Abstract
The large conductance calcium- and voltage-activated potassium channel (BKCa) is widely expressed at the plasma membrane. This channel is involved in a variety of fundamental cellular functions including excitability, smooth muscle contractility, and Ca2+ homeostasis, as well as in pathological situations like proinflammatory responses in rheumatoid arthritis, and cancer cell proliferation. Immunochemical, biochemical and pharmacological studies from over a decade have intermittently shown the presence of BKCa in intracellular organelles. To date, intracellular BKCa (iBKCa) has been localized in the mitochondria, endoplasmic reticulum, nucleus and Golgi apparatus but its functional role remains largely unknown except for the mitochondrial BKCa whose opening is thought to play a role in protecting the heart from ischaemic injury. In the nucleus, pharmacology suggests a role in regulating nuclear Ca2+, membrane potential and eNOS expression. Establishing the molecular correlates of iBKCa, the mechanisms defining iBKCa organelle-specific targeting, and their modulation are challenging questions. This review summarizes iBKCa channels, their possible functions, and efforts to identify their molecular correlates.
Harpreet Singh (left) is Research Assistant Professor at UCLA. He obtained his PhD from the University of Edinburgh with Dr Richard Ashley and Prof. Michael Cousin, and pursued his postdoctoral training at UCLA on intracellular BKCa channels with Prof. Toro. His research focuses on the cell biology of intracellular ion channels and their role in cardiac function. He developed an interest in superresolution microscopy while working with Prof. Stefani. In 2011, Harpreet was awarded the National Scientist Development Grant by the American Heart Association. Enrico Stefani (middle) obtained his MD from University of Buenos Aires and a PhD from University College, London. He is Distinguished Professor of Anesthesiology and Physiology at UCLA, and John Bartley Dillon Endowed Chair in Anesthesiology. With 254 publications, chapters and reviews in the biophysics field, he is now developing superresolution fluorescence microscopy that can reach a resolution of 20–40 nm in biological samples. His custom-built state-of-the-art microscope is shared with the academic community facilitating its reproduction (http://www.anes.ucla.edu/sted/index.html). Ligia Toro (right) received her PhD in Physiology and Biophysics from the Centro de Investigación y Estudios Avanzados del IPN, Mexico, and postdoctoral training at Baylor College of Medicine, Houston, TX, USA. She is Professor of Anesthesiology and of Molecular & Medical Pharmacology at UCLA, and has 126 publications, reviews and book chapters with the main focus on the biology of BKCa channels. Her current interests include mitochondrial BKCa channels in the healthy and failing heart, and BKCa channel interactions with angiotensin II receptors in the kidney.
 |
Introduction
Ion channels are present at the plasma membrane and in all intracellular organelles including mitochondria (O’Rourke, 2007), nucleus (Mazzanti et al. 1990; Singh, 2010), Golgi complex (Thompson et al. 2002) and endoplasmic reticulum (ER) (Osman et al. 2003; Ashrafpour et al. 2008). In intracellular organelles, they modulate the concentration of ions and play important roles in physiological events such as the voltage-dependent anion channel (VDAC) in apoptosis (Chacko et al. 2010), Ca2+-release-activated Ca2+ channels (CRACs) in Ca2+ signalling (Yeromin et al. 2006), and mitochondrial K+ channels in cardioprotection (Xu et al. 2002). In this review, we will particularly address the large conductance calcium- and voltage-activated K+ channel (BKCa) found intracellularly but will first discuss some general properties that may define its intracellular targeting.
BKCa channels are ubiquitously expressed at the plasma membrane of nervous and non-nervous cells including smooth muscle, sensory and epithelial cells where they couple membrane potential and intracellular calcium concentration. An interesting exception is the adult cardiomyocyte which lacks BKCa at the cell surface but expresses intracellular BKCa (iBKCa) particularly in the mitochondria.
The α-subunit of BKCa channel is encoded by a single gene Kcnma1 or Slo1 that undergoes extensive pre-mRNA splicing (Butler et al. 1993). Four α-subunits assemble to form a functional ion channel pore (Fig. 1). BKCa channels can be in complex with several modulatory subunits with one or two transmembrane domains (Fig. 1B) that greatly modify the channel kinetics and voltage/Ca2+ sensitivities. β1–β4 have two transmembrane domains and also affect channel pharmacology and its response to lipids (Knaus et al. 1994; Wallner et al. 1999; Xia et al. 1999; Brenner et al. 2000; Meera et al. 2000; Uebele et al. 2000; Vaithianathan et al. 2008), while leucine-rich repeat-containing proteins (LRRC) 26, LRRC38, LRRC52 and LRRC55 are single pass membrane proteins with LRRC26 being the most potent activator producing a negative shift of ∼140 mV of the voltage dependence of activation (Yan & Aldrich, 2010, 2012).
Figure 1. Topology of BKCa and modulatory subunits.
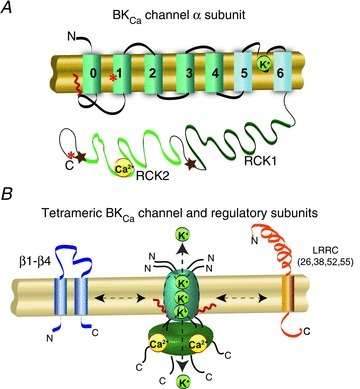
A, topology of BKCaα-subunit. N-terminus and C-terminus are located in opposite sides of the membrane. At the plasma membrane the N-terminus is extracellular and the C-terminus is intracellular. Orientation in organelles is unknown. S0–S4 transmembrane domains are involved in voltage sensing. The S5–S6 linker lines the K+-selective pore. The C-terminus has two RCK (regulator of potassium conductance) domains. RCK2 contains the Ca2+-bowl. For crystallographic information see Yuan et al. (2010). The S0–S1 linker can be palmitoylated or myristoylated (red zig-zag line). *, sites of splice variation that can result in ER retention. Stars, sites containing export signals. B, diagram of BKCa channel and regulatory subunits. Four α-subunits are needed to form a functional channel. β1–β4 subunits have two transmembrane domains. N- and C-termini are facing the same side of the membrane. LRRC-subunits have a single transmembrane domain. N- and C-termini face opposite sides of the membrane.
Increasing evidence suggests that splicing of BKCaα or β-subunits can govern the ‘normal’ traffic of the channel to the plasma membrane, consequently defining its subcellular distribution at a given time. BKCa variants originating from N- and C-terminal alternative splicing as well as C-terminal exon skipping are retained in the ER serving as repressors of BKCa channel expression at the plasmalemma (Zarei et al. 2004; Chen et al. 2005; Ma et al. 2007). On the other hand, β1– and β2-subunits can increase removal from the plasma membrane via endocytosis to a prelysosomal compartment (Toro et al. 2006; Zarei et al. 2007), while β4-subunits retain BKCa channels in the ER reducing its plasmalemmal localization (Shruti et al. 2012). Consistent with these findings, in hair cells, β1 and β4 expression reduce BKCa channels at the cell surface (Bai et al. 2011). Post-translational modifications can also affect the targeting of BKCa channels to the plasma membrane. Palmitoylation of intracellular loop 1 promotes cell surface expression (Jeffries et al. 2010), whereas internal myristoylation of loops 1 or 3 has the opposite effect (Alioua et al. 2011). Palmitoylation favours the exit of the channel from the ER and the trans-Golgi network (Tian et al. 2012) while myristoylation seems to favour endocytosis via clathrin-rich compartments (Alioua et al. 2011).
Most of the above studies have been performed in heterologous expression systems, which have been valuable in allowing the dissection of molecular mechanisms regulating the targeting of BKCa channels to the plasma membrane but only a few have been carried out in native cells. In astrocytes, transportation to the plasma membrane involves the microtubule network as fully assembled BKCa was found to be intracellularly associated with this cytoskeletal structure. When the Ca2+ concentration of the cytosol was elevated either pharmacologically or with thromboxane A2, iBKCa was translocated to the plasma membrane implying that microtubule-associated iBKCa was a readily available pool for astrocytes (Ou et al. 2009). In smooth muscle cells from pregnant mouse myometrium, iBKCa was found in the perinuclear region resulting in diminished plasma membrane expression. Possible explanations for this phenomenon are that retention in the perinuclear region and decreased plasma membrane expression is a mechanism preparing the uterine muscle for effective contractions during delivery (Eghbali et al. 2003), or that in addition, iBKCa within the perinuclear region serves an unknown functional role. In fibroblast-like synoviocytes from patients with rheumatoid arthritis, BKCa is observed at the plasma membrane but also in the nucleus (Hu et al. 2012) opening the intriguing question of whether BKCa localized to the nucleus may play a role in diseased states. In line with the view that iBKCa channels are also targeted to intracellular organelles for a specific function – unrelated to the overall regulation of cell surface expression – in neonatal cardiomyocytes, iBKCa has been visualized in the mitochondria (mitoBKCa) coincident with VDAC1 signals (Redel et al. 2008), and pharmacological evidence supports its role in protecting the heart from ischaemic insult as will be discussed in the following section.
Thus, it appears that there are at least three types of iBKCa channels present inside the cells: (1) a pool related to the normal traffic to the plasma membrane and its regulation, (2) a pool awaiting to be translocated to the plasma membrane, and (3) another set specifically targeted to organelles. In this regard, several groups have shown the functional activity of iBKCa channels in the mitochondria and nucleus (Table 1). Mechanisms that may define iBKCa localization could include splice variation, β-subunit association, and/or cell-specific mechanisms.
Table 1.
iBKCa biophysical properties in mitochondria and nucleus
| Organelle and method used | Cell type/ organ | Conductance and pharmacology | Recording solution [K+] (mm),[Ca2+] (μm) | V1/2 or open probability (Po) | EC50 for Ca2+ | Reference |
|---|---|---|---|---|---|---|
| Mitochondria Patch clamp (mitoplast) | Human glioma cell line (LN229) | 295 pS ChTx-sensitive | Pipette/bath 150 K+/150 K+ Nominal or no Ca2+/variable Ca2+ | V1/2=−33 ± 19 mV at 8.7 μm Ca2+; V1/2= 41 ± 23 mV at 1 μm Ca2+ | 6.9 μm at −20 mV | Siemen et al. (1999) |
| Mitochondria Patch clamp (mitoplast) | Guinea-pig ventricular myocytes | 307 pS ChTx-sensitive | 150 K+/150 K+ 0.512 Ca2+/0.512 and 40 Ca2+ | Po∼0.9 at +60 mV and 0.512 μm Ca2+* | N/A | Xu et al. (2002) |
| Mitochondria Patch clamp (mitoplast) | Rat ventricular myocytes | 270 pS Paxilline-sensitive | 140 K+/140 K+ 0.5 Ca2+/0.5 Ca2+ | Po= 0.0087 at +40 mV and 0.5 μm Ca2+ | N/A | Ohya et al. (2005) |
| Mitochondria Patch clamp (mitoplast) | Human glioma cell line (LN229 and LN405) | 276 pS ChTx-sensitive | 150 K+/150 K+ 0 Ca2+/100–400 Ca2+ | V1/2∼−42 mV at 200 μm Ca2+* | N/A | Gu et al. (2007) |
| Mitochondria Patch clamp (mitoplast) | Rat astrocytes | 295–296 pS IbTx-sensitive; Bax-sensitive | 150 K+/150 K+ 200 Ca2+/200 Ca2+ | V1/2∼−50 mV at 200 μm Ca2+* | N/A | Cheng et al. (2008, 2011) |
| Mitochondria Lipid bilayers | Rat whole brain | 265 pS ChTx sensitive | (cis/trans) 50 K+/450 K+ 300–500 Ca2+/300–500 Ca2+ | Po= 0.50 at 0 Ca2+ Po= 0.77 at +70 mV and 300 μm Ca2+ | N/A | Skalska et al. (2009) |
| Mitochondria Lipid bilayers | Rat whole brain | 211 pS IbTx-, 4-AP-sensitive; ChTx-insensitive | 200 K+/50 K+‘Contaminant’ Ca2+ | Po= 0.9 ± 0.01 at +40 mV V1/2= 11 ± 1 mV | N/A | Fahanik-Babaei et al. (2011a) |
| Mitochondria Lipid bilayers | Rat whole brain | 565 pS ChTx-, IbTx-, 4-AP-sensitive | 200 K+/50 K+ 100 Ca2+/100 Ca2+ 10 Ca2+/10 Ca2+‘Ca2+-free’/‘Ca2+-free’ | At 100 μm Ca2+, Po= 0.9 ± 0.05 at −40 to +40 mV At 0 Ca2+, Po= 0.8 at +20 mV and Po= 0.07 at −40 mV | N/A | Fahanik-Babaei et al. (2011b) |
| Nucleus Patch clamp | Rat pancreatic acinar cells | 200 pS | Pipette/bath 148 K+/148 K+ 200 Ca2+/0.1, 200 Ca2+ | Po=∼0.5 at +40 mV* at 200 μm Ca2+ | N/A | Maruyama et al. (1995) |
Calculated from published figure. Abbreviations: V1/2, half-activation potential or potential where an open probability of 0.5 is achieved; EC50, concentration of half-maximal effect; ChTx, charybdotoxin; IbTx, iberiotoxin; 4-AP, 4-aminopyridine; N/A, not available.
Mitochondrial BKCa channels (mitoBKCa)
mitoBKCa channels were first identified by patch clamping of mitoplasts prepared from human glioma cells LN229 (Siemen et al. 1999) and later they were shown by several groups to be involved in cardioprotection against ischaemic injury by using pharmacological agents to open and block the channel. Preconditioning hearts with BKCa openers like NS1619 or NS11021 reduced myocardial infarction or heart function and these beneficial effects could be antagonized by coadministration with paxilline, a commonly used BKCa inhibitor (Xu et al. 2002; Wang et al. 2004; Stowe et al. 2006; Bentzen et al. 2009, 2010). Also, stimulating mitoBKCa activity with β-oestradiol resulted in decreased cardiomyocyte death due to ischaemic insult (Ohya et al. 2005). mitoBKCa has also been proposed to mediate the cardioprotective effects of the anaesthetic desflurane, the peptide adrenomedullin, and the tumour necrosis factor-α (Gao et al. 2005; Nishida et al. 2008; Redel et al. 2008). However, recent studies using BKCa knockout (Slo1−/− or Kcnma1−/−) mice have challenged the role of mitoBKCa in isoflurane-mediated cardioprotection from ischaemia/reperfusion injury, and proposed a role for a large conductance K+ channel that is activated by Na+ (Slo2). Importantly, the reduction in infarct size by isoflurane preconditioning was abolished by paxilline in wild-type as well as in Slo1−/− hearts (Wojtovich et al. 2011) raising serious concerns about the usage of this drug as a specific blocker of BKCa. It would be interesting to test whether different anaesthetics use distinct cardioprotective pathways.
Mechanisms triggered by the putative opening of mitoBKCa by NS1619 include regulation of reactive oxygen species (ROS) production and calcium retention capacity (CRC). In isolated mitochondria from brain and heart, mitoBKCa is known to reduce ROS production on activation with NS1619 and CGS7184 (Heinen et al. 2007; Kulawiak et al. 2008), while in brain mitochondria the opening of the mitochondrial permeability transition pore (mPTP) by Ca2+ (indirectly measured as mitochondrial depolarization in response to Ca2+ pulses) is accelerated by blocking mitoBKCa with iberiotoxin (Cheng et al. 2008). Moreover, in isolated hearts, preconditioning with NS1619 reduces ROS and mitochondrial Ca2+ (Stowe et al. 2006). Thus, it is tempting to hypothesize that reduced mitoBKCa channel activity favours the opening of mPTP and vice versa. Consistent with this idea, the putative inhibition of mitoBKCa with paxilline, induced the release of cytochrome c, a signature of mPTP opening and initiation of apoptosis. That inhibition of mitoBKCa favours mPTP opening and apoptosis is further substantiated by the fact that the proapoptopic protein Bax can directly inhibit mitoBKCa single channel activity recorded in astrocyte mitoplasts (Cheng et al. 2011). Conversely, the opening of mitoBKCa with NS11021 improves cardiac mitochondria function by enhancing K+ uptake without a significant change in mitochondrial membrane potential (ΔΨm) and improving its energetic performance (Aon et al. 2010).
In addition to pharmacological evidence, immunochemistry and immunogold electron microscopy have also placed BKCa in the mitochondria. Western blot analysis using antibodies raised against the C-terminus of BKCa channel showed a signal at ∼55 kDa (Xu et al. 2002) or at ∼125 kDa (Shi et al. 2007) in isolated cardiac mitochondria. Double immunostaining and confocal microscopy showed that in the cerebellum, neuronal BKCa signals coincide with signals of mitochondrial proteins, OP4–1, ANT, IMM, the heat shock protein 60 (hsp60) and TIM23 (Douglas et al. 2006). mitoBKCa signals have also been reported in rat neonatal cardiomyocytes together with VDAC1 (Redel et al. 2008), and data from our lab shows that it colocalizes with mitotracker in the rat embryonic heart cell line H9c2 (Fig. 2). Note that in this embryonic cell line, the majority of BKCa signals are localized to mitochondria and fewer but clear signals are also observed at the cell periphery.
Figure 2. BKCa localization in mitochondria.
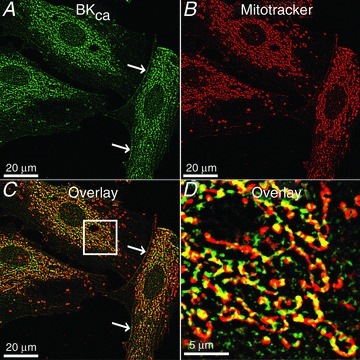
Cardiac H9c2 cells (rat embryonic heart cell line) were labelled with a specific BKCa antibody raised against plasma membrane BKCa channels and mitotracker. A, signals of BKCa were readily observed inside the cell and at the cell periphery indicating plasma membrane expression (arrows). Note that in contrast to adult cardiomyocytes where BKCa is absent at the plasma membrane, embryonic heart cells are known to express BKCa at the plasma membrane. B, mitotracker labelling. C, overlay showing a high coincidence between BKCa and mitotracker signals. D, square in C at higher magnification.
Efforts to identify the molecular correlate of mitoBKCa have been carried out by several groups but with limited success. A full length mRNA was cloned from mouse cardiomyocytes but the protein failed to localize to the mitochondria (Ko et al. 2009). Since BKCa is coded by a single gene, it is possible that a splice variant is responsible for its mitochondrial localization. In fact, in mouse cochlea where BKCa was found in the mitochondria (in addition to the cytoplasm and plasma membrane), a BKCa isoform containing four splice sequences along the C-terminus was cloned (IYF, 27 amino acids, ATRMTRMGQ, which is upstream of 50 C-terminal amino acids ending in VEDEC) (GenBank accession no. FJ872117). This cochlea clone when expressed in Chinese hamster ovary cells was observed in mitochondria with some expression at the plasma membrane (Kathiresan et al. 2009). However, it is not clear whether any of these splice inserts can target BKCa to the mitochondria. In silico analysis of the cochlea variant using MitoProt (Claros & Vincens, 1996) indicate a probability of 0.0175 for mitochondrial targeting. However, this engine searches for classical N-terminal mitochondrial signal peptides (‘presequences’). It is now known that mitochondrial targeted proteins may possess internal signals at multiple sites within the protein including the C-terminus and that inner membrane proteins may contain an internal ‘presequence-like’ signal. Scrutinizing the role of each of the cochlea BKCa splice sequences in their ability to target mitoBKCa to the mitochondria is an open topic of research.
From the functional point of view, mitoBKCa conductance ranges from ∼211 pS to 565 pS depending on the biological system and experimental conditions (Table 1). Typically mitoBKCa is inhibited by blockers iberiotoxin, charybdotoxin and paxilline. However, in brain mitochondrial inner membranes, mitoBKCa with distinct pharmacological profiles have been detected after reconstitution into lipid bilayers, a voltage-dependent 211 pS channel that is insensitive to charybdotoxin but sensitive to iberiotoxin, and a 565 pS channel that is sensitive to both toxins. Notably, both conductances were sensitive to 10 mm 4-aminopyridine (Fahanik-Babaei et al. 2011a,b), a drug that does not affect cloned BKCa channels (Wallner et al. 1995). At a high Ca2+ concentration (100 μm Ca2+), the open probability of the channel is maintained near ∼0.9 from −40 to +40 mV; however, under Ca2+-free solutions the voltage dependency of the channel becomes evident as the channel open probability changes from 0.8 at 0 mV to 0.07 at −40 mV. It is known that splice variation as well as β-subunits can confer different voltage/Ca2+ sensitivities to the BKCa channel. For example, in the presence of the β1-subunit and Ca2+ near 25 μm, the open probability of the channel is around 0.9 from −50 mV onwards with a half-activation potential, V1/2, around −100 mV (Meera et al. 1996). Also, β-subunits can change BKCa pharmacology, for example the β4-subunit that makes BKCa channels resistant to iberiotoxin (Meera et al. 2000). These factors could explain the variability in the single channel properties of the reported mitoBKCa. Whether mitoBKCa isoforms with different pore properties, pharmacology and voltage sensitivities originate from splice variation and/or association with known or unknown β-subunits are relevant problems to solve.
An important question is how does K+ flux via mitoBKCa affect mitochondrial function? In other words what is the physiological role of mitoBKCa? Attempts to answer this question have been carried out with BKCa openers like NS1619 and the higher affinity analogue NS11021 using isolated cardiac mitochondria. Low doses of NS11021 (e.g. 50 nm) increase charybdotoxin-sensitive K+ influx and swelling in the presence of permeable anions like acetate (passively diffused) and dihydrogen phosphate (carrier-mediated transport) but with very limited change (5–10 mV) in mitochondrial membrane potential (ΔΨm). Moreover, this K+ influx is accompanied by a better mitochondrial respiratory control due to a decrease in state 4 respiration without a change in state 3 respiration (Aon et al. 2010). These properties may explain how the specific opening of mitoBKCa may promote cardioprotection. Paradoxically, micromolar concentrations of NS11021 that protect the heart from ischemia and reperfusion (Bentzen et al. 2009) cause non-specific deleterious effects on mitochondria such as decreased respiratory control that is insensitive to charybdotoxin and a large drop in membrane potential of near 30 mV even in the absence of K+ (Aon et al. 2010). Similarly, concentrations of NS1619 that cause cardioprotection (∼10 μm) (Shi et al. 2007) have been reported to decrease light scattering in K+-free medium accompanied by respiration uncoupling in liver mitochondria (Bednarczyk et al. 2008). One possible explanation is that in whole heart experiments the effective concentration of NS1619 is actually lower due to limited diffusion of the drug to its site of action, and thus, it promotes cardioprotection instead of mitochondrial damage and heart stress. Experiments using BKCa knockout animals should shed some light on these questions.
BKCa channels in the nucleus
The nuclear genome and the molecular machinery required for DNA replication as well as transcription are present in the nucleus, which is sheathed by a nuclear envelope. There are two membranes in the nuclear envelope: the inner nuclear membrane (INM) interacting with the nuclear skeleton, and the outer nuclear membrane (ONM), continuous with the ER and also studded with ribosomes. The perinuclear space between ONM and in INM is continuous with the ER lumen, so it is likely to be rich in Ca2+ ions. Proteins made in the ER and in the perinuclear space are transported to the lumen of ER for further trafficking in the cell. INM and ONM fuse together to form large nuclear pore complexes (NPCs) which allow bidirectional flow of large molecules. Several fundamental processes such as cell replication and differentiation, ageing, regeneration, cell cycles, and enzyme activity are governed by nuclear ionic concentrations.
K+ channels with conductances of 55 pS and 200 pS were first recorded on the nuclear envelope from murine zygotes (Mazzanti et al. 1990). BKCa currents were recorded later in the ONM of rat pancreatic acinar cells (Maruyama et al. 1995) and observed with immunocytochemistry in chick retinal nuclei (Yamashita et al. 2006), the perinuclear region of isolated nuclei of brain endothelial cells (Gobeil et al. 2002), and in nuclei of fibroblast-like synoviocytes from patients with rheumatoid arthritis (Hu et al. 2012). Consistent with the electrophysiological recordings, analysis of the plasma membrane BKCa constitutive sequence by ‘Nucleo’, a nuclear protein localization predictor (Hawkins et al. 2007), yielded a score of 0.72 (1 being the perfect signal), while NucPred gave a score of 0.65 (Brameier et al. 2007). These in silico analyses suggest that BKCa might carry an intrinsic nuclear localization signal, which can target it to the nuclear membrane. However, since the same BKCa gene is present in all cells, there must be additional mechanisms targeting it to the nucleus that are cell-type specific or cell physiological-status specific.
Experiments in the nuclei of brain endothelial cells utilizing 100 nm NS1619 as BKCa opener and 100 nm iberiotoxin as a specific channel blocker indicate that nuclear iBKCa is coupled to the activity of perinuclear prostaglandin receptors (EP3) regulating nuclear Ca2+, membrane potential and eNOS expression. Specifically in isolated nuclei, Ca2+ transients, K+-dependent membrane potential changes and eNOS transcript expression induced by the activation of EP3 agonist M&B28767 were all abolished by iberiotoxin; while NS1619 produced Ca2+ transients and changes in membrane potential in 100 mm K+ but not in 1 mm K+ that were iberiotoxin sensitive. Further, the EP3 agonist-induced increase of eNOS expression was completely abolished by iberiotoxin mimicking the effect of Ca2+ chelators (Gobeil et al. 2002). Whether nuclear voltage-dependent R-type Ca2+ channels (Bkaily et al. 2012) are functionally coupled to iBKCa, whether iBKCa plays a role in regulating nuclear Ca2+ transients that occur in other cell types such as contracting chick embryonic cardiomyocytes (Bkaily et al. 2009), or what is the orientation and molecular nature of iBKCa in the nucleus, are open questions.
iBKCa channels in other organelles
Endoplasmic reticulum
Proteins present in the membrane of the endoplasmic reticulum (ER) are involved in protein synthesis, protein processing, protein folding, and ionic homeostasis. Enzymes working in protein synthesis and processing also require ionic homeostasis which is maintained by ion channels and transporters. Disruption in homeostasis results in accumulation of misfolded or unfolded proteins in the ER lumen. This results in ER stress which can be restored by the unfolded protein response but when this mechanism fails to remove unfolded or misfolded proteins it can result in apoptosis (Kaufman, 1999; Jing et al. 2012).
Similar to other proteins encoded by nuclear DNA, BKCa channels are also synthesized in the ER. Whether the iBKCa channel is active in the ER is not yet established. However, the α-subunit protein can be retained in the ER if it includes splice sequences SV1 (Zarei et al. 2001, 2004) or DEC (Ma et al. 2007). SV1 contains the ER retention motif CVLF at its first intracellular loop. This motif found in rat myometrium retains/retrieves the channel in/to the ER and also prevents BKCa surface expression. The surface expression of the protein is controlled by multiple signals in the C-terminus including an acidic cluster-like motif present in the RCK1 and RCK2 linker region DDXXDXXXI that accelerates exit from the ER (Chen et al. 2010) as well as six amino acids DLIFCL located near the C-terminal end (Kwon & Guggino, 2004) (Fig. 1). However, the presence of these sequences cannot override the ER-retention signal CVLF (Zarei et al. 2004). Interestingly, a human splice variant (hSloΔ579–664) where the DDXXDXXXI motif is excluded is expressed in multiple tissues and in heterologous expression fails to form a functional ion channel at the surface and localizes the channel protein to the ER. These data strongly support the idea that one molecular mechanism defining iBKCa fate and localization to the ER (or any other organelle) is splice variation. Another mechanism contributing to BKCa localization to the ER is the presence of the β4-subunit that possesses the ER retention signal KKRKFS at its C-terminus (Shruti et al. 2012). Further work is needed to determine whether iBKCa localized to the ER plays a functional role.
Golgi apparatus
During protein synthesis, proteins refold and pass through the Golgi apparatus where they undergo post-translational modifications. Proteins and lipids are sorted as they exit the Golgi apparatus and are sent to their final destinations. The Golgi apparatus has an acidic environment inside the lumen which increases from the cis (entry face) to the trans (exit face) (Anderson & Pathak, 1985); the pH in the Golgi cisternae has been estimated at 6.45 and in the trans-Golgi at 5.91–5.95 (Demaurex et al. 1998; Paroutis et al. 2004). The acidic environment inside the Golgi apparatus is maintained by the vacuolar-type H+-ATPase (V-ATPase), and is essential for post-translational modifications of proteins and disruption in pH results in improperly glycosylated and unsorted proteins (Maeda & Kinoshita, 2010).
Although the V-ATPase is electrogenic in nature and would generate a positive potential inside the Golgi (Paroutis et al. 2004), experiments making the Golgi membrane mainly permeable to K+ (with valinomycin) revealed that the Golgi membrane potential must be near zero under physiological conditions. This conclusion was reached after finding that valinomycin failed to change Golgi pH indicating that the Golgi membrane potential was already near the potential expected by clamping the potential with valinomycin (equilibrium potential, EK=−59log[K+]cytosol/[K+]Golgi=−59log140/107 =−6.9 mV) (Schapiro & Grinstein, 2000). Thus, to neutralize the membrane potential generated by the V-ATPase, H+ or K+ ions would need to flow out of the lumen or Cl− ions to flow into the lumen (Paroutis et al. 2004). In fact, several Cl− channels have been shown to be active in the Golgi apparatus (Nordeen et al. 2000; Thompson et al. 2002; Maeda et al. 2008) but so far no functional K+ channel has been identified even though K+ channels pass through the Golgi apparatus en route to the plasma membrane.
BKCa also traffics to the plasma membrane via the cis- and trans-Golgi networks where it may undergo palmitoylation/depalmitoylation cycles with palmitoylation favouring forward traffic to the plasma membrane of HEK293 cells (Tian et al. 2012). In native systems, accumulation of iBKCa in perinuclear organelles (that might include the Golgi apparatus) can be observed in myometrial cells of pregnant mouse (Eghbali et al. 2003) where the channel may be localized until it is needed at the plasma membrane or else playing an unknown physiological role. It is known that lowering pH can block BKCa channel unitary currents (Brelidze & Magleby, 2004) and this could indicate that if iBKCa channels are present in the Golgi, they should be most active at the trans-Golgi as compared to the cis-Golgi.
Role of iBKCa channels
In neurons, plasma membrane BKCa channels act as Ca2+ sensors participating in the regulation of cellular excitability and neurotransmitter release (Gribkoff et al. 2001). Similarly, we predict that iBKCa channels could also be working as Ca2+ sensors in intracellular organelles. Possibilities for iBKCa channels to get activated are either via an increase in Ca2+ ion concentration or by a positive shift in the membrane potential. Additionally and resembling plasma membrane channels, iBKCa could also be modulated by β-subunits (Piwonska et al. 2008) or G-protein-coupled receptors such as angiotensin II type 2 receptors which are present in mitochondria (Abadir et al. 2011) or the angiotensin II type 1 receptor present in nuclear membranes (Bkaily et al. 2012).
BKCa channels have a large conductance and can ideally transport ∼108 ions per second (assuming an open probability of 1, and 25 pA at 100 mV for a 250 pS channel). The electrochemical driving force for ion movement across membranes varies with intracellular organelles; according to the calculated Gibbs free energy it is high in mitochondria (see legend of Fig. 3). The K+ concentration in the nucleus is higher than in the cytoplasm but in all other organelles either it is equal (ER), or lower (mitochondria, Golgi) (Fig. 3). The flow of K+ via potassium channels like iBKCa is essential to maintain this ionic homeostasis for cellular functions. Since the opening of iBKCa can result in a significant change in [K+] within organelles, in organelles with a sizeable driving force for K+ they would be expected to be present in low abundance and/or not to fully open upon activation so organelle ionic homeostasis is not greatly disturbed during channel activation. On the other hand, if these channels were highly expressed and/or fully opened upon activation, to avoid damage they would need to be tightly modulated to bring them back to baseline or organelles would need to have alternative mechanisms to regulate their ionic homeostasis. For example in mitochondria, if BKCa were fully activated, K+ influx could depolarize its membrane potential unless the channel open probability is tightly regulated along with coupling to other ions. In fact, a recent report indicates that the opening of BKCa does not significantly modify mitochondrial membrane potential but improves mitochondria respiratory function depending on anion usage (KH2PO4 vs. KCl) (Aon et al. 2010). A more complete understanding of the direct relationship between iBKCa and the modulation of membrane potential is required. In addition, iBKCa could be playing a role as a signalling molecule. It is known that BKCa interacts directly or indirectly with other proteins which can either affect the channel activity or participate in cell signalling (Lu et al. 2006). In line with this view, several mitochondrial, nuclear, ER, Golgi, ribosomal and peroxisomal-related proteins were also reported to be interacting with BKCa (Kathiresan et al. 2009). These intracellular proteins associated with iBKCa channels may be participating in organelle signalling much like those associated with its membrane counterpart.
Figure 3. Intracellular organelles and potassium flow.
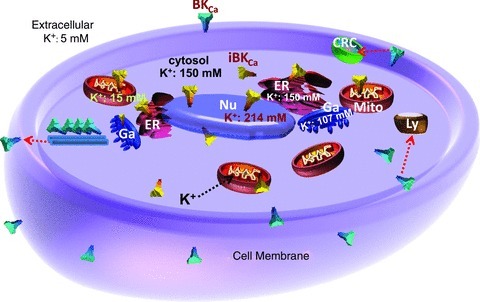
A schematic diagram depicting the major intracellular organelles with their inner K+ concentrations [K+]. [K+] inside the mitochondrion (Mito) is ∼15 mm (Zoeteweij et al. 1994), in the nucleus (Nu) is estimated as ∼214 mm (Nagy et al. 1981), and in the Golgi apparatus (Ga) is estimated as ∼107 mm (Schapiro & Grinstein, 2000). [K+] in the ER is assumed to be similar to that in the cytoplasm, in analogy to the concentrations found in toadfish (Somlyo et al. 1977). Extracellular K+ is 5 mm and cytosolic K+ is 150 mm. BKCa channels are also shown at the plasma membrane and associated with microtubules. Red arrows show translocation to clathrin reach compartments (CRC), lysosomes (Ly), and plasma membrane. Black arrow indicates the direction of K+ ion flux upon channel opening as predicted by calculating the Gibbs free energy, ΔG, for mitochondria: ΔG=ΔGK+ΔGV, where ΔGK is the free energy dependent on free K+ ions and ΔGV is the free energy dependent on the mitochondrial potential, according to: ΔGK=–RT ln([K+cytosol]/[K+matrix]) =−5609 J mol−1 where, R is the gas constant = 8.314 J (°K mol)−1, and T is the absolute temperature = 293°K at 20°C, [K+cytosol]= 150 mm, [K+matrix]= 15 mm and, ΔGV=FΔΨm=–17367 J mol−1 where, F is the Faraday constant = 96485 J (mol V)−1 and ΔΨm is the mitochondrial membrane potential, which is typically −180 mV (Kamo et al. 1979). Thus, ΔG=ΔGK+ΔGV=−22976 J mol−1 and K+ influx to the mitochondrial matrix is thermodynamically favoured. These equations can also be used to calculate ionic movements in other organelles.
Concluding remarks
iBKCa channels have been functionally and pharmacologically characterized by several independent groups. The variability in electrophysiological properties (Table 1) of iBKCa indicates that these channels are either splice variant isoforms and/or they are associated with modulatory subunits which can alter their biophysical properties. The presence of BKCa channels in the mitochondria is best established amongst the iBKCa channels. They are involved in physiological cellular functions such as cardioprotection. If the protection mechanism against ischaemic injury is via the opening of mitoBKCa, it could also serve as a promising pharmacological target for transplant medicine where various transplantable organs are continuously susceptible to ischaemic injury. The main challenge now is to define the molecular identity of these iBKCa channels, and their regulation and functional roles in distinct cell types.
Acknowledgments
This work was supported by NIH grants HL107418, HL096740 (L.T., E.S.) and HL088640 (E.S.), and an American Heart Association National Scientist Development Grant 11SDG7230059 (H.S.). The authors thank Dr Riccardo Olcese for helpful discussion.
References
- Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioua A, Li M, Wu Y, Stefani E, Toro L. Unconventional myristoylation of large-conductance Ca2+-activated K+ channel (Slo1) via serine/threonine residues regulates channel surface expression. Proc Natl Acad Sci U S A. 2011;108:10744–10749. doi: 10.1073/pnas.1008863108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RG, Pathak RK. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985;40:635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Wei AC, Grunnet M, O’Rourke B. Energetic performance is improved by specific activation of K+ fluxes through KCa channels in heart mitochondria. Biochim Biophys Acta. 2010;1797:71–80. doi: 10.1016/j.bbabio.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafpour M, Eliassi A, Sauve R, Sepehri H, Saghiri R. ATP regulation of a large conductance voltage-gated cation channel in rough endoplasmic reticulum of rat hepatocytes. Arch Biochem Biophys. 2008;471:50–56. doi: 10.1016/j.abb.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Bai JP, Surguchev A, Navaratnam D. β4-subunit increases Slo responsiveness to physiological Ca2+ concentrations and together with β1 reduces surface expression of Slo in hair cells. Am J Physiol Cell Physiol. 2011;300:C435–C446. doi: 10.1152/ajpcell.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk P, Barker GD, Halestrap AP. Determination of the rate of K+ movement through potassium channels in isolated rat heart and liver mitochondria. Biochim Biophys Acta. 2008;1777:540–548. doi: 10.1016/j.bbabio.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Bentzen BH, Andersen RW, Olesen SP, Grunnet M, Nardi A. Synthesis and characterisation of NS13558: a new important tool for addressing KCa1.1 channel function ex vivo. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:271–283. doi: 10.1007/s00210-009-0456-2. [DOI] [PubMed] [Google Scholar]
- Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M. Activation of big conductance Ca2+-activated K+ channels (BK) protects the heart against ischemia-reperfusion injury. Pflugers Arch. 2009;457:979–988. doi: 10.1007/s00424-008-0583-5. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Avedanian L, Al-Khoury J, Ahmarani L, Perreault C, Jacques D. Receptors and ionic transporters in nuclear membranes: new targets for therapeutical pharmacological interventions. Can J Physiol Pharmacol. 2012;90:953–965. doi: 10.1139/y2012-077. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Avedanian L, Jacques D. Nuclear membrane receptors and channels as targets for drug development in cardiovascular diseases. Can J Physiol Pharmacol. 2009;87:108–119. doi: 10.1139/Y08-115. [DOI] [PubMed] [Google Scholar]
- Brameier M, Krings A, MacCallum RM. NucPred–Predicting nuclear localization of proteins. Bioinformatics. 2007;23:1159–1160. doi: 10.1093/bioinformatics/btm066. [DOI] [PubMed] [Google Scholar]
- Brelidze TI, Magleby KL. Protons block BK channels by competitive inhibition with K+ and contribute to the limits of unitary currents at high voltages. J Gen Physiol. 2004;123:305–319. doi: 10.1085/jgp.200308951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding ‘maxi’ calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Chacko AD, Liberante F, Paul I, Longley DB, Fennell DA. Voltage dependent anion channel-1 regulates death receptor mediated apoptosis by enabling cleavage of caspase-8. BMC Cancer. 2010;10:380. doi: 10.1186/1471-2407-10-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jeffries O, Rowe IC, Liang Z, Knaus HG, Ruth P, Shipston MJ. Membrane trafficking of large conductance calcium-activated potassium channels is regulated by alternative splicing of a transplantable, acidic trafficking motif in the RCK1-RCK2 linker. J Biol Chem. 2010;285:23265–23275. doi: 10.1074/jbc.M110.139758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Tian L, MacDonald SH, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) α–subunits generated from a single site of splicing. J Biol Chem. 2005;280:33599–33609. doi: 10.1074/jbc.M505383200. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Gu XQ, Bednarczyk P, Wiedemann FR, Haddad GG, Siemen D. Hypoxia increases activity of the BK-channel in the inner mitochondrial membrane and reduces activity of the permeability transition pore. Cell Physiol Biochem. 2008;22:127–136. doi: 10.1159/000149790. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Gulbins E, Siemen D. Activation of the permeability transition pore by Bax via inhibition of the mitochondrial BK channel. Cell Physiol Biochem. 2011;27:191–200. doi: 10.1159/000327944. [DOI] [PubMed] [Google Scholar]
- Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241:779–786. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Furuya W, D'Souza S, Bonifacino JS, Grinstein S. Mechanism of acidification of the trans-Golgi network (TGN). In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J Biol Chem. 1998;273:2044–2051. doi: 10.1074/jbc.273.4.2044. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Lai JC, Bian S, Cummins L, Moczydlowski E, Haddad GG. The calcium-sensitive large-conductance potassium channel (BK/MAXI K) is present in the inner mitochondrial membrane of rat brain. Neuroscience. 2006;139:1249–1261. doi: 10.1016/j.neuroscience.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Toro L, Stefani E. Diminished surface clustering and increased perinuclear accumulation of large conductance Ca2+-activated K+ channel in mouse myometrium with pregnancy. J Biol Chem. 2003;278:45311–45317. doi: 10.1074/jbc.M306564200. [DOI] [PubMed] [Google Scholar]
- Fahanik-Babaei J, Eliassi A, Jafari A, Sauve R, Salari S, Saghiri R. Electro-pharmacological profile of a mitochondrial inner membrane big-potassium channel from rat brain. Biochim Biophys Acta. 2011a;1808:454–460. doi: 10.1016/j.bbamem.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Fahanik-Babaei J, Eliassi A, Saghiri R. How many types of large conductance Ca2+-activated potassium channels exist in brain mitochondrial inner membrane: evidence for a new mitochondrial large conductance Ca2+-activated potassium channel in brain mitochondria. Neuroscience. 2011b;199:125–132. doi: 10.1016/j.neuroscience.2011.09.055. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhang SZ, Cao CM, Bruce IC, Xia Q. The mitochondrial permeability transition pore and the Ca2+-activated K+ channel contribute to the cardioprotection conferred by tumor necrosis factor-α. Cytokine. 2005;32:199–205. doi: 10.1016/j.cyto.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Jr, Dumont I, Marrache AM, Vazquez-Tello A, Bernier SG, Abran D, Hou X, Beauchamp MH, Quiniou C, Bouayad A, Choufani S, Bhattacharya M, Molotchnikoff S, Ribeiro-Da-Silva A, Varma DR, Bkaily G, Chemtob S. Regulation of eNOS expression in brain endothelial cells by perinuclear EP3 receptors. Circ Res. 2002;90:682–689. doi: 10.1161/01.res.0000013303.17964.7a. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Starrett JE, Jr, Dworetzky SI. Maxi-K potassium channels: form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist. 2001;7:166–177. doi: 10.1177/107385840100700211. [DOI] [PubMed] [Google Scholar]
- Gu XQ, Siemen D, Parvez S, Cheng Y, Xue J, Zhou D, Sun X, Jonas EA, Haddad GG. Hypoxia increases BK channel activity in the inner mitochondrial membrane. Biochem Biophys Res Commun. 2007;358:311–316. doi: 10.1016/j.bbrc.2007.04.110. [DOI] [PubMed] [Google Scholar]
- Hawkins J, Davis L, Boden M. Predicting nuclear localization. J Proteome Res. 2007;6:1402–1409. doi: 10.1021/pr060564n. [DOI] [PubMed] [Google Scholar]
- Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG, Camara AK. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2007;293:H1400–H1407. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- Hu X, Laragione T, Sun L, Koshy S, Jones KR, Ismailov II, Yotnda P, Horrigan FT, Gulko PS, Beeton C. KCa1.1 potassium channels regulate key proinflammatory and invasive properties of fibroblast-like synoviocytes in rheumatoid arthritis. J Biol Chem. 2012;287:4014–4022. doi: 10.1074/jbc.M111.312264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries O, Geiger N, Rowe IC, Tian L, McClafferty H, Chen L, Bi D, Knaus HG, Ruth P, Shipston MJ. Palmitoylation of the S0-S1 linker regulates cell surface expression of voltage- and calcium-activated potassium (BK) channels. J Biol Chem. 2010;285:33307–33314. doi: 10.1074/jbc.M110.153940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo N, Muratsugu M, Hongoh R, Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979;49:105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Kathiresan T, Harvey M, Orchard S, Sakai Y, Sokolowski B. A protein interaction network for the large conductance Ca2+-activated K+ channel in the mouse cochlea. Mol Cell Proteomics. 2009;8:1972–1987. doi: 10.1074/mcp.M800495-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of β-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- Ko JH, Ibrahim MA, Park WS, Ko EA, Kim N, Warda M, Lim I, Bang H, Han J. Cloning of large-conductance Ca2+-activated K+ channel α-subunits in mouse cardiomyocytes. Biochem Biophys Res Commun. 2009;389:74–79. doi: 10.1016/j.bbrc.2009.08.087. [DOI] [PubMed] [Google Scholar]
- Kulawiak B, Kudin AP, Szewczyk A, Kunz WS. BK channel openers inhibit ROS production of isolated rat brain mitochondria. Exp Neurol. 2008;212:543–547. doi: 10.1016/j.expneurol.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Guggino WB. Multiple sequences in the C terminus of MaxiK channels are involved in expression, movement to the cell surface, and apical localization. Proc Natl Acad Sci U S A. 2004;101:15237–15242. doi: 10.1073/pnas.0404877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol. 2006;570:65–72. doi: 10.1113/jphysiol.2005.098913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett. 2007;581:1000–1008. doi: 10.1016/j.febslet.2007.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Ide T, Koike M, Uchiyama Y, Kinoshita T. GPHR is a novel anion channel critical for acidification and functions of the Golgi apparatus. Nat Cell Biol. 2008;10:1135–1145. doi: 10.1038/ncb1773. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Kinoshita T. The acidic environment of the Golgi is critical for glycosylation and transport. Methods Enzymol. 2010;480:495–510. doi: 10.1016/S0076-6879(10)80022-9. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Shimada H, Taniguchi J. Ca2+-activated K+-channels in the nuclear envelope isolated from single pancreatic acinar cells. Pflugers Arch. 1995;430:148–150. doi: 10.1007/BF00373851. [DOI] [PubMed] [Google Scholar]
- Mazzanti M, DeFelice LJ, Cohn J, Malter H. Ion channels in the nuclear envelope. Nature. 1990;343:764–767. doi: 10.1038/343764a0. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between α (hslo) and β subunits (KV,Caβ) of maxi K channels. FEBS Lett. 1996;382:84–88. doi: 10.1016/0014-5793(96)00151-2. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal β subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy IZ, Lustyik G, Nagy VZ, Zarandi B, Bertoni-Freddari C. Intracellular Na+:K+ ratios in human cancer cells as revealed by energy dispersive x-ray microanalysis. J Cell Biol. 1981;90:769–777. doi: 10.1083/jcb.90.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Sato T, Miyazaki M, Nakaya H. Infarct size limitation by adrenomedullin: protein kinase A but not PI3-kinase is linked to mitochondrial KCa channels. Cardiovasc Res. 2008;77:398–405. doi: 10.1016/j.cardiores.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Nordeen MH, Jones SM, Howell KE, Caldwell JH. GOLAC: an endogenous anion channel of the Golgi complex. Biophys J. 2000;78:2918–2928. doi: 10.1016/S0006-3495(00)76832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya S, Kuwata Y, Sakamoto K, Muraki K, Imaizumi Y. Cardioprotective effects of estradiol include the activation of large-conductance Ca2+-activated K+ channels in cardiac mitochondria. Am J Physiol Heart Circ Physiol. 2005;289:H1635–H1642. doi: 10.1152/ajpheart.00016.2005. [DOI] [PubMed] [Google Scholar]
- O’Rourke B. Mitochondrial ion channels. Annu Rev Physiol. 2007;69:19–49. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman AA, Saito M, Makepeace C, Permutt MA, Schlesinger P, Mueckler M. Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. J Biol Chem. 2003;278:52755–52762. doi: 10.1074/jbc.M310331200. [DOI] [PubMed] [Google Scholar]
- Ou JW, Kumar Y, Alioua A, Sailer C, Stefani E, Toro L. Ca2+- and thromboxane-dependent distribution of MaxiK channels in cultured astrocytes: from microtubules to the plasma membrane. Glia. 2009;57:1280–1295. doi: 10.1002/glia.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroutis P, Touret N, Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 2004;19:207–215. doi: 10.1152/physiol.00005.2004. [DOI] [PubMed] [Google Scholar]
- Piwonska M, Wilczek E, Szewczyk A, Wilczynski GM. Differential distribution of Ca2+-activated potassium channel β4 subunit in rat brain: immunolocalization in neuronal mitochondria. Neuroscience. 2008;153:446–460. doi: 10.1016/j.neuroscience.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Redel A, Lange M, Jazbutyte V, Lotz C, Smul TM, Roewer N, Kehl F. Activation of mitochondrial large-conductance calcium-activated K+ channels via protein kinase A mediates desflurane-induced preconditioning. Anesth Analg. 2008;106:384–391. doi: 10.1213/ane.0b013e318160650f. [DOI] [PubMed] [Google Scholar]
- Schapiro FB, Grinstein S. Determinants of the pH of the Golgi complex. J Biol Chem. 2000;275:21025–21032. doi: 10.1074/jbc.M002386200. [DOI] [PubMed] [Google Scholar]
- Shi Y, Jiang MT, Su J, Hutchins W, Konorev E, Baker JE. Mitochondrial big conductance KCa channel and cardioprotection in infant rabbit heart. J Cardiovasc Pharmacol. 2007;50:497–502. doi: 10.1097/FJC.0b013e318137991d. [DOI] [PubMed] [Google Scholar]
- Shruti S, Urban-Ciecko J, Fitzpatrick JA, Brenner R, Bruchez MP, Barth AL. The brain-specific beta4 subunit downregulates BK channel cell surface expression. PLoS One. 2012;7:e33429. doi: 10.1371/journal.pone.0033429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem Biophys Res Commun. 1999;257:549–554. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- Singh H. Two decades with dimorphic Chloride Intracellular Channels (CLICs) FEBS Lett. 2010;584:2112–2121. doi: 10.1016/j.febslet.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Skalska J, Bednarczyk P, Piwonska M, Kulawiak B, Wilczynski G, Dolowy K, Kudin AP, Kunz WS, Szewczyk A. Calcium ions regulate K+ uptake into brain mitochondria: The evidence for a novel potassium channel. Int J Mol Sci. 2009;10:1104–1120. doi: 10.3390/ijms10031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AV, Shuman H, Somlyo AP. Composition of sarcoplasmic reticulum in situ by electron probe X-ray microanalysis. Nature. 1977;268:556–558. doi: 10.1038/268556a0. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol. 2006;290:H434–H440. doi: 10.1152/ajpheart.00763.2005. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Nordeen MH, Howell KE, Caldwell JH. A large-conductance anion channel of the Golgi complex. Biophys J. 2002;83:278–289. doi: 10.1016/S0006-3495(02)75168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, McClafferty H, Knaus HG, Ruth P, Shipston MJ. Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium (BK) channels. J Biol Chem. 2012;287:14718–14725. doi: 10.1074/jbc.M111.335547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro B, Cox N, Wilson RJ, Garrido-Sanabria E, Stefani E, Toro L, Zarei MM. KCNMB1 regulates surface expression of a voltage and Ca2+-activated K+ channel via endocytic trafficking signals. Neuroscience. 2006;142:661–669. doi: 10.1016/j.neuroscience.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, Austin CP, Bennett PB, Swanson R. Cloning and functional expression of two families of β-subunits of the large conductance calcium-activated K+ channel. J Biol Chem. 2000;275:23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- Vaithianathan T, Bukiya A, Liu J, Liu P, Suncion-Chin M, Fan Z, Dopico A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol. 2008;132:13–28. doi: 10.1085/jgp.200709913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Meera P, Ottolia M, Kaczorowski GJ, Latorre R, Garcia ML, Stefani E, Toro L. Characterization of and modulation by a beta-subunit of a human maxi KCa channel cloned from myometrium. Receptors Channels. 1995;3:185–199. [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane β-subunit homolog. Proc Natl Acad Sci U S A. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yin C, Xi L, Kukreja RC. Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2070–H2077. doi: 10.1152/ajpheart.00431.2004. [DOI] [PubMed] [Google Scholar]
- Wojtovich AP, Sherman TA, Nadtochiy SM, Urciuoli WR, Brookes PS, Nehrke K. SLO-2 is cytoprotective and contributes to mitochondrial potassium transport. PLoS One. 2011;6:e28287. doi: 10.1371/journal.pone.0028287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Sugioka M, Ogawa Y. Voltage- and Ca2+-activated potassium channels in Ca2+ store control Ca2+ release. FEBS J. 2006;273:3585–3597. doi: 10.1111/j.1742-4658.2006.05365.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 2010;466:513–516. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- Yan J, Aldrich RW. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc Natl Acad Sci U S A. 2012;109:7917–7922. doi: 10.1073/pnas.1205435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Pico AR, Hsiung Y, MacKinnon R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 Å resolution. Science. 2010;329:182–186. doi: 10.1126/science.1190414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MM, Eghbali M, Alioua A, Song M, Knaus HG, Stefani E, Toro L. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proc Natl Acad Sci U S A. 2004;101:10072–10077. doi: 10.1073/pnas.0302919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MM, Song M, Wilson RJ, Cox N, Colom LV, Knaus HG, Stefani E, Toro L. Endocytic trafficking signals in KCNMB2 regulate surface expression of a large conductance voltage and Ca2+-activated K+ channel. Neuroscience. 2007;147:80–89. doi: 10.1016/j.neuroscience.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Zarei MM, Zhu N, Alioua A, Eghbali M, Stefani E, Toro L. A novel MaxiK splice variant exhibits dominant-negative properties for surface expression. J Biol Chem. 2001;276:16232–16239. doi: 10.1074/jbc.M008852200. [DOI] [PubMed] [Google Scholar]
- Zoeteweij JP, van de WB, de Bont HJ, Nagelkerke JF. Mitochondrial K+ as modulator of Ca2+-dependent cytotoxicity in hepatocytes. Novel application of the K+-sensitive dye PBFI (K+-binding benzofuran isophthalate) to assess free mitochondrial K+ concentrations. Biochem J. 1994;299:539–543. doi: 10.1042/bj2990539. [DOI] [PMC free article] [PubMed] [Google Scholar]


