Abstract
Dystonia is a movement disorder characterized by repetitive twisting muscle contractions and postures1,2. Its molecular pathophysiology is poorly understood, in part due to limited knowledge of the genetic basis of the disorder. Only three genes for primary torsion dystonia (PTD), TOR1A (DYT1)3, THAP1 (DYT6)4, and CIZ15 have been identified. Using exome sequencing in two PTD families we identified a novel causative gene, GNAL, with a nonsense p.S293X mutation resulting in premature stop codon in one family and a missense p.V137M mutation in the other. Screening of GNAL in 39 PTD families, revealed six additional novel mutations in this gene. Impaired function of several of the mutations was shown by bioluminescence resonance energy transfer (BRET) assays.
PTD is a subgroup of dystonia that was originally considered idiopathic. It is defined by the presence of dystonia (with or without tremor) as the only neurologic sign, as well as the absence of historical, imaging or laboratory findings suggesting an acquired cause or non-primary form of dystonia (e.g. Wilsons ’s disease)6. PTD is clinically and causally heterogeneous with a significant genetic component7. Analyses of clinical subgroups i.e. focal adult and early-onset generalized, are consistent with autosomal dominant inheritance8–10, but with reduced penetrance, ranging from 15% for focal PTD to 30–60% in generalized PTD10–12. The incomplete penetrance, together with the genetic and clinical heterogeneity complicates the identification of PTD genes by traditional linkage analysis. As an alternative, we employed exome sequencing to systematically search for causative genes for PTD.
We performed exome sequencing in individuals from two families with PTD (Supplementary Fig. 1, indicated by asterisks). Clinical features of the affected individuals in these families are summarized in Table 1 and Supplementary Table 1 (Fams D1 and P)13–15. Exome sequencing produced about 50 million paired reads per sample, more than 97% of which were mapped to the genome. The average coverage was 52X with more than 80% of target bases covered at 20X. The reads were mapped to the human reference genome sequence (assembly GRCh37/hg19) and allelic variants were detected. About 60,000 variants were called per individual (Supplementary Table 2). Since dystonia is rare and inherited in an AD manner in these pedigrees, the causative mutation is expected to be an extremely rare heterozygous variant, shared by all affected family members. Comparing sequenced family members, we found 11,124 heterozygous shared variants in Fam P and 4,578 in Fam D1. These variants were further compared to dbSNP release132 with 458 and 208 novel variants identified, respectively. After annotation by both SIFT16 and SeattleSeq Annotation servers, 68 missense and 1 nonsense variants remained in Fam P while 20 missense variants were defined in Fam D1 (Supplementary Table 2). Identification of insertion/deletion variants were performed in a similar fashion for Fam P only (see Materials and Methods and Supplemenary Table 3).
Table 1.
Clinical Characteristics of 28 GNAL Patients from 8 Families
| Gender | Age onset (yrs) | Age exam (yrs) | Dystonia distribution | Site of onset | Allele variant$ | Protein variant$ | |
|---|---|---|---|---|---|---|---|
| FAMILY D1* |
c.409G>A V137M |
p.V137M | |||||
| 1 | M | 31 | 75 | S | Neck | ||
| 2 | M | 44 | 63 | S | Neck,Larynx,Trunk | ||
| 3 | F | 26 | 62 | S | Neck | ||
| 4^ | M | 7 | 69 | G | Legs | ||
| 5 | F | 50 | 60 | S | Neck | ||
| 6 | F | 22 | 49 | S | Neck | ||
| 7 | F | 19 | 51 | S | Neck, Face | ||
| FAMILY P * | c.878C>A | p.S293X | |||||
| 1 | F | 48 | 68 | F | Neck | ||
| 2 | M | 35 | 38 | G | Neck | ||
| 3 | M | 47 | 48 | F | Neck | ||
| 4^ | F | 25 | 47 | G | Leg | ||
| 5 | M | 32 | 44 | S | Neck | ||
| 6 | M | 35 | 41 | F | Neck | ||
| FAMILY S* | c.463G>A | p.E155K | |||||
| 1^ | M | 18 | 38 | F | Neck | ||
| 2 | M | 17 | 38 | S | Neck | ||
| FAMILY D2 | c.283-284insT | p.S95fsX110 | |||||
| 1 | F | 33 | 46 | S | Neck | ||
| 2^ | F | 39 | 44 | S | Jaw | ||
| 3 | F | 18 | 31 | S | Neck | ||
| 4 | F | 11 | 19 | F | Tongue | ||
| FAMILY H | c.591-592insA | p.R198fsX210 | |||||
| 1 | M | 47 | 72 | F | Neck | ||
| 2^ | F | 38 | 39 | F | Neck | ||
| 3 | F | 31 | 41 | F | Neck | ||
| FAMILY B | g.chr18:11,753,820# | ||||||
| 1 | M | 54 | 59 | F | Neck | c.274-5T>C | |
| 2^ | F | 36 | 50 | S | Neck | ||
| FAMILY T | c.61C>T | p.R21X | |||||
| 1^ | M | 25 | 45 | F | Neck | ||
| 2 | M | 42 | 54 | F | Neck | ||
| 3 | F | 25 | 33 | S | Larynx | ||
| FAMILY N | c.304-312delCCTCCAGTT | p.P102-V104del | |||||
| 1^ | M | 20 | 56 | F | Neck | ||
Gender: F – female, M – male; Dystonia distribution: F – focal, S – segmental, M – multifocal, G – generalized
Denoted probands
reported previously and updated here: Fam D1 in13,14,58, Fam P in14 and15, Fam S in59. The following are the corresponding identifiers from13 for the individuals in D1: 1=individual 207, 2=302, 3=307, 4=304, 5=317, 6=315, 7=403.
numbering based on NM_001142339 and NP_001135811
splice mutation, genomic position from hg19 assembly
Fam P was previously subjected to a whole genome scan that identified 3 regions of potential linkage, with LOD scores ranging from 1.5 to 2.1 (data not shown). Within these regions, we found 7 novel coding variants: 4 single nucleotide substitutions and 3 insertion/deletions that were shared by all three affected individuals of Fam P. Among these, the p.S293X variant in the GNAL gene co-segregated with dystonia in the remaining members of Fam P (Supplementary Fig. 1). Furthermore, an additional variant in this gene, p.V137M, was found among the 20 missense variants shared by all 4 members in Fam D1; it segregated with the disease in this family (Supplementary Fig. 1) and was not observed in 572 control chromosomes we tested. In addition, neither p.S293X nor p.V137M variant was found in ~3,500 European exomes in the NHLBI Exome Sequencing Project database.
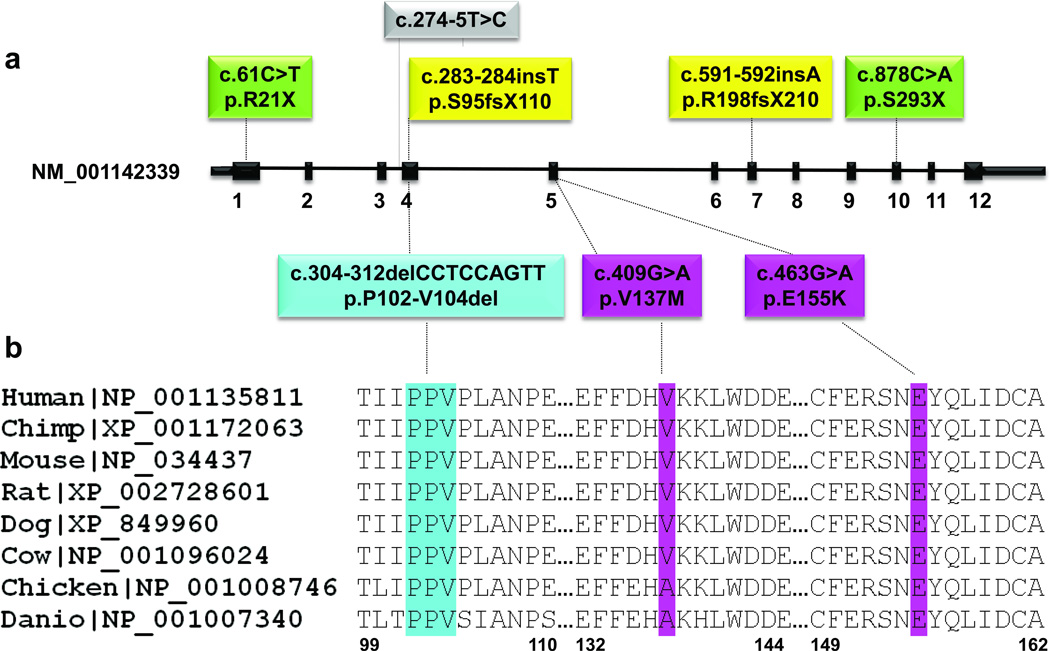
To confirm GNAL as a causative gene for dystonia, we performed Sanger sequencing of the entire coding region in 39 additional multiplex PTD families of mixed European origin who were mutation negative for DYT1 and DYT6. We detected novel mutations in the GNAL gene in six additional families (19%), including one nonsense (p.R21X), two frameshift (p.S95fsX110 and p.R198fsX210), one missense (p.E155K), one in-frame deletion of 3 amino acids (p.P102-V104del) and one possible splice site mutation at position chr18:11,753,820 (hg19) (c.274-5T>C) upstream of exon 4 of GNAL (Fig. 1a, Supplementary Fig 1, and Table 1). Bioinformatics analysis enumerating all possible splice sites in the human genome suggests that the wild type sequence is 10 times more likely to be found near an acceptor splice site than the mutant sequence17. However, we were unable to prove this hypothesis in patient samples, as lymphoblasts are the only tissue available, and GNAL expression is undetectable by semi-quantitative PCR and western blot analysis (data not shown). None of the variants were detected in dbSNP135, ~3,500 European exomes or 572 control chromosomes that we tested. Segregation analyses confirmed transmission of the GNAL mutations with the disease phenotype in all families for which DNA from multiple affected individuals was available (Supplementary Fig. 1). The p.V137 and p.E155 residues were completely conserved in GNAL orthologs (Fig. 1b). In addition to the coding variants in dystonia patients, we found one novel synonymous variant, p.E18E, and one novel non-synonymous substitution, p.V16F, each in a single control subject and not in any database.
Figure 1.
Mutations identified in GNAL in PTD patients. a. Schematic representation of exon:intron structure of the short isoform of GNAL (NM_001142339) with mutations indicated. Missense mutations are depicted in pink, in-frame deletion is in blue, nonsense mutations are in green, frame-shift mutations are in yellow and the tentative splice mutation is in grey. b. Protein sequence alignment of Gαolf orthologs from vertebrate species. Protein sequences were obtained from RefSeq database and aligned using ClustalW57. The regions of alignment corresponding to the in-frame deletion and missense mutations are shown. The mutations are colored as in panel a. RefSeq accession numbers are indicated.
Among the 8 families with mutations there were 28 individuals with complete clinical information who had definite dystonia (Table 1 and Supplementary Table 1). Average age of dystonia onset in the carriers was 31.3, and ranged from 7–54 years. Most carriers (82%) had onset in the neck, and 93% had neck involvement at final exam, however most progressed to have dystonia at other sites, and only 46% had focal dystonia at last exam. Further, cranial involvement was present in 57% of carriers, and 44% had speech involvement. Brachial onset was not observed and eventual arm involvement was seen in only 32%, distinguishing GNAL from DYT6 (Supplementary Table 4)14,18. All carriers were Caucasian of mixed European ancestry. Because it has been suggested that GNAL may be imprinted 19 we examined the parental origin of the mutations. Dystonia was inherited from maternal and paternal sides equally, and there were no apparent phenotypic differences between maternally and paternally inherited cases. However, further study is required to fully determine whether parental origin of the mutation affects penetrance or expression. In addition, no genotype/phenotype correlation could be discerned with regard to mutation type and, as seen in Table 1 and Supplementary Table 1, phenotypes varied within families with the same mutation.
GNAL is located on chromosome 18p centromeric to the DYT7 locus for focal dystonia20 and the DYT15 locus for myoclonus dystonia21,22. Dystonia occurs in patients with 18p deletions23–28, and absence of GNAL may be contributing to dystonia in these cases. GNAL encodes the stimulatory alpha subunit, Gαolf, first identified as a G-protein (guanine nucleotide-binding proteins) mediating odorant signaling in the olfactory epithelium29. G-proteins link seven transmembrane domain receptors to downstream effector molecules and function as heterotrimers composed of alpha, beta and gamma subunits30. The predominant stimulatory G protein subunit in the brain is Gαs, but Gαolf replaces Gαs in the striatal medium spiny neurons (MSNs)31,32. In MSNs, Gαolf couples dopamine type 1 receptors (D1R) of the direct pathway and adenosine A2A receptors (A2AR) of the indirect pathway to the activation of adenylyl cyclase type 5 (AC5)33–35. Relevant to dystonia, A2AR and Gαolf are also expressed in the striatal cholinergic interneurons (reviewed in36).
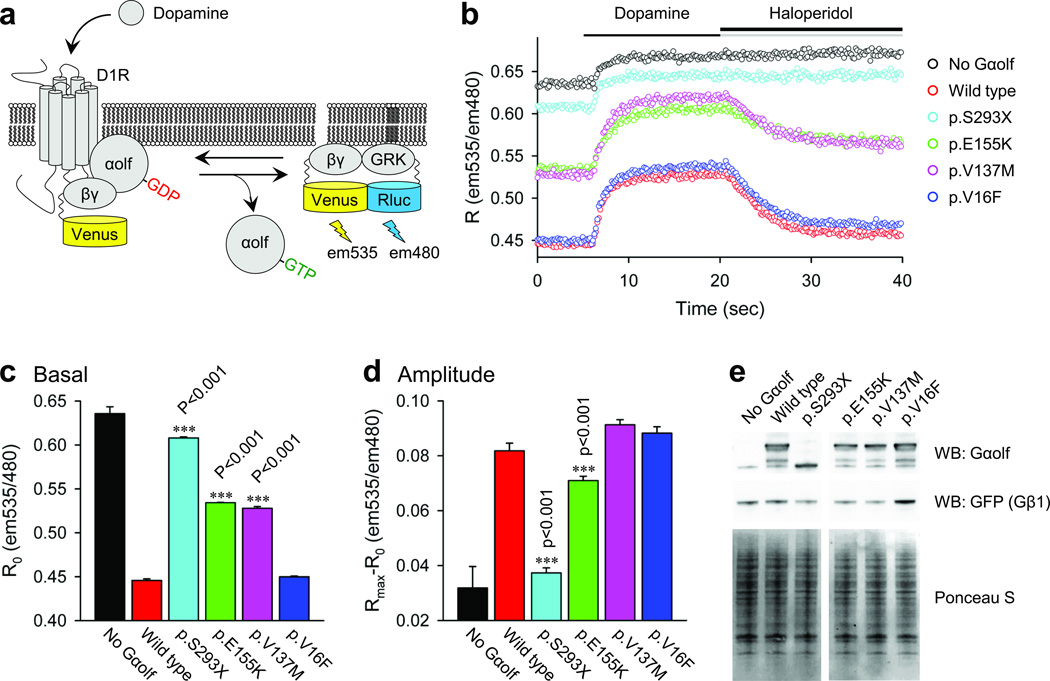
Gαolf shares 80% amino acid identity with Gαs29, and based on the crystal structure of Gαs37, Gαolf is predicted to harbor, a Ras-like domain (RD) that mediates GTP binding and hydrolysis and a helical domain (HD) that interfaces with the RD and stabilizes nucleotide binding. We thus predicted that early truncating mutations would remove essential functional regions of Gαolf resulting in a loss-of-function phenotype (p.R21X, p.S95fsX110 and p.R198fsX210). In addition, p.R21X is likely degraded by nonsense mediated decay thus producing no protein. To investigate the impact of small deletions and more subtle missense mutations (Fig. 2) we used a cell-based BRET reporter system in which Gαolf function is assessed by its ability to interact with Gβγ subunits during the receptor initiated cycle of nucleotide binding and hydrolysis. Introduction of wild type Gαolf resulted in significant reduction of the Gβγ interaction with the effector-based reporter, indicating efficient formation of the Gαolf-Gβγ heterotrimer. Stimulation of the D1R resulted in rapid increase in the BRET signal reflecting Gαolf activation and resultant release of the Gβγ subunits. Conversely, inactivation of D1R rapidly brought the signal to the baseline due to GTP hydrolysis and heterotrimer re-association. The p.S293X deletion mutant failed to support any D1R driven responses while p.V16F, a variant found in a single control sample showed normal wild-type like behavior. Two mutants found in dystonia patients, p.E155K and p.V137M, showed intermediate phenotypes largely consistent with impaired association with the Gβγ subunits (Fig. 2).
Figure 2.
Effect of mutations on Gαolf coupling to D1R. a. Schematics of the assay design. Stimulation of the D1R by dopamine results in the dissociation of Gαolf from the heterotrimer. Released Gβγ subunits tagged with Venus become available for the interaction with Rluc8-tagged GRK reporter producing the BRET signal which is determined by the change in the emission ratio at wavelengths 535nm and 480nm. b. Time course of the BRET signal change upon stimulation of cells with dopamine and subsequent deactivation by haloperidol. c. Basal BRET ratios calculated before the application of dopamine that reflect the extent of the Gαolf-Gβγ heterotrimer formation. d. Changes in the BRET ratio from basal signal to maximal response that reflect the amplitude of the response. e. Analysis of the expression levels of Gαolf and Gβγ (detected by anti-GFP antibodies) subunits by Western blotting. Ponceau S staining of total cell lysates was used as a loading control. Results represent the mean of quadruplicate wells from a typical experiment. Similar results were seen in two independent experiments. Error bars represent the standard error of the mean. One way ANOVA followed by the Holm–Sidak method were performed to determine statistically significant differences. Asterisks indicate statistical significance from wild type control: ***, p < 0.001. The Gαolf vectors were based on transcript NM_001142339.
Although dysfunction of the basal ganglia is not the exclusive etiology of dystonia, abundant evidence supports the involvement of its many circuits, including dopamine pathways38–42. Further, in the basal ganglia, an imbalance between the indirect (dopamine type 2 receptors, D2R) and direct (D1R) pathways has been hypothesized, initiated by primary abnormalities of either the D2R42 or cholinergic systems43. Identification of causative mutations in GNAL points to primary abnormalities of D1R and/or A2AR transmission as possibly leading to dystonia.
The level of Gαolf is rate-limiting in the activation of AC5 following stimulation of the D1R36,44. Heterozygote GNAL-null mice demonstrate a muted response to acute psychostimulant and caffeine exposure44. Homozygote null mice are anosmic, hyperactive at baseline and fail to respond to acute psychostimulant exposure, but do not manifest an overt movement disorder 44–46. As a rate-limiting mediator of the D1R signal transduction system, Gαolf has been physiologically linked to levodopa-induced dyskinesias (LID) 47,48 a debilitating disorder arising from chronic administration of L-DOPA in patients with Parkinson’s disease (reviewed in49). We have previously posited compensatory alterations in the D1R signal transduction system in transgenic mice overexpressing mutant torsinA in dopaminergic neurons, and suggested a shared molecular abnormality between dystonia and LID based on the DA release deficit in mice expressing the DYT1 mutation41,50. Within the striatum, Gαolf is enriched in the striosomal compartment51, and Crittenden and Greybiel hypothesize that an imbalance in striosomal activity, relative to the matrix compartment, is an etiological factor in the development of hyperkinetic movement disorders 52. Genetically, Gαolf has been associated with hyperactivity in attention deficit hyperactivity disorder and schizophrenia, although mutations have not been identified in patients with these disorders 53–55.
In conclusion, we have identified eight different mutations in GNAL, a novel causative primary dystonia gene. The phenotype in the 8 multiplex families is predominantly one of cervical dystonia, with a relatively broad range in age onset and spread to other muscles, especially the facial muscles, in over one half of subjects. Refinement of this phenotype as well as the role of GNAL in sporadic PTD awaits further screening in both familial and sporadic cohorts. A functional assay testing several of the mutations is suggestive of a loss of function. Along with mutations in the tyrosine hydroxylase (TH) biosynthetic pathway in dopa-responsive dystonia (DRD)56, GNAL directly points to the dopamine and/or adenosine signal transduction pathways as the origin of dystonia pathophysiology. However, unlike the DRD mutations, GNAL mutations place the primary abnormality post-synaptically in striatal dopaminoceptive neurons and/or cholinergic interneurons.
Materials and Methods
Patients
Families were recruited through advertisement or referral from movement disorder physicians. We included only individuals from families having three or more affected individuals by exam, medical record or reliable history, and for whom the proband did not carry a TOR1A or THAP1 mutation. One family (family P) with mixed Russian and Swiss Mennonite heritage was also screened for a founder mutation in A-T15. Three families, D1, P, and S, have been previously reported13–15,58,59. All study subjects gave informed consent prior to participation, and the study was approved by all institutional review boards. Videotaped examinations and determination of affected status was undertaken as previously published11. Human DNA control samples from European Caucasian blood donors (n=376) were purchased from Sigma-Aldrich.
Exome Sequencing, variant calling and analysis
Genomic DNA was extracted from white blood cells using the Purgene procedure (Gentra Systems Inc). Both exome capture and sequencing were performed by Perkin Elmer. Briefly, the Agilent SureSelect Human All Exon 50 MB library was used for exome capture as described by the manufacturer. The exome library was sequenced using Illumina HiSeq 2000 paired end module. The reads were mapped to the human reference genome sequence (assembly GRCh37/hg19) using Burrows-Wheeler Alignment Tool (BWA) version 0.5.8c60 and allelic variants were detected using the Genome Analysis Toolkit (GATK)61. GATK was also used for base-quality recalibration, local sequence realignment and variant filtering to minimize base calling and mapping errors. Allelic variants were annotated using SIFT 16 and SeattleSeq Annotation tools. Allelic/indel variant comparison between samples, comparison to the SNP databases and variant selection was done using in-house Perl scripts. NCBI dbSNP (versions 132 to 135) and exome variant database were utilized for novel variants selection. The called indels in Family P were compared among three affected individuals (Supplementary Table 3) and shared variants were selected. The shared indels residing in linkage regions were selected and annotated using SNPnexus62. Since we found a missense mutation in GNAL as a dystonia cause in Family D1, no analysis of indels was performed in this family.
PCR amplification and sequencing
Sanger sequencing was performed to confirm variants found by exome sequencing and to search for additional variants in the GNAL gene. Intron based, exon-specific primers were designed from the UCSC human genome assembly sequence (hg19) using Integrated DNA Technologies Primer Quest online server which is derived from Primer3 software (release 0.9)63. Primers were designed to cover the following GNAL transcripts, NM_001142339, NM_001261443 and NM_182978. Standard PCR amplification was performed using primers in Supplementary Table 5. The amplified fragments were cleaned and sequenced as described4.
Analysis of Gαolf cycle by BRET
Gαolf function in living cells was analyzed by monitoring the kinetics of its association and dissociation with Gβ1γ2 subunits following activation of D1R by agonist. The assay measures agonist-dependent changes in bioluminescence resonance energy transfer (BRET) between Gβ1γ2 tagged with Venus and its effector GRK3 tagged with Rluc8 and was conducted as previously described64,65. Briefly, N-terminal 3xHA-tagged dopamine D1 receptor, EE-tagged Gαolf, Venus155–239-Gβ1, Venus1-155-Gγ2, masGRKct-Rluc8, and Flag-tagged Ric-8B constructs were transfected into HEK293 cells at a 1:6:1:1:1:2 ratio with 5 ug total DNA delivered per 4 × 106 cells. 16 h post transfection cells were stimulated with 100 uM dopamine followed by treatment with 100 uM haloperidol that has reported 63 nM Kd for D1 receptor66. The EE-tagged Gαolf vectors were based on transcript NM_001142339.
Immunoblotting
Proteins resolved by SDS-PAGE were transferred to nitrocellulose membranes (GE Healthcare). Ponceau S staining of blots after transfer revealed equivalent loading of total protein, which was later demonstrated by blotting with antibody to actin. Membranes were blocked with 5% nonfat dry milk diluted in Tris-buffered saline-0.2% Tween and incubated successively with the primary antibodies (anti body to GNAL, 1:2000; antibody to GFP, 1:2,000; antibody to actin, 1:2500 in blocking buffer) overnight at 4 °C and horseradish peroxidase-conjugated secondary antibodies (1:3,000 in block buffer) for 1 h at room temperature. Proteins were visualized using ECL-Plus (GE Healthcare). Secondary antibodies were purchased from GE Healthcare. Rabbit polyclonal antibody to GNAL (146/49) was provide by Dr. Herve and purified as described previously35. Rabbit polyclonal antibodies to GFP (ab290) and beta-actin (ab8227) were obtained from Abcam.
Supplementary Material
Acknowledgements
We wish to thank all patients and family members who participated in this study. We are indebted to the following physicians for referring dystonia families included in this manuscript: S. Reich, J. Hammerstadt, D. Hobson, D. Truong, F. Danisi, M. Hutchinson, S. O'Riordan, T. Lynch, J. Rogers. We would also like to thank the following physicians who examined study subjects during their movement disorder fellowships: R. Tabamo, E. Chai, K. Blatt, P. Kavanagh, P. Kapoor, G. Petzinger. We thank R. Sachidanandam for the bioinformatic analysis related to the splice mutation; H. Lederman for technical help; N.A. Lambert (Georgia Health Sciences University) for sharing Venus155-239-Gβ1, Venus1-155-Gγ2 and B. Malnic (Universidade de São Paulo) for the gift of the Ric-8B construct. This work was supported by research grants from the Dystonia Medical Research Foundation (TF), the Bachmann-Strauss Dystonia and Parkinson Foundation (LO), the Lockwood Family Foundation (NS, LJO), the National Institute of Neurological Disorders and Stroke (NS26656 SBB, RSP and LJO; NS037409 NS, LJO; K02-NS073836, RSP) the National Institute on Drug Abuse (DA021743 and DA026405, KAM) and Agence Nationale de la Recherche (DH, ANR09-MNPS-014). The authors would like to thank the National Heart Lung Blood Institute GO Exome Sequencing Project and its ongoing studies which produced and provided exome variant calls for comparison: the Lung GO Sequencing Project (HL-102923), the WHI Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010).
Footnotes
URLs
SeattleSeq Annotation http://snp.gs.washington.edu/SeattleSeqAnnotation/
Exome Variant Server http://evs.gs.washington.edu/EVS/
Author contributions
TF developed the computational analysis pipeline, analyzed the next generation and Sanger sequencing data and wrote the paper; SW and EA performed molecular experiments; NS provided funding for the linkage studies; DH provided the GNAL antibody; DR collected samples and provided clinical information for the subjects; MSL and RSP performed the statistical analysis related to the phenotype; SF, AEL, TWL, RMT, RSP and SBB examined subjects; SBB and RSP supervised the acquisition of clinical data and blood samples and assigned final clinical status; KM and MEE formulated the functional assay; IM performed the assays of Gαolf function; KM and IM analyzed and interpreted the functional data, LJO designed and supervised the genetic studies; TF, IM, AEL, DH, DR, RSP, MSL, NS, KM, MEE, SBB, and LJO edited the manuscript.
References
- 1.Fahn S. Concept and classification of dystonia. Adv Neurol. 1988;50:1–8. [PubMed] [Google Scholar]
- 2.Fahn S, Bressman SB, Marsden CD. Classification of dystonia. Adv Neurol. 1998;78:1–10. [PubMed] [Google Scholar]
- 3.Ozelius LJ, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs T, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–288. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- 5.Xiao J, et al. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71:458–469. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albanese A, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 7.Ozelius LJ, Bressman SB. Genetic and clinical features of primary torsion dystonia. Neurobiol Dis. 2011;42:127–135. doi: 10.1016/j.nbd.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risch NJ, et al. Segregation analysis of idiopathic torsion dystonia in Ashkenazi Jews suggests autosomal dominant inheritance. Am J Hum Genet. 1990;46:533–538. [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad F, et al. Evidence for locus heterogeneity in autosomal dominant torsion dystonia. Genomics. 1993;15:9–12. doi: 10.1006/geno.1993.1003. [DOI] [PubMed] [Google Scholar]
- 10.Defazio G, Livrea P, Guanti G, Lepore V, Ferrari E. Genetic contribution to idiopathic adult-onset blepharospasm and cranial-cervical dystonia. Eur Neurol. 1993;33:345–350. doi: 10.1159/000116969. [DOI] [PubMed] [Google Scholar]
- 11.Bressman SB, et al. Idiopathic dystonia among Ashkenazi Jews: evidence for autosomal dominant inheritance. Ann Neurol. 1989;26:612–620. doi: 10.1002/ana.410260505. [DOI] [PubMed] [Google Scholar]
- 12.Waddy HM, Fletcher NA, Harding AE, Marsden CD. A genetic study of idiopathic focal dystonias. Ann Neurol. 1991;29:320–324. doi: 10.1002/ana.410290315. [DOI] [PubMed] [Google Scholar]
- 13.Bressman SB, et al. A study of idiopathic torsion dystonia in a non-Jewish family: evidence for genetic heterogeneity. Neurology. 1994;44:283–287. doi: 10.1212/wnl.44.2.283. [DOI] [PubMed] [Google Scholar]
- 14.Bressman SB, et al. Mutations in THAP1 (DYT6) in early-onset dystonia: a genetic screening study. Lancet Neurol. 2009;8:441–446. doi: 10.1016/S1474-4422(09)70081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders-Pullman R, et al. Variant ataxia-telangiectasia presenting as primary-appearing dystonia in Canadian Mennonites. Neurology. 2012;78:649–657. doi: 10.1212/WNL.0b013e3182494d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 17.Sheth N, et al. Comprehensive splice-site analysis using comparative genomics. Nucleic Acids Res. 2006;34:3955–3967. doi: 10.1093/nar/gkl556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanchard A, et al. DYT6 dystonia: review of the literature and creation of the UMD Locus-Specific Database (LSDB) for mutations in the THAP1 gene. Hum Mutat. 2011;32:1213–1224. doi: 10.1002/humu.21564. [DOI] [PubMed] [Google Scholar]
- 19.Corradi JP, et al. Alternative transcripts and evidence of imprinting of GNAL on 18p11.2. Mol Psychiatry. 2005;10:1017–1025. doi: 10.1038/sj.mp.4001713. [DOI] [PubMed] [Google Scholar]
- 20.Leube B, et al. Idiopathic torsion dystonia: assignment of a gene to chromosome 18p in a German family with adult onset, autosomal dominant inheritance and purely focal distribution. Hum Mol Genet. 1996;5:1673–1677. doi: 10.1093/hmg/5.10.1673. [DOI] [PubMed] [Google Scholar]
- 21.Grimes DA, et al. A novel locus for inherited myoclonus-dystonia on 18p11. Neurology. 2002;59:1183–1186. doi: 10.1212/wnl.59.8.1183. [DOI] [PubMed] [Google Scholar]
- 22.Han F, Racacho L, Lang AE, Bulman DE, Grimes DA. Refinement of the DYT15 locus in myoclonus dystonia. Mov Disord. 2007;22:888–892. doi: 10.1002/mds.21400. [DOI] [PubMed] [Google Scholar]
- 23.Tezzon F, Zanoni T, Passarin MG, Ferrari G. Dystonia in a patient with deletion of 18p. Ital J Neurol Sci. 1998;19:90–93. doi: 10.1007/BF02427563. [DOI] [PubMed] [Google Scholar]
- 24.Klein C, et al. Genetic analysis of three patients with an 18p- syndrome and dystonia. Neurology. 1999;52:649–651. doi: 10.1212/wnl.52.3.649. [DOI] [PubMed] [Google Scholar]
- 25.Nasir J, et al. Unbalanced whole arm translocation resulting in loss of 18p in dystonia. Mov Disord. 2006;21:859–863. doi: 10.1002/mds.20846. [DOI] [PubMed] [Google Scholar]
- 26.Postma AG, Verschuuren-Bemelmans CC, Kok K, van Laar T. Characteristics of dystonia in the 18p deletion syndrome, including a new case. Clin Neurol Neurosurg. 2009;111:880–882. doi: 10.1016/j.clineuro.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Graziadio C, et al. Dystonia, autoimmune disease and cerebral white matter abnormalities in a patient with 18p deletion. Arq Neuropsiquiatr. 2009;67:689–691. doi: 10.1590/s0004-282x2009000400021. [DOI] [PubMed] [Google Scholar]
- 28.Kowarik MC, et al. Myoclonus-dystonia in 18p deletion syndrome. Mov Disord. 2011;26:560–561. doi: 10.1002/mds.23446. [DOI] [PubMed] [Google Scholar]
- 29.Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244:790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- 30.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 31.Drinnan SL, Hope BT, Snutch TP, Vincent SR. G(olf) in the basal ganglia. Mol Cell Neurosci. 1991;2:66–70. doi: 10.1016/1044-7431(91)90040-u. [DOI] [PubMed] [Google Scholar]
- 32.Herve D, et al. G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13:2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kull B, Svenningsson P, Fredholm BB. Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol Pharmacol. 2000;58:771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- 34.Herve D, et al. Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J Neurosci. 2001;21:4390–4399. doi: 10.1523/JNEUROSCI.21-12-04390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corvol JC, Studler JM, Schonn JS, Girault JA, Herve D. Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76:1585–1588. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 36.Herve D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat. 2011;5:48. doi: 10.3389/fnana.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sunahara RK, Tesmer JJ, Gilman AG, Sprang SR. Crystal structure of the adenylyl cyclase activator Gsalpha. Science. 1997;278:1943–1947. doi: 10.1126/science.278.5345.1943. [DOI] [PubMed] [Google Scholar]
- 38.Berardelli A, et al. The pathophysiology of primary dystonia. Brain. 1998;121(Pt 7):1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- 39.Breakefield XO, et al. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 40.Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page ME, et al. Cell-autonomous alteration of dopaminergic transmission by wild type and mutant (DeltaE) TorsinA in transgenic mice. Neurobiol Dis. 2010;39:318–326. doi: 10.1016/j.nbd.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sciamanna G, et al. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia. Neurobiol Dis. 2009;34:133–145. doi: 10.1016/j.nbd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonsi P, et al. Centrality of striatal cholinergic transmission in Basal Ganglia function. Front Neuroanat. 2011;5:6. doi: 10.3389/fnana.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corvol JC, et al. Quantitative changes in Galphaolf protein levels, but not D1 receptor, alter specifically acute responses to psychostimulants. Neuropsychopharmacology. 2007;32:1109–1121. doi: 10.1038/sj.npp.1301230. [DOI] [PubMed] [Google Scholar]
- 45.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang X, Belluscio L, Hen R. G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci. 2000;20:RC91. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corvol JC, et al. Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J Neurosci. 2004;24:7007–7014. doi: 10.1523/JNEUROSCI.0676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alcacer C, et al. Galpha(olf) mutation allows parsing the role of cAMP-dependent and extracellular signal-regulated kinase-dependent signaling in L-3,4-dihydroxyphenylalanine-induced dyskinesia. J Neurosci. 2012;32:5900–5910. doi: 10.1523/JNEUROSCI.0837-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Del Sorbo F, Albanese A. Levodopa-induced dyskinesias and their management. J Neurol. 2008;255(Suppl 4):32–41. doi: 10.1007/s00415-008-4006-5. [DOI] [PubMed] [Google Scholar]
- 50.Balcioglu A, et al. Dopamine release is impaired in a mouse model of DYT1 dystonia. J Neurochem. 2007;102:783–788. doi: 10.1111/j.1471-4159.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- 51.Sako W, Morigaki R, Nagahiro S, Kaji R, Goto S. Olfactory type G-protein alpha subunit in striosome-matrix dopamine systems in adult mice. Neuroscience. 2010;170:497–502. doi: 10.1016/j.neuroscience.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 52.Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamnasaran D. Genetic analysis of psychiatric disorders associated with human chromosome 18. Clin Invest Med. 2003;26:285–302. [PubMed] [Google Scholar]
- 54.Laurin N, et al. Investigation of the G protein subunit Galphaolf gene (GNAL) in attention deficit/hyperactivity disorder. J Psychiatr Res. 2008;42:117–124. doi: 10.1016/j.jpsychires.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DasBanerjee T, et al. A comparison of molecular alterations in environmental and genetic rat models of ADHD: a pilot study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1554–1563. doi: 10.1002/ajmg.b.30877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozelius LJ, Lubarr N, Bressman SB. Milestones in dystonia. Mov Disord. 2011;26:1106–1126. doi: 10.1002/mds.23775. [DOI] [PubMed] [Google Scholar]
- 57.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarman PR, et al. Primary torsion dystonia: the search for genes is not over. J Neurol Neurosurg Psychiatry. 1999;67:395–397. doi: 10.1136/jnnp.67.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Factor SA. Cervical dystonia in twins. Mov Disord. 2002;17:846–847. doi: 10.1002/mds.10215. [DOI] [PubMed] [Google Scholar]
- 60.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKenna A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dayem Ullah AZ, Lemoine NR, Chelala C. SNPnexus: a web server for functional annotation of novel and publicly known genetic variants (2012 update) Nucleic Acids Res. 2012 doi: 10.1093/nar/gks364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 64.Hollins B, Kuravi S, Digby GJ, Lambert NA. The c-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell Signal. 2009;21:1015–1021. doi: 10.1016/j.cellsig.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gopalakrishna KN, et al. Interaction of transducin with uncoordinated 119 protein (UNC119): implications for the model of transducin trafficking in rod photoreceptors. J Biol Chem. 2011;286:28954–28962. doi: 10.1074/jbc.M111.268821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niznik HB, et al. Dopamine D1 receptors characterized with [3H]SCH 23390. Solubilization of a guanine nucleotide-sensitive form of the receptor. J Biol Chem. 1986;261:8397–8406. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.