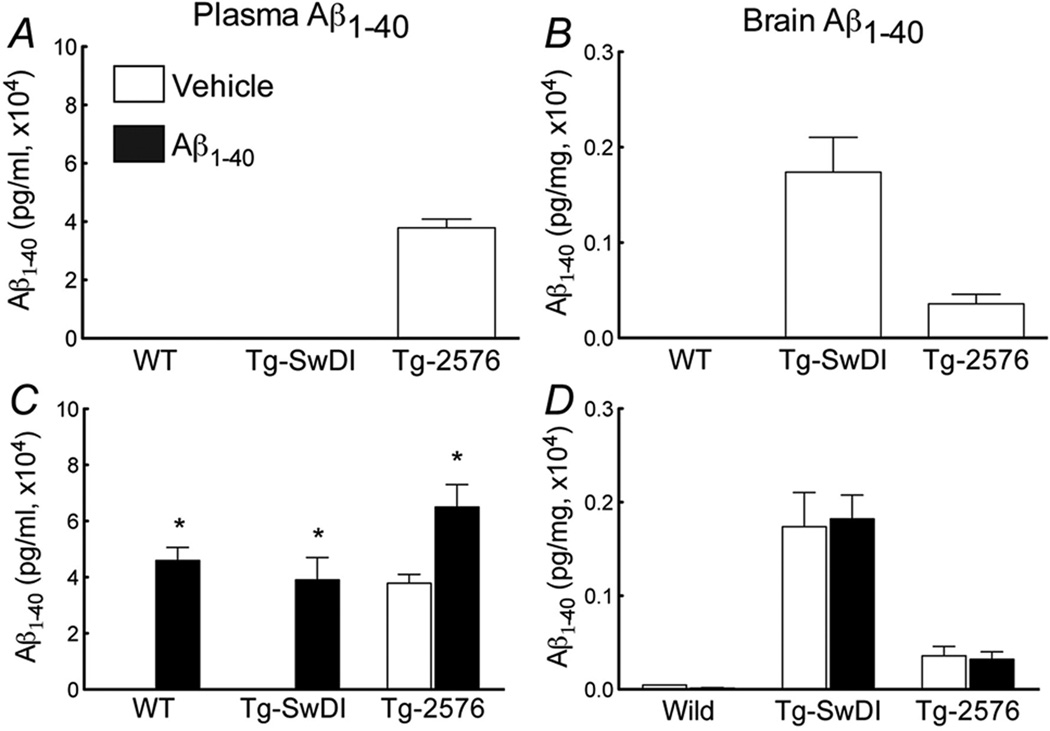
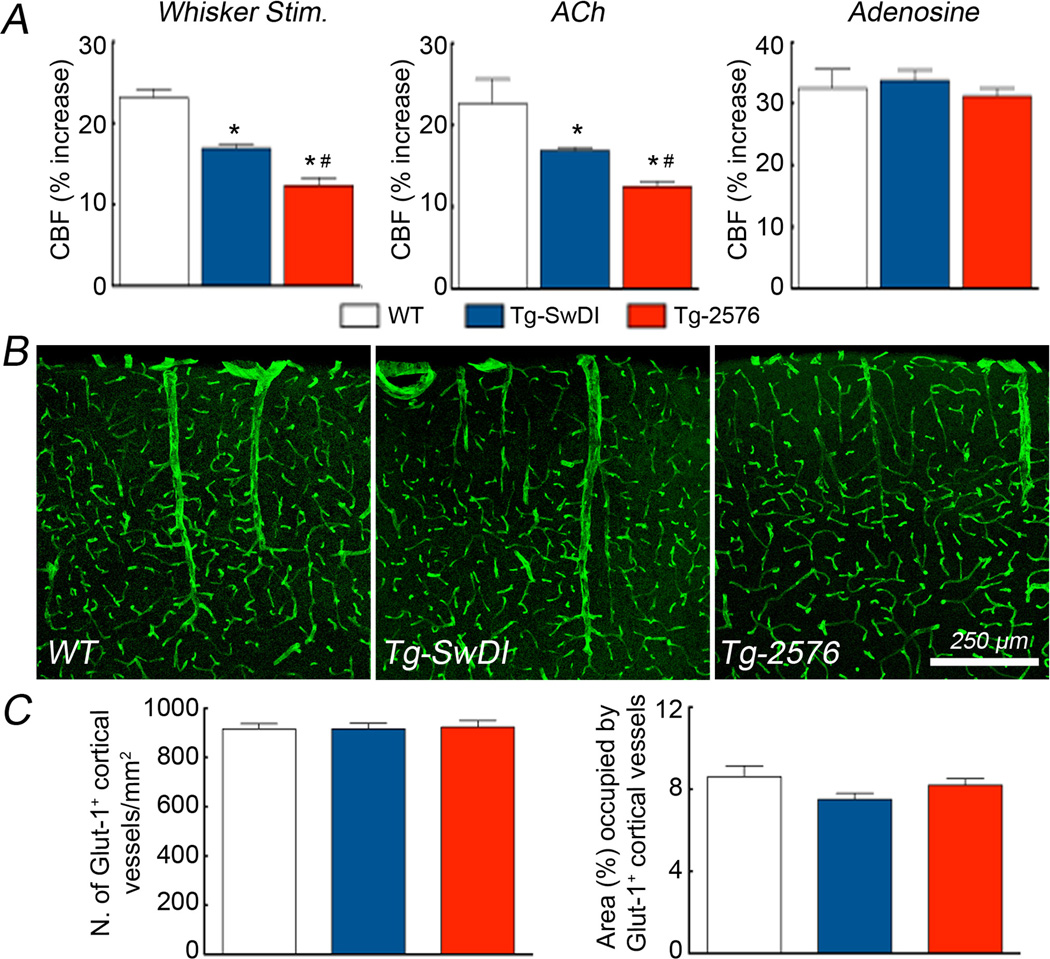
In agreement with previous studies, Aβ1–40 levels were elevated in brain and plasma in 3–4 month-old Tg-2576 mice (fig. 2). However, in comparably aged Tg-SwDI mice Aβ1–40 levels were elevated only in brain, an increase more pronounced than in Tg-2576 (fig. 2B). The increases in CBF induced by whisker stimulation or endothelium-dependent vasodilators (ACh, A23187, and bradykinin) were attenuated in Tg-SwDI mice (fig. 3A,B; suppl. fig. 1), but the attenuation was less pronounced than in Tg-2576 mice (fig. 3A,B; suppl. fig. 1). The CBF response to adenosine was not altered in either transgenics (fig. 3C), suggesting that the attenuation in vasomotor responses was not due to a non-specific impairment of vascular smooth muscle reactivity or vascular damage. In support for this conclusion, no differences in the morphology and number of cerebral microvessels were observed in the somatosensory cortex of Tg-SwDI and Tg-2576 mice (fig. 3D-H).
Figure 2.
Aβ1–40 levels in plasma (A) and brain (B) of Tg-SwDI and Tg-2576 mice. Effect i.c. infusion of Aβ1–40 on plasma (C) and brain (D) levels in WT, Tg-SwDI and Tg-2576 mice (*p<0.05; from vehicle; Analysis of variance and Tukey’s test; n=5/group).
Figure 3.
Increases in CBF elicited by whisker stimulation (A), ACh (B) and Adenosine (C) in WT, Tg-SwDI and Tg-2576 mice (*p<0.05 from WT; # p<0.05 from WT and Tg-SwDI; Analysis of variance and Tukey’s test; n=5/group). Glut-1 immunoreactivity in the somatosensory cortex of WT (D), Tg-SwDI (E) and Tg-2576 mice (F). Number of vascular profiles (G) and % area occupied by blood vessels (H) do not differ among the groups (p>0.05; n=4–5/group).
Elevation in plasma Aβ1–40 induces cerebrovascular dysfunction in WT mice
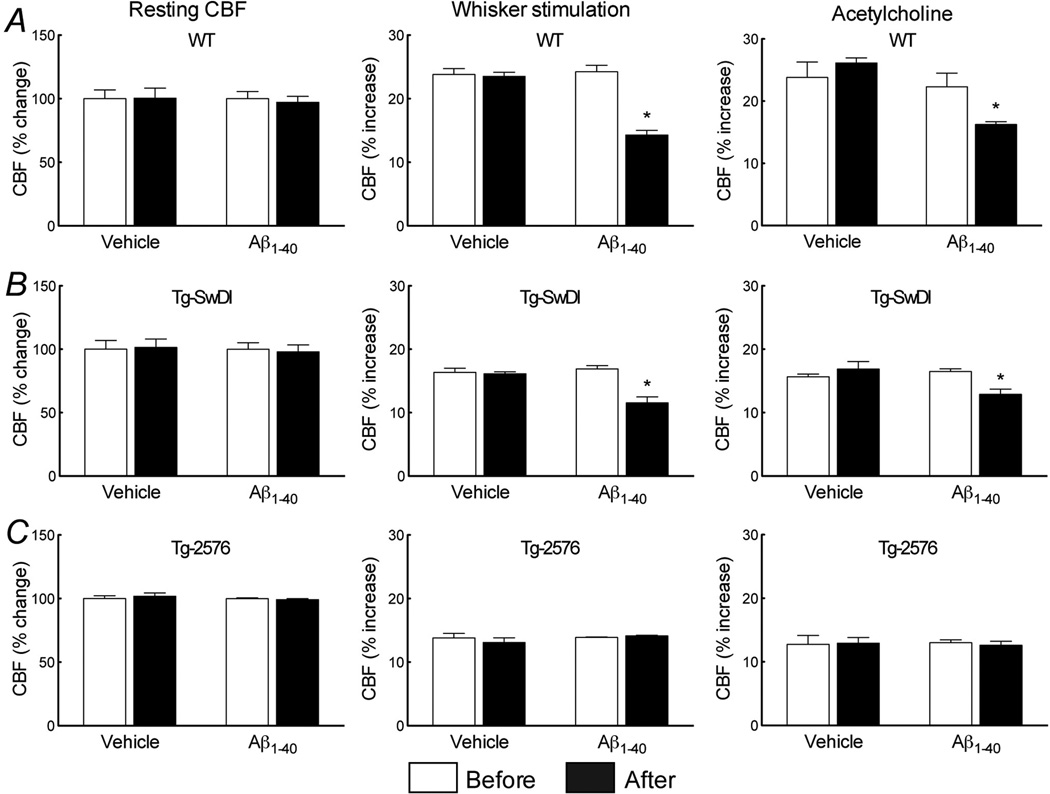
Next, we investigated the role of plasma Aβ1–40 in the cerebrovascular dysfunction. In WT mice, i.c. infusion of human Aβ1–40 elevated plasma Aβ1–40 to levels comparable to those observed in Tg-2576, without increasing brain Aβ1–40 (fig. 2C,D). Aβ1–40 i.c. infusion attenuated the increase in CBF induced by whisker stimulation and ACh (fig. 4B,C) (p>0.05), but did not alter resting CBF or the CBF response to adenosine (fig. 4A; suppl. fig. 2A). Therefore, circulating Aβ1–40 is sufficient to induce cerebrovascular dysfunction.
Figure 4.
Effect of i.c. infusion of Aβ1–40 on resting CBF (A, D, G) and on the increase in CBF evoked by whisker stimulation (B, E, H) or ACh (C, F, I) in WT (A, B, C), Tg-SwDI (D,E,F), and Tg-2576 (G, H, I). (*p<0.05 from before; Analysis of variance and Tukey’s test; n=5/group).
Elevation of plasma Aβ1–40 aggravates cerebrovascular dysfunction in Tg-SwDI, but not in Tg-2576 mice
To determine whether circulating Aβ1–40 and brain Aβ1–40 act synergistically on cerebrovascular function, we examined the cerebrovascular effects of elevation of plasma Aβ1–40 in Tg-SwDI mice. In Tg-SwDI mice, Aβ1–40 i.c. infusion induced plasma Aβ1–40 elevations comparable to those of Tg-2576 mice (fig. 2C) and attenuated cerebrovascular responses to levels not different from Tg-2576 mice (fig. 4E,F). In contrast, in Tg-2576 mice Aβ1–40 infusion did not aggravate the cerebrovascular dysfunction, despite a substantial increase in plasma Aβ1–40 (fig. 2C; fig. 4H,I). Infusion of Aβ1–40 did not affect brain levels of Aβ1–40 in Tg-SwDI or Tg-2576 mice (fig. 2D). Similarly, the Aβ infusion did not affect resting CBF or the CBF increase produced by adenosine (fig. 4D,G; suppl. fig. 2B,C).
Discussion
Novel findings of the study
We found that elevations in brain Aβ1–40, as observed in Tg-SwDI mice, are sufficient to induce cerebrovascular alterations. However, the cerebrovascular dysfunction is less marked than that of Tg-2576 mice despite higher brain Aβ1–40 concentrations. Elevation in circulating Aβ1–40 in Tg-SwDI mice enhances the vasomotor dysfunction to levels comparable to those of Tg-2576 mice. In contrast, further elevations in circulating Aβ1–40 in Tg-2576 mice do not aggravate the cerebrovascular dysfunction. These novel observations demonstrate that: (a) both brain and circulating Aβ1–40 are capable of inducing cerebrovascular dysfunction, (b) their effects are distinct and additive, and (c) reach a maximum at the concentrations achieved in Tg-2576 mice.
Exclusion of potential sources of artifacts
The findings of the present study cannot be attributed to differences in the physiological variables of the mice, because arterial pressure, blood gases and body temperature were monitored and did not differ among the groups studied. Similarly, the differences in the cerebrovascular responses between Tg-2576 and Tg-SwDI mice are unlikely to be a consequence of differences in smooth muscle relaxation because the CBF response to the smooth muscle relaxant adenosine was preserved in both transgenics. Tg-SwDI mice exhibit amyloid deposition primarily in cerebral microvessels 16, whereas Tg-2576 mice develop amyloid deposition in pial and meningeal vessels 23–25. However, such differences in Aβ deposition are not relevant to the present study because mice were studied at 3 months of age, prior to development of amyloid angiopathy 5, 16, 17. Similarly, gross morphological alterations of the cerebral microvasculature are unlikely to explain the observed differences in vascular reactivity because no differences were observed in the microvessels involved in the vascular responses studied.
Contribution of plasma and brain Aβ1–40 to the cerebrovascular dysfunction
We found that the alterations in functional hyperemia and endothelium-dependent responses in Tg-SwDI mice were less marked than in Tg-2576 mice, despite ≈40% higher brain Aβ levels. Considering that plasma Aβ1–40 is not measurable in Tg-SwDI but is elevated in Tg-2576, we hypothesized that the absence of circulating Aβ1–40 could explain the difference in the cerebrovascular dysfunction. Consistent with this prediction, i.c. infusion of Aβ1–40 raised plasma Aβ1–40 up to the concentration observed in Tg-2576 mice and enhanced the dysfunction in Tg-SwDI mice to levels identical to those observed in Tg-2576 mice. Aβ1–40 can cross the blood-brain barrier (BBB) in both direction 26 and administration of exogenous Aβ1–40 into the circulation could conceivably enter the brain especially if the BBB is altered 27. However, in our experiments the observed effects were not due to changes in brain levels because infusion of Aβ1–40 did not augment brain Aβ1–40 levels. Interestingly, infusion of Aβ1–40 in Tg-2576 mice increased plasma Aβ1–40 further, but failed to aggravate the cerebrovascular dysfunction. These observations, collectively, indicate that although brain or blood Aβ1–40 are sufficient to induce cerebrovascular dysfunction, their effects are additive and maximal at the concentrations reached in Tg-2576 mice.
Cellular mechanisms of the cerebrovascular effects of brain and plasma Aβ1–40
The present findings provide evidence that circulating and brain Aβ1–40 act on distinct luminal and abluminal sites to induce cerebrovascular dysfunction. However, the cellular substrates underlying such effects on opposite sides of the vessel wall remain to be defined. Increasing evidence implicates oxidative stress mediated by CD36-induced activation of a Nox2-containing NADPH oxidase 28. In Tg-2576, in which both brain and plasma levels of Aβ1–40 are elevated, deletion of CD36 or Nox2 rescues the cerebrovascular alterations completely 19, 20, 22, suggesting that ROS are involved in the cerebrovascular effects of both blood and brain Aβ1–40. However, the cellular localization of CD36 and Nox2 has not been clarified in full. Studies with i.c. administration of Aβ1–40 and in endothelial cell cultures suggest that the effects of circulating Aβ1–40 involve activation of CD36 and Nox2 in cerebral endothelial cells leading to vascular oxidative stress 19. However, it remains unclear how brain Aβ1–40 exerts its vascular action from the abluminal side of the vessel. One possibility is that parenchymal Aβ1–40, which is cleared through the perivascular space 29, acts on perivascular cells expressing CD36, i.e., microglia and macrophages 30, 31, which, in turn, contribute to vascular oxidative stress. In this case, brain and circulating Aβ1–40 would act on different targets on opposite sides of the vessels wall to induce cerebrovascular dysfunction. Another scenario is that circulating Aβ1–40 acts on circumventricular organs, which are devoid of BBB, and exert their cerebrovascular effects through release of vasopressin from the paraventricular hypothalamus and cerebrovascular endothelin upregulation, as recently described for angiotensin-II 32. These possibilities need to be examined in future studies.
Does soluble Aβ cause cognitive dysfunction?
The deleterious cognitive effects of vascular and parenchymal amyloid deposition are well established 33, but the clinical correlates of soluble Aβ remain less clear. Amyloid deposits and their attendant vascular and parenchymal effects are detectable in patients by imaging 33, 34, but is not yet possible to monitor soluble Aβ in its different states of aggregation. However, studies of human cerebral arteries have demonstrated that soluble Aβ induces alteration in vascular tone 35, 36. Considering that in AD patients, as in APP mice, soluble Aβ is present in brain and cerebral blood vessels prior to amyloid deposition 11, 18, it is conceivable that soluble Aβ has vascular effects also in humans. Indeed, soluble Aβ in low-order aggregation states (monomer, dimers, etc.) have emerged as key pathogenic factors in AD 37, and oligomeric Aβ is likely to alter both neuronal and vascular function.
Potential limitations of the study
One limitation of the present study is that the levels of exogenous Aβ1–40 in plasma producing cerebrovascular dysfunction and observed in Tg-2576 are higher than those observed in AD, small vessel disease, cerebral amyloid angiopathy or Down syndrome 12–14, 18, 38, 39. Therefore, it remains unclear whether the levels of Aβ present in AD patients would be sufficient to induce vascular dysfunction. However, cerebral blood vessels of AD patients are exposed to elevated plasma Aβ levels for years, and lower concentrations may be effective with a more prolonged exposure. Interestingly, AD immunotherapy can increase plasma Aβ levels up to 1000 folds, resulting in Aβ levels closer to those observed in Tg-2576 mice 15, 40, 41. Alterations in vascular structure and function are well known to occur in patients treated with Aβ antibodies, which has called for developing a better understanding of the intravascular effects of Aβ 42. The present findings demonstrate that circulating Aβ1–40 aggravates the cerebrovascular dysfunction induced by brain Aβ, potentially impeding the clearance of brain Aβ through the vascular pathway 29. Therefore, our data raise the possibility that increasing the clearance of plasma Aβ or counteracting its deleterious vascular actions could enhance the potential beneficial effects of Aβ immunotherapy. A caveat, however, is that after immunotherapy most plasma Aβ is antibody bound and it is unclear whether it retains its vasoactivity. Further studies are needed to address this important issue.
Conclusions
We used mice overexpressing mutated forms of APP to investigate the relative contribution of plasma and brain Aβ1–40 in the cerebrovascular dysfunction. We found that the cerebrovascular alterations are also present in Tg-SwDI mice, which have elevated levels of Aβ1–40 only in brain. However, the dysfunction is less marked than in Tg-2576 mice, in which both plasma and brain Aβ1–40 are increased. Intravascular administration of exogenous Aβ1–40 aggravates the cerebrovascular function in Tg-SwDI, but not Tg-2576 mice. The data indicate that plasma and brain Aβ1–40, acting on distinct targets on opposite sides of the vessels wall, exert additive effects on cerebrovascular regulation, and have implications for clinical conditions in which plasma levels of Aβ are elevated.
Supplementary Material
Acknowledgments
Grant support
Supported by grants from the NIH (CI: NS037853; WEVN: NS55118), the AHA (LP: 09SDG2060701), and the Alzheimer’s Association (CI: Zenith Fellow Award).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: Molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta neuropathologica. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, et al. Cerebral hypoperfusion and clinical onset of dementia: The rotterdam study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 4.Pimentel-Coelho PM, Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer's disease. Eur J Neurosci. 2012;35:1917–1937. doi: 10.1111/j.1460-9568.2012.08126.x. [DOI] [PubMed] [Google Scholar]
- 5.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, et al. Sod1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- 6.Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, et al. Abeta 1–40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niwa K, Carlson GA, Iadecola C. Exogenous a beta1–40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20:1659–1668. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol. 2002;283:H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- 9.Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato K, et al. Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009;54:1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586. [DOI] [PubMed] [Google Scholar]
- 10.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Head E, Doran E, Nistor M, Hill M, Schmitt FA, Haier RJ, et al. Plasma amyloid-beta as a function of age, level of intellectual disability, and presence of dementia in down syndrome. J Alzheimers Dis. 2011;23:399–409. doi: 10.3233/JAD-2010-101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma beta-amyloid and white matter lesions in ad, mci, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 14.Gomis M, Sobrino T, Ois A, Millan M, Rodriguez-Campello A, Perez de la Ossa N, et al. Plasma beta-amyloid 1–40 is associated with the diffuse small vessel disease subtype. Stroke. 2009;40:3197–3201. doi: 10.1161/STROKEAHA.109.559641. [DOI] [PubMed] [Google Scholar]
- 15.Levites Y, Das P, Price RW, Rochette MJ, Kostura LA, McGowan EM, et al. Anti-abeta42- and anti-abeta40-specific mabs attenuate amyloid deposition in an alzheimer disease mouse model. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, et al. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic dutch/iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 18.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, csf, and plasma amyloid (beta) protein in the tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, et al. Scavenger receptor cd36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci U S A. 2011;108:5063–5068. doi: 10.1073/pnas.1015413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iadecola C. Nitric oxide participates in the cerebrovasodilation elicited from cerebellar fastigial nucleus. Am J Physiol. 1992;263:R1156–R1161. doi: 10.1152/ajpregu.1992.263.5.R1156. [DOI] [PubMed] [Google Scholar]
- 22.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, et al. Nadph-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, et al. Apolipoprotein e markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J Neurosci. 2003;23:7889–7896. doi: 10.1523/JNEUROSCI.23-21-07889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han BH, Zhou ML, Abousaleh F, Brendza RP, Dietrich HH, Koenigsknecht-Talboo J, et al. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: Contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J Neurosci. 2008;28:13542–13550. doi: 10.1523/JNEUROSCI.4686-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kara F, Dongen ES, Schliebs R, Buchem MA, Groot HJ, Alia A. Monitoring blood flow alterations in the tg2576 mouse model of Alzheimer's disease by in vivo magnetic resonance angiography at 17.6 t. NeuroImage. 2012;60:958–966. doi: 10.1016/j.neuroimage.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 26.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Clifford PM, Zarrabi S, Siu G, Kinsler KJ, Kosciuk MC, Venkataraman V, et al. Abeta peptides can enter the brain through a defective blood-brain barrier and bind selectively to neurons. Brain Res. 2007;1142:223–236. doi: 10.1016/j.brainres.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 28.Iadecola C, Park L, Capone C. Threats to the mind: Aging, amyloid, and hypertension. Stroke. 2009;40:S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer's disease and their potential impact on therapy. Acta neuropathologica. 2009;118:87–102. doi: 10.1007/s00401-009-0498-z. [DOI] [PubMed] [Google Scholar]
- 30.Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, et al. Cd36, a class b scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverstein RL, Febbraio M. Cd36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, et al. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin ii hypertension. J Neurosci. 2012;32:4878–4886. doi: 10.1523/JNEUROSCI.6262-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auriel E, Greenberg SM. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Curr Atheroscler Rep. 2012;14:343–350. doi: 10.1007/s11883-012-0254-z. [DOI] [PubMed] [Google Scholar]
- 34.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paris D, Quadros A, Humphrey J, Patel N, Crescentini R, Crawford F, et al. Nilvadipine antagonizes both abeta vasoactivity in isolated arteries, and the reduced cerebral blood flow in appsw transgenic mice. Brain Res. 2004;999:53–61. doi: 10.1016/j.brainres.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 36.Paris D, Humphrey J, Quadros A, Patel N, Crescentini R, Crawford F, et al. Vasoactive effects of a beta in isolated human cerebrovessels and in a transgenic mouse model of Alzheimer's disease: Role of inflammation. Neurol Res. 2003;25:642–651. doi: 10.1179/016164103101201940. [DOI] [PubMed] [Google Scholar]
- 37.Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma abeta(1–40) and abeta(1–42) and the risk of dementia: A prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 39.Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, et al. Plasma a[beta]40 and a[beta]42 and Alzheimer's disease: Relation to age, mortality, and risk. Neurology. 2003;61:1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 40.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-a beta antibody alters cns and plasma a beta clearance and decreases brain a beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siemers ER, Friedrich S, Dean RA, Gonzales CR, Farlow MR, Paul SM, et al. Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid beta monoclonal antibody in subjects with alzheimer disease. Clin Neuropharmacol. 2010;33:67–73. doi: 10.1097/WNF.0b013e3181cb577a. [DOI] [PubMed] [Google Scholar]
- 42.Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer's association research roundtable workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.