Abstract
Purpose
We developed three absolute risk models for second primary thyroid cancer to assist with long-term clinical monitoring of childhood cancer survivors.
Patients and Methods
We used data from the Childhood Cancer Survivor Study (CCSS) and two nested case-control studies (Nordic CCSS; Late Effects Study Group). Model M1 included self-reported risk factors, model M2 added basic radiation and chemotherapy treatment information abstracted from medical records, and model M3 refined M2 by incorporating reconstructed radiation absorbed dose to the thyroid. All models were validated in an independent cohort of French childhood cancer survivors.
Results
M1 included birth year, initial cancer type, age at diagnosis, sex, and past thyroid nodule diagnosis. M2 added radiation (yes/no), radiation to the neck (yes/no), and alkylating agent (yes/no). Past thyroid nodule was consistently the strongest risk factor (M1 relative risk [RR], 10.8; M2 RR, 6.8; M3 RR, 8.2). In the validation cohort, 20-year absolute risk predictions for second primary thyroid cancer ranged from 0.04% to 7.4% for M2. Expected events agreed well with observed events for each model, indicating good calibration. All models had good discriminatory ability (M1 area under the receiver operating characteristics curve [AUC], 0.71; 95% CI, 0.64 to 0.77; M2 AUC, 0.80; 95% CI, 0.73 to 0.86; M3 AUC, 0.75; 95% CI, 0.69 to 0.82).
Conclusion
We developed and validated three absolute risk models for second primary thyroid cancer. Model M2, with basic prior treatment information, could be useful for monitoring thyroid cancer risk in childhood cancer survivors.
INTRODUCTION
The incidence of childhood cancers in developed nations has been increasing at a modest but consistent rate.1,2 Because of therapeutic advances, this rise in the pediatric cancer rate has coincided with a significant decline in mortality, and 85% of 5-year childhood cancer survivors diagnosed after 1970 are expected to survive another 30 years or more.3 Despite its curative benefit, the treatment for childhood cancers can have adverse late effects on the health of long-term survivors, including an increased risk of primary malignancies and cardiac- or pulmonary-related mortality.4–7
Approximately 10% of subsequent primary malignancies among childhood cancer survivors are cancers of the thyroid gland.8 This excess risk is largely attributable to prior radiotherapy. The risk of second primary thyroid cancer (SPTC) persists throughout the adult life of irradiated survivors and is highest for those who received a radiation absorbed dose of 15 to 30 Gy to the thyroid.9,10
Previous studies11,12 of SPTC among childhood cancer survivors have focused on overall incidence, excess risk,9,10 or measures of relative risk (RR).13–19 No study has yet quantified the absolute risk of SPTC. Absolute risk is the probability that an individual with a specific risk profile will develop disease by a given age in the presence of competing events.20,21 A validated risk prediction tool could be important for clinical practice, as exemplified by established absolute risk models for breast cancer and cardiovascular disease.22–24
There is debate among experts about best practices for monitoring thyroid cancer risk in childhood cancer survivors who received radiotherapy. The Children's Oncology Group recommends a yearly thyroid examination,25,26 but other expert groups recommend limiting physical examination of the thyroid to survivors with a self-reported thyroid enlargement or nodule.27 A risk-prediction tool for SPTC could help clinicians appropriately match the intensity of monitoring to a patient's individual risk and thereby reduce the harms associated with unnecessary screening and false-positive diagnoses.28,29
In this article, we report, to the best of our knowledge, the first absolute risk models for SPTC in 5-year survivors of a childhood cancer. Because it may not always be possible to obtain detailed information about treatment during early childhood, we developed three models: one model included self-reported risk factors only, a second model included risk factors from self-report and medical record abstraction, and the third model considered all available risk information, including a reconstructed radiation absorbed dose to the thyroid gland. We validated each model in an independent cohort of childhood cancer survivors and compared each model's ability to correctly classify low- and high-risk individuals.
PATIENTS AND METHODS
Patients
Study populations.
For model development, we combined data from a large ongoing cohort, the Childhood Cancer Survivor Study (CCSS),30,31 and two case-control studies, the Late Effects Study Group (LESG)15 and the Nordic Childhood Cancer Survivor Study (Nordic).11,32 CCSS participants were 5-year survivors of a childhood cancer diagnosed between 1970 and 1986 at 26 medical centers in the United States and Canada, with follow-up to January 1, 2010, for this analysis. LESG was a nested case-control study of cancer survivors diagnosed before age 18 between 1936 and 1979 at 13 US medical centers. Nordic was a nested case-control study of survivors diagnosed between 1960 and 1987 who were identified through national cancer registries for Denmark, Finland, Iceland, Norway, and Sweden. Patients were eligible for inclusion in the study analysis if they were alive and at risk of developing SPTC 5 years after a first primary cancer (FPC) diagnosed before age 21 and had a reconstructed radiation absorbed dose to the thyroid gland.
For model validation, we used data from the 3,254 French patients in the France/United Kingdom Childhood Cancer Survivor Study (CCSS-France) cohort, consisting of childhood cancer survivors identified from national registries between 1942 and 1986 who had information on benign thyroid conditions and had a reconstructed radiation absorbed dose to the thyroid gland.14
Outcome definition.
SPTC was defined as the first occurrence of a pathologically confirmed thyroid malignancy by using the International Classification of Diseases (9th revision; ICD-9) codes 193.0 to 193.9 or ICD-Oncology (3rd revision; ICD-O-3) site code 73.9 (morphology less than 9000).
Competing risks.
Competing events for SPTC were death, self-reported complete removal of the thyroid gland, and other second primary cancers, which were determined from pathology reports with follow-up to January 1, 2010.
Predictors.
Patient self-reported variables included demographic information, medical conditions, and health behaviors (Data Supplement). Factors obtained from medical records included use of radiation therapy, body regions irradiated, and use of each one of five classes of chemotherapeutic agents. The treatment period was the first 10 years following the childhood cancer diagnosis. One reconstructed risk factor we considered was the radiation absorbed dose to the thyroid gland, which was estimated from dosimetric models that used anthropometric characteristics, water phantoms, and data abstracted from radiotherapy records; CCSS and LESG used the dose reconstruction methods of Stovall et al,33 and Nordic and CCSS-France used the methods of Diallo et al.34
Taking the imprecision of the self-reported age at thyroid nodule diagnosis into consideration, thyroid nodule diagnoses made within 12 months of the SPTC diagnosis were considered coincident with the SPTC diagnosis and were, therefore, not counted as predictive factors.
Statistical Methods
Model development.
We developed three absolute risk models for SPTC by using data from self-report (model 1 [M1]), self-report and medical records (model 2 [M2]), and all available sources including the reconstructed radiation absorbed dose to the thyroid (model 3 [M3]). Estimates of absolute risk combined semiparametric estimates of baseline incidences and RRs for SPTC and competing risk comprising death, thyroid removal, or other second primary cancers (Data Supplement). Baseline incidences were estimated from the observed event times in the CCSS cohort. RRs for SPTC were estimated from the pooled cohort and case-control studies, but competing event RRs were estimated from the CCSS cohort only. Hazard models for SPTC under M1 and M2 and competing event models for M1, M2, and M3 followed a Cox proportional hazards model. For the M3 model of SPTC, we used a nonlinear excess relative risk (ERR) model,35,36 with separate radiation dose-response curves by age at childhood cancer diagnosis (Data Supplement). Starting from a base model, risk factors were selected by using a stepwise forward regression procedure with a 10% significance level using the CCSS data only. The base model for M1 included sex and age at diagnosis of the childhood cancer, M2 added radiation (yes/no), and M3 replaced radiation-related variables from M2 with the reconstructed dose model (Data Supplement).
The case-control studies did not have data for thyroid nodules, neck irradiation, and birth year (for 75% of LESG only). A multiple imputation37,38 procedure handled missing data for these variables (Data Supplement).
Validation.
Each model was validated in the independent cohort of French 5-year childhood cancer survivors. We assessed model calibration by comparing the expected to the observed number of events overall and in selected subgroups. The projection length for each individual was the time from study entry to the end of study follow-up on January 1, 2010.39 Discriminatory performance was evaluated with the area under the receiver operating characteristic curve (AUC), and 95% CIs for differences between model AUCs were constructed with bootstrap methods. We contrasted each model's ability to correctly assign more cases to higher-risk categories and more noncases to lower-risk categories with the net reclassification index (NRI)40 using 20-year SPTC risk projections. For the NRI calculations, we defined absolute risk estimates less than 0.5%, 0.5% to 1.5%, and more than 1.5% as low-, intermediate-, and high-risk categories on the basis of consultation with our study's clinicians.
RESULTS
Development Data
The analysis included 12,150 individuals comprising 11,997 participants (124 cases) from the CCSS and 153 participants (35 cases) from the case-control studies. Compared with noncases, cases in each study were significantly more likely to have been treated with radiation or with an alkylating agent; to have had radiation treatment including the neck and absorbed doses greater than 5 Gy to the thyroid gland; and to have a diagnosis of hypothyroidism, thyroid enlargement, or thyroid nodule (Table 1). Of the 159 SPTC cases, 131 (82%) were papillary (Data Supplement).
Table 1.
Summary Characteristics of the Analytic Sample Stratified by Study and SPTC Case Status
| Characteristic | Noncases |
SPTC Cases |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCSS (n = 11,873) |
LESG (n = 82) |
Nordic (n = 36) |
CCSS (n = 124) |
LESG (n = 22) |
Nordic (n = 13) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Femaleab | 5,609 | 47 | 54 | 66 | 27 | 75 | 86 | 69 | 14 | 64 | 10 | 77 |
| Type of first cancerabc | ||||||||||||
| Bone cancer | 1,000 | 8 | 4 | 5 | 3 | 8 | 11 | 9 | 0 | 0 | 1 | 8 |
| CNS | 1,540 | 13 | 3 | 4 | 7 | 19 | 16 | 13 | 1 | 5 | 0 | 0 |
| HL | 1,514 | 13 | 15 | 18 | 1 | 3 | 39 | 31 | 5 | 23 | 5 | 38 |
| Kidney (Wilms tumor) | 1,045 | 9 | 21 | 26 | 2 | 6 | 2 | 2 | 4 | 18 | 1 | 8 |
| Leukemia | 4,058 | 34 | 0d | 7 | 29 | 33 | 27 | 0 | 2 | 15 | ||
| Neuroblastoma | 805 | 7 | 27 | 33 | 1 | 3 | 8 | 6 | 7 | 32 | 0 | 0 |
| NHL | 878 | 7 | 5 | 6 | 1 | 3 | 6 | 5 | 2 | 9 | 1 | 8 |
| Soft tissue sarcoma | 1,033 | 9 | 3 | 4 | 2 | 6 | 9 | 7 | 1 | 5 | 0 | 0 |
| Other | 0 | 0 | 4 | 5 | 12 | 33 | 0 | 0 | 2 | 9 | 3 | 23 |
| Age at diagnosis, yearsabc | ||||||||||||
| ≤ 5 | 4,846 | 41 | 51 | 62 | 12 | 33 | 33 | 27 | 11 | 50 | 3 | 23 |
| 5-9 | 2,599 | 22 | 15 | 18 | 6 | 17 | 27 | 22 | 5 | 23 | 2 | 15 |
| 10-14 | 2,346 | 20 | 11 | 13 | 4 | 11 | 50 | 40 | 4 | 18 | 2 | 15 |
| 15+ | 2,082 | 18 | 5 | 6 | 14 | 39 | 14 | 11 | 2 | 9 | 6 | 46 |
| Year of birthabc | ||||||||||||
| Before 1970 | 4,435 | 37 | —e | 30 | 83 | 55 | 44 | — | 11 | 85 | ||
| 1970-1986 | 7,438 | 63 | — | 6 | 17 | 69 | 56 | — | 2 | 15 | ||
| Radiationabf | 7,896 | 67 | 71 | 87 | 17 | 47 | 112 | 90 | 22 | 100 | 12 | 92 |
| Radiation, Gyabfg | ||||||||||||
| Median | 1.1 | 3.3 | 0.72 | 20.35 | 11.7 | 7.43 | ||||||
| IQR | 0.47-20.20 | 0.88-19.97 | 0.17-3.68 | 10.00-30.69 | 4.41-26.03 | 1.75-21.23 | ||||||
| Chemotherapyacf | 9,569 | 81 | 42 | 51 | 16 | 44 | 102 | 82 | 12 | 55 | 9 | 69 |
| Alkylating agentabcf | 6,405 | 54 | 27 | 33 | 8 | 22 | 84 | 68 | 9 | 41 | 5 | 38 |
| Other chemotherapyf | ||||||||||||
| Bleomycinf | 702 | 6 | 10 | 8 | ||||||||
| Anthracyclinesf | 4,886 | 41 | 43 | 35 | ||||||||
| Platinum agentf | 725 | 6 | 6 | 5 | ||||||||
| Epipodophyllotoxinsf | 1,125 | 9 | 12 | 10 | ||||||||
| Radiation to neckbf | ||||||||||||
| Yes | 2,888 | 24 | 88 | 71 | ||||||||
| No | 8,984 | 76 | 36 | 29 | ||||||||
| Missing | 1 | 0 | 0 | 0 | ||||||||
| Age at last known vital status, yearsb | ||||||||||||
| ≤ 21 | 1,357 | 11 | 20 | 16 | ||||||||
| 21-34 | 6,015 | 51 | 81 | 65 | ||||||||
| 35-44 | 3,537 | 30 | 21 | 17 | ||||||||
| 45+ | 964 | 8 | 2 | 2 | ||||||||
| No. of visits to physicianbh | ||||||||||||
| None | 4 | 0 | 0 | 0 | ||||||||
| 1-6 | 6,677 | 56 | 65 | 52 | ||||||||
| 7-20 | 2,077 | 18 | 34 | 27 | ||||||||
| 20+ | 1,372 | 12 | 9 | 7 | ||||||||
| Missing | 1,743 | 15 | 16 | 13 | ||||||||
| Years since last physical examinationbh | ||||||||||||
| < 1 | 5,306 | 45 | 63 | 51 | ||||||||
| 1-4 | 3,253 | 28 | 32 | 26 | ||||||||
| 5+ | 904 | 8 | 12 | 10 | ||||||||
| Never | 575 | 5 | 4 | 3 | ||||||||
| Missing | 1,835 | 15 | 13 | 10 | ||||||||
| Ever smokedb | ||||||||||||
| Yes | 2,601 | 22 | 29 | 23 | ||||||||
| No | 8,691 | 73 | 94 | 76 | ||||||||
| Unsure | 81 | 1 | 1 | 1 | ||||||||
| Missing | 500 | 4 | 0 | 0 | ||||||||
| Use of any thyroid medicationbf | ||||||||||||
| Yes | 1,021 | 9 | 49 | 40 | ||||||||
| No | 10,335 | 87 | 68 | 55 | ||||||||
| Unsure | 76 | 1 | 0 | 0 | ||||||||
| Missing | 441 | 4 | 7 | 6 | ||||||||
| Overactive thyroid (in lifetime)bi | ||||||||||||
| Yes | 301 | 3 | 12 | 10 | ||||||||
| No | 11,372 | 96 | 105 | 85 | ||||||||
| Unsure | 176 | 2 | 6 | 6 | ||||||||
| Missing | 24 | 0 | 1 | 1 | ||||||||
| Underactive thyroid (in lifetime)bi | ||||||||||||
| Yes | 1,314 | 11 | 45 | 36 | ||||||||
| No | 10,338 | 87 | 63 | 56 | ||||||||
| Unsure | 198 | 2 | 8 | 8 | ||||||||
| Missing | 23 | 0 | 1 | 1 | ||||||||
| Thyroid nodules (in lifetime)bi | ||||||||||||
| Yes | 478 | 4 | 70 | 56 | ||||||||
| No | 11,181 | 94 | 46 | 37 | ||||||||
| Unsure | 182 | 2 | 7 | 6 | ||||||||
| Missing | 32 | 0 | 1 | 1 | ||||||||
| Thyroid enlargement (in lifetime)bi | ||||||||||||
| Yes | 390 | 3 | 56 | 45 | ||||||||
| No | 11,261 | 95 | 62 | 50 | ||||||||
| Unsure | 178 | 2 | 5 | 4 | ||||||||
| Missing | 50 | 0 | 1 | 1 | ||||||||
Abbreviations: CCSS, Childhood Cancer Survivor Study; HL, Hodgkin lymphoma; LESG, Late Effects Study Group; NHL, non-Hodgkin lymphoma; SPTC, second primary thyroid cancer.
Comparing noncases between studies, P < .05; Wilcoxon test for continuous variables; χ2 test for categorical variables.
Comparing cases between studies, P < .05; Wilcoxon test for continuous variables; χ2 test for categorical variables.
Comparing combined cases and noncases, P < .05; Wilcoxon test for continuous variables; χ2 test for categorical variables.
In LESG, there were no second thyroid cancers for survivors of childhood leukemia.
Data not collected. In LESG, 26 patients (25%; seven cases and 19 controls) were older than age 8 years at the childhood cancer diagnosis and were therefore known to have been born after 1970.
During 10 years following first cancer, determined from medical record.
Reconstructed dose.
During previous 2 years; self-report on baseline questionnaire.
Combined self-report on baseline questionnaire and 2007 follow-up questionnaire.
RR Models
M1.
The self-report RR model included the following significant risk factors of SPTC: birth after 1970, age younger than 15 years at FPC, an FPC diagnosis of Hodgkin lymphoma, female sex, and prior diagnosis of a thyroid nodule (Table 2). A prior diagnosis of thyroid nodules was the strongest risk factor (RR, 10.8; 95% CI, 6.5 to 18.0); the RRs from all other factors ranged from 1.5 to 2.8. An FPC diagnosis of Hodgkin lymphoma (RR, 1.7; 95% CI, 1.6 to 1.9) and any prior diagnosis of a thyroid nodule (RR, 1.7; 95% CI, 1.3 to 2.2) were also found to be significant prognostic variables for competing events in the self-report-only model (Data Supplement).
Table 2.
Multivariable Relative Risk Estimates for Models of SPTC in Childhood Cancer Survivors (N = 12,150; 159 events)
| Risk Factor | M1 |
M2 |
M3 |
Subgroup Characteristics |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | No. of Events | % | No. of Patients | % Irradiated | Mean Dose (Gy) | |
| Birth year after 1970 | 1.54 | 1.00 to 2.37 | 1.67 | 1.05 to 2.64 | 1.77 | 1.08 to 2.90 | 69 | 0.9 | 7,507 | 62.9 | 6.44 |
| Age at FPC < 15 years | 2.78 | 1.53 to 5.06 | 3.17 | 1.63 to 6.16 | 137 | 1.4 | 10,027 | 66.4 | 8.5 | ||
| Hodgkin lymphoma FPC | 2.58 | 1.71 to 3.91 | 49 | 3.1 | 1,579 | 93.9 | 34.95 | ||||
| Female | 1.75 | 1.23 to 2.49 | 2.28 | 1.56 to 3.32 | 2.12 | 1.34 to 3.37 | 110 | 1.9 | 5,800 | 65.6 | 11.24 |
| Thyroid nodules (in lifetime)* | 10.81 | 6.51 to 17.98 | 6.83 | 4.06 to 11.48 | 8.22 | 4.59 to 14.74 | 28 | 5.5 | 506 | 90.5 | 26.49 |
| Any alkylating agent† | 1.56 | 1.07 to 2.28 | 1.49 | 1.08 to 2.06 | 98 | 1.5 | 6,538 | 71.2 | 12.12 | ||
| Any radiation† | 1.92 | 0.92 to 4.02 | 146 | 1.8 | 8,130 | 100 | 11.17 | ||||
| Radiation to neck† | 5.57 | 3.33 to 9.33 | 88 | 3 | 2,967 | 100 | 28.11 | ||||
| Radiation dose (linear term)‡ | |||||||||||
| By age at FPC, years§ | |||||||||||
| < 5 | 1.58 | 0.58 to 4.32 | |||||||||
| 5-9 | 1.66 | 0.85 to 3.26 | |||||||||
| 10-14 | 2.33 | 1.10 to 4.94 | |||||||||
| ≥15 | 0.87 | 0.34 to 2.22 | |||||||||
| Radiation dose (exponential term) | −0.60 | −0.043 to −0.085 | |||||||||
Abbreviations: FPC, first primary cancer; M1, model 1; M2, model 2; M3, model 3; RR, relative risk; SPTC, second primary thyroid tumor.
Self-reported “Yes” at baseline or 2007 follow-up; diagnoses within 12 months of SPTC excluded.
Within 10 years of FPC.
Computationally reconstructed radiation dose (Gy) to thyroid.
Likelihood ratio test against model with homogeneous linear term, P = .08.
M2.
When treatment variables from medical records were considered, indicators of radiation, radiation to the neck, and the use of an alkylating agent were strong risk factors. Birth after 1970, age younger than 15 years at FPC, female sex, and prior diagnosis of a thyroid nodule were also significant. The magnitudes of the RRs were similar to M1 but the RR of thyroid nodules was reduced (RR, 6.8; 95% CI, 4.1 to 11.5). In the competing events model, the presence of treatment variables with large RRs—1.6 (95% CI, 1.5 to 1.7) for alkylating agents (yes/no), 2.1 (95% CI, 1.8 to 2.3) for radiation (yes/no), and 1.8 (95% CI, 1.6 to 1.9) for radiation to the neck (yes/no)—resulted in an attenuation of the RR associated with thyroid nodules (RR, 1.4; 95% CI, 1.1 to 1.8; Data Supplement).
M3.
In an ERR model that included the radiation absorbed dose to the thyroid gland (with separate dose-response curves by age at diagnosis), birth year, sex, and treatment with an alkylating agent were significant risk factors. The risk association for thyroid nodules was slightly stronger than in the M2 model (RR, 8.2; 95% CI, 4.6 to 14.7). The dose-response parameters of M3 indicate an increasing RR up to 15 Gy and a declining risk at higher doses. Given the same radiation absorbed dose, the effect modification by age (P = .08) suggests that survivors diagnosed after age 14 had the lowest excess risk. All treatment-related factors were strongly associated with the competing risk model for M3; the risk association for thyroid nodules (RR, 1.5; 95% CI, 1.1 to 1.9) was unchanged from M2 (Data Supplement). We found no significant interactions between absorbed dose and use of an alkylating agent, treatment era, or years from the FPC diagnosis.
Validation cohort.
Among the 2,966 French survivors who met inclusion criteria, there were 39 SPTC cases, 261 other second primary malignancies, and 400 deaths before a second cancer diagnosis. SPTC cases and noncases of the CCSS and CCSS-France cohorts differed on most baseline characteristics (Table 3). SPTC cases in the validation cohort were all younger than age 15 years when diagnosed with a childhood cancer, had a significantly lower median radiation absorbed dose to the thyroid, and had significantly fewer diagnoses of thyroid nodules.
Table 3.
Summary Characteristics of the Model Development (CCSS, LESG, Nordic) and Validation (CCSS-France) Samples Stratified by SPTC Status
| Characteristic | Noncases |
SPTC Cases |
||||||
|---|---|---|---|---|---|---|---|---|
| Model Development (n= 11,991) |
CCSS-France (n= 2,927) |
Model Development (n= 159) |
CCSS-France (n= 39) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Female | 5,690 | 47 | 1,279 | 44 | 110 | 69 | 24 | 62 |
| Type of first cancerabc | ||||||||
| CNS | 1,550 | 13 | 426 | 15 | 17 | 11 | 2 | 5 |
| HL | 1,530 | 13 | 198 | 7 | 49 | 31 | 11 | 28 |
| Kidney (Wilms tumor) | 1,068 | 9 | 621 | 21 | 7 | 4 | 7 | 18 |
| Neuroblastoma | 833 | 7 | 416 | 14 | 15 | 9 | 7 | 18 |
| NHL | 884 | 7 | 328 | 11 | 9 | 6 | 5 | 13 |
| Other | 6,126 | 51 | 938 | 32 | 76 | 45 | 7 | 18 |
| Age at diagnosis, yearsb | ||||||||
| ≤ 5 | 4,909 | 41 | 1,542 | 53 | 47 | 30 | 11 | 28 |
| 5-9 | 2,620 | 22 | 699 | 24 | 34 | 21 | 14 | 36 |
| 10-14 | 2,361 | 20 | 592 | 20 | 56 | 35 | 13 | 36 |
| 15+ | 2,101 | 18 | 90 | 3 | 22 | 14 | 0 | 0 |
| Year of birthbc | ||||||||
| Before 1970 | 4,465 | 37 | 1,341 | 46 | 66 | 42 | 24 | 62 |
| 1970-1986 | 7,444 | 63 | 1,575 | 54 | 71 | 45 | 15 | 38 |
| Missing | 11 | 0 | 0 | 0 | 22 | 14 | 0 | 0 |
| Radiation, Gybcde | ||||||||
| Median | 1.1 | 0.7 | 19.0 | 6.5 | ||||
| IQR | 0.5-20.3 | 0.2-5.6 | 6.9-29.0 | 1.1-16.3 | ||||
| Radiationbe | 7,984 | 67 | 2,049 | 70 | 146 | 92 | 36 | 92 |
| Radiation to necke | 2,888 | 24 | 761 | 26 | 88 | 55 | 29 | 74 |
| Missing | 119 | 1 | 0 | 0 | 35 | 22 | 0 | 0 |
| Chemotherapybe | 9,627 | 80 | 2,173 | 74 | 123 | 77 | 28 | 72 |
| Missing | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alkylating agentbe | 6,440 | 54 | 1,437 | 49 | 98 | 62 | 19 | 49 |
| Other chemotherapye | ||||||||
| Bleomycinbe | 702 | 6 | 161 | 6 | 10 | 6 | 5 | 13 |
| Anthracyclinesbe | 4,886 | 41 | 933 | 32 | 43 | 27 | 11 | 28 |
| Platinum agentbe | 725 | 6 | 181 | 6 | 6 | 4 | 2 | 5 |
| Epipodophyllotoxinsbe | 1,125 | 9 | 80 | 3 | 12 | 8 | 1 | 3 |
| Ever smokedbcf | 2,601 | 22 | 947 | 32 | 29 | 18 | 17 | 44 |
| Use of hypothyroid medicationbg | 948 | 8 | 179 | 6 | 43 | 27 | 14 | 58 |
| Overactive thyroidbf | 301 | 3 | 46 | 2 | 12 | 8 | 5 | 21 |
| Underactive thyroidbf | 1,314 | 11 | 115 | 4 | 45 | 28 | 4 | 17 |
| Thyroid nodulesf | 478 | 4 | 121 | 4 | 70 | 44 | 11 | 46 |
| Thyroid enlargementcfh | 390 | 3 | 39 | 1 | 56 | 35 | 2 | 8 |
Abbreviations: CCSS, Childhood Cancer Survivor Study; HL, Hodgkin lymphoma; IQR, interquartile range; LESG, Late Effects Study Group; NHL, non-Hodgkin lymphoma; SPTC, second primary thyroid cancer.
Leukemia cases were excluded from CCSS-France.
Comparing noncases, P < .05; Wilcoxon test for continuous variables; χ2 test for categorical variables.
Comparing cases, P < .05; Wilcoxon test for continuous variables; χ2 test for categorical variables.
Reconstructed dose.
During 10 years following first cancer, determined from medical record.
In lifetime; self-report on baseline questionnaire.
CCSS-France collected only treatment information for hypothyroidism; same restriction applied to CCSS cohort.
CCSS-France assessed goiter; CCSS enlargement or swelling.
Example projections.
For illustrative purposes, we report 20-year SPTC risk for three selected childhood cancer survivors from CCSS-France (Table 4). The SPTC risk difference between profile A (low risk) and profile C (high risk) is as large as 10%. M1 gave a more similar predicted risk for patients B and C than either M2 or M3, which demonstrates the limited discriminatory ability of M1.
Table 4.
20-Year Absolute Risk Projections (%) for Three Selected Patients From CCSS-France
| Risk Factor | Factor in M1, M2, M3 | Patient A* |
Patient B† |
Patient C†
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Gy | %‡ | Subgroup | Gy | % | Subgroup | Gy | % | ||
| Age at FPC < 15 years | Y,Y,N | Y | Y | |||||||
| Birth year after 1970 | Y,Y,Y | N | Y | Y | ||||||
| Age 5 years at FPC | Y,Y,Y | Y | Y | Y | ||||||
| Hodgkin lymphoma FPC | Y,N,N | N | Y | N | ||||||
| Female | Y,Y,Y | N | Y | Y | ||||||
| Thyroid nodules (in lifetime) | Y,Y,Y | N | N | N | ||||||
| Any alkylating agent for FPC | N,Y,Y | N | Y | Y | ||||||
| Any radiation | N,Y,N | N | Y | Y | ||||||
| Radiation treatment to neck | N,Y,N | N | N | Y | ||||||
| Radiation dose to thyroid | N,N,Y | 0 | 0.2 | 15.7 | ||||||
| M1 | 0.21 | 5.6 | 2.2 | |||||||
| M2 | 0.05 | 1.6 | 7.1 | |||||||
| M3 | 0.04 | 1.0 | 10.6 | |||||||
Abbreviations: CCSS, Childhood Cancer Survivor Study; FPC, first primary cancer; M1, model 1; M2, model 2; M3, model 3; N, no; Y, yes.
Projection interval for Patient A is 6 to 26 years.
Projection intervals for Patient B and Patient C are 17 to 37 years.
Absolute risk.
Validation.
There was no significant bias in the overall calibration of models M1 to M3 (Table 5). In general, M2 had the least evidence of bias across subgroups defined by demographic and treatment-related variables. Ratios of the observed numbers of cases to expected numbers of cases computed from the models during follow-up for the risk categories less than 0.5%, 0.5% to 0.749%, 0.75% to 0.99%, 1.0% to 1.24%, 1.25% to 1.49%, and 1.5%+ were 0/1, 2/3, 9/6, 1/4, 6/4, and 21/19 for M1; 3/4, 2/2, 3/3, 2/2, 1/0.5, and 28/25 for M2; and 8/4.5, 7/2, 2/2, 1/1, 0/1, and 21/18 for M3. The agreement between the observed and expected counts across risk groups provides further support that the models were unbiased.
Table 5.
Comparison of Model Calibration Based on the CCSS-France Validation Cohort (n = 2,966; events = 39)
| Factor | Subgroup | No. of Patients | Events Observed | M1 |
M2 |
M3 |
|||
|---|---|---|---|---|---|---|---|---|---|
| Expected/Observed | 95% CI | Expected/Observed | 95% CI | Expected/Observed | 95% CI | ||||
| Overall | 2,966 | 39 | 1.06 | 0.77 to 1.47 | 1.08 | 0.78 to 1.50 | 1.33 | 0.92 to 1.91 | |
| Birth year after 1970 | Y | 1,589 | 14 | 1.36 | 0.81 to 2.30 | 1.30 | 0.77 to 2.20 | 1.09 | 0.64 to 1.84 |
| N | 1,377 | 25 | 0.71 | 0.48 to 1.05 | 0.71 | 0.48 to 1.06 | 0.57 | 0.38 to 0.84 | |
| Age at FPC < 15 years | Y | 2,876 | 39 | 0.93 | 0.68 to 1.27 | 0.91 | 0.67 to 1.25 | 0.74 | 0.54 to 1.01 |
| Hodgkin lymphoma FPC | Y | 209 | 11 | 0.55 | 0.30 to 0.99 | 0.52 | 0.29 to 0.95 | 0.51 | 0.28 to 0.92 |
| N | 2,757 | 28 | 1.10 | 0.76 to 1.59 | 1.08 | 0.75 to 1.57 | 0.85 | 0.59 to 1.23 | |
| Female | Y | 1,303 | 24 | 0.92 | 0.62 to 1.38 | 0.96 | 0.64 to 1.43 | 0.75 | 0.50 to 1.11 |
| N | 1,663 | 15 | 0.97 | 0.59 to 1.61 | 0.87 | 0.53 to 1.45 | 0.76 | 0.46 to 1.26 | |
| Thyroid nodules* | N | 2,965 | 39 | 0.94 | 0.69 to 1.29 | 0.92 | 0.66 to 1.26 | 0.75 | 0.55 to 1.03 |
| Any alkylating agent | Y | 1,456 | 19 | 0.99 | 0.63 to 1.55 | 1.10 | 0.70 to 1.72 | 0.92 | 0.59 to 1.44 |
| N | 1,510 | 20 | 0.90 | 0.58 to 1.39 | 0.76 | 0.49 to 1.18 | 0.60 | 0.38 to 0.92 | |
| Any radiation | Y | 2,085 | 36 | 0.77 | 0.56 to 1.07 | 0.92 | 0.66 to 1.27 | 0.74 | 0.54 to 1.03 |
| N | 881 | 3 | 3.01 | 0.97 to 9.33 | 1.05 | 0.34 to 3.24 | 0.88 | 0.28 to 2.71 | |
| Radiation to neck | Y | 786 | 29 | 0.43 | 0.30 to 0.62 | 0.84 | 0.59 to 1.21 | 0.62 | 0.43 to 0.90 |
| N | 2,180 | 10 | 2.42 | 1.30 to 4.51 | 1.16 | 0.62 to 2.16 | 1.13 | 0.61 to 2.09 | |
| Radiation dose > 10 Gy | Y | 405 | 11 | 0.67 | 0.37 to 1.21 | 1.15 | 0.64 to 2.08 | 1.11 | 0.62 to 2.01 |
| N | 2,561 | 28 | 1.05 | 0.72 to 1.52 | 0.84 | 0.58 to 1.21 | 0.61 | 0.42 to 0.89 | |
NOTE. Factors included in a given model appear in bold.
Abbreviations: CCSS, Childhood Cancer Survivor Study; FPC, first primary cancer; M1, model 1; M2, model 2; M3, model 3; N, no; Y, yes.
Diagnosis status at the beginning of the projection interval.
Discrimination significantly improved with the inclusion of treatment risk factors: M2 had an AUC of 0.80 (95% CI, 0.73 to 0.86), a 0.09 (95% CI, 0.02 to 0.15) statistically significant improvement over M1 (AUC, 0.71; 95% CI, 0.64 to 0.77), and a statistically significant improvement of 0.05 (95% CI, 0.01 to 0.10) over M3 (AUC, 0.75; 95% CI, 0.69 to 0.82).
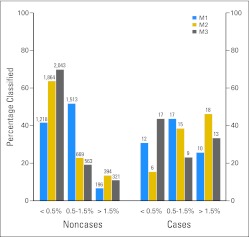
When compared with M1, both M2 and M3 significantly improved the 20-year SPTC risk classification of noncases (M2 v M1 NRI for noncases, 0.15; 95% CI, 0.13 to 0.18; M3 v M1 NRI for noncases, 0.24; 95% CI, 0.21 to 0.26). There was a small statistically significant improvement in classification using M3 versus M2 (NRI for noncases, 0.07; 95% CI, 0.05 to 0.09). M1 categorized 42% (95% CI, 40% to 43%) of noncases in the lowest-risk group, M2 categorized 64% (95% CI, 62% to 65%), and M3 categorized 69% (95% CI, 68% to 71%; Fig 1).
Fig 1.
Comparison of model risk classification for 20-year projected risk of second primary thyroid cancer in the France/United Kingdom Childhood Cancer Survivor Study validation cohort (n = 2,966; events = 39). M1, model 1; M2, model 2; M3, model 3.
M2 also significantly improved classification of cases when compared with M1 (NRI for cases, 0.36; 95% CI, 0.12 to 0.59) and M3 (NRI for cases, 0.35; 95% CI, 0.16 to 0.56). There was no difference in M1 and M3 case classification (NRI for cases, −0.03; 95% CI, −0.24 to 0.19). M2 categorized 46% (95% CI, 30% to 63%) of cases in the highest-risk group, whereas M1 categorized 26% (95% CI, 14% to 42%) and M3 categorized 33% (95% CI, 20% to 50%). The substantive conclusions of these comparisons were unchanged when a six-category or continuous NRI was used (data not shown).
DISCUSSION
We have developed the first absolute risk prediction models for SPTC in 5-year survivors of a childhood cancer, differing by the treatment information they included. In an independent validation cohort, all three models were well calibrated and had greater discriminatory ability than established prediction models for incidence of other cancers.41 A model that included sex, birth after 1970, age younger than 15 years when diagnosed with a childhood cancer, prior diagnosis of a thyroid nodule, history of radiation therapy, radiation therapy including the neck, and history of treatment with an alkylating agent (M2) had the best overall discriminatory performance, with an AUC of 0.80. This model significantly improved discrimination and risk classification compared with a model without treatment information (M1) and performed as well as a model that included radiation absorbed dose to the thyroid (M3).
Because of its potential clinical utility for managing thyroid cancer risk in childhood cancer survivors, we have written software to compute risk projections using model M2 (Data Supplement; http://dceg.cancer.gov/tools/riskassessment) and provided selected tabulations with 95% CIs constructed from an influence-based variance42 as a Data Supplement. Despite its somewhat inferior performance to M2, M1 had good discriminatory ability and might be considered for SPTC risk assessment when sufficient treatment data are unavailable.43
In its recent review, the National Council on Radiation Protection and Measurement recommended the development of risk prediction models for second primary cancers for cancer survivors to facilitate risk-based clinical monitoring.44 As the first absolute risk model for second primary cancers following a childhood cancer, we chose to focus on thyroid cancer because it is a leading second cancer affecting survivors,8 and its risk has high individual variation. Risk-guided monitoring for thyroid cancer could, therefore, have an important impact on optimizing screening and interventional decision making during the long-term follow-up of childhood cancer survivors.
Our study had several limitations. We imputed missing data on thyroid nodules, radiation to the neck, and birth year in the case-control studies on the basis of information from the CCSS cohort. This approach assumes that the joint distribution of these variables, given all observed ones in the CCSS cohort, well approximates this distribution in the case-control studies. SPTC numbers were too small to determine risk differences by histology. Further, M3 was based on a reconstructed measure of radiation absorbed dose to the thyroid gland, and different reconstruction methods were used in the CCSS and LESG studies33 than in the Nordic and CCSS-France studies.34 The possibility of measurement error and study differences in dosimetry methods might have limited the ability of M3 to serve as a standard of reference for M1 and M2 risk-prediction performance. Although an increased risk of SPTC has been found to persist 30 to 40 years after treatment of childhood cancer,9,17 given the length of follow-up in the CCSS cohort at the time of our analysis, our models do not allow one to estimate SPTC risk beyond age 50 years. However, as CCSS patients age and more data become available, the models can be updated accordingly to allow longer-term projections.
Independent validation is the strongest test of model performance. However, the validation based on the CCSS-France cohort had some shortcomings. Cancer cases diagnosed after age 14 years were excluded from the CCSS-France study, which prevented external validation of our models for this age group. Further validation for individuals diagnosed between age 14 and 21 years is therefore needed. There were also noticeable differences in the distributions of reconstructed radiation absorbed dose in the CCSS-France cohort and the model development data set (Data Supplement), which raises the possibility that the radiation dose-response relationship in this cohort was distinct from what was estimated from the model development data. However, in a pooled analysis that included participants from the CCSS, LESG, Nordic, and CCSS-France studies, Veiga et al36 found no statistically significant differences between studies based on a linear-exponential-linear ERR dose-response model for subsequent thyroid cancer risk.
In summary, we have developed the first absolute risk models for SPTC and validated them in an independent cohort of childhood cancer survivors. Our analysis was strengthened by the inclusion of a multinational data set of patients with childhood cancer who were diagnosed before the 1990s identified through academic medical centers and national disease registries. These models will need to be updated as more outcome data become available for survivors in older adulthood. It will also be important to determine how changes in treatment modalities after the 1990s might have an impact on the applicability of these models to later cohorts of childhood cancer survivors.
Supplementary Material
Acknowledgment
We thank Alina Brenner, MD, PhD, and Jay H. Lubin, PhD, for their valuable comments.
Footnotes
Supported by the Intramural Research Program of the National Cancer Institute (NCI), National Institutes of Health. The Childhood Cancer Survivor Study was supported by Grant No. U24 CA55727 from the NCI (L.L.R.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Stephanie A. Kovalchik, Cécile M. Ronckers, Peter D. Inskip, Parveen Bhatti, Ann C. Mertens, Ruth M. Pfeiffer
Financial support: Leslie L. Robison
Administrative support: Alice J. Sigurdson
Provision of study materials or patients: Leslie L. Robison
Collection and assembly of data: Stephanie A. Kovalchik, Alice J. Sigurdson, Peter D. Inskip, Florent de Vathaire, Harald Anderson, Sue Hammond, Wendy M. Leisenring, Susan A. Smith, Marilyn Stovall, Margaret A. Tucker, Rita E. Weathers, Leslie L. Robison, Ruth M. Pfeiffer
Data analysis and interpretation: Stephanie A. Kovalchik, Cécile M. Ronckers, Lene H.S. Veiga, Alice J. Sigurdson, Peter D. Inskip, Charles A. Sklar, Sarah S. Donaldson, Sue Hammond, Susan A. Smith, Marilyn Stovall, Rita E. Weathers, Ruth M. Pfeiffer
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Steliarova-Foucher E, Stiller C, Kaatsch P, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): An epidemiological study. Lancet. 2004;364:2097–2105. doi: 10.1016/S0140-6736(04)17550-8. [DOI] [PubMed] [Google Scholar]
- 2.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004) Cancer. 2008;112:416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GT. Long-term survivors of childhood central nervous system malignancies: The experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 2010;14:298–303. doi: 10.1016/j.ejpn.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: Findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305:2311–2391. doi: 10.1001/jama.2011.747. [DOI] [PubMed] [Google Scholar]
- 9.Bhatti P, Veiga LH, Ronckers CM, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: An update from the Childhood Cancer Survivor Study. Radiat Res. 2010;174:741–752. doi: 10.1667/RR2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veiga LH, Bhatti P, Ronckers CM, et al. Chemotherapy and thyroid cancer risk: A report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2012;21:92–101. doi: 10.1158/1055-9965.EPI-11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen JH, Möller T, Anderson H, et al. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst. 2009;101:806–813. doi: 10.1093/jnci/djp104. [DOI] [PubMed] [Google Scholar]
- 12.Schwenn MR, Brill AB. Childhood cancer 10 years after the Chernobyl accident. Curr Opin Pediatr. 1997;9:51–54. doi: 10.1097/00008480-199702000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Sklar C, Whitton J, Mertens A, et al. Abnormalities of the thyroid in survivors of Hodgkin's disease: Data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2000;85:3227–3232. doi: 10.1210/jcem.85.9.6808. [DOI] [PubMed] [Google Scholar]
- 14.de Vathaire F, Hardiman C, Shamsaldin A, et al. Thyroid carcinomas after irradiation for a first cancer during childhood. Arch Intern Med. 1999;159:2713–2719. doi: 10.1001/archinte.159.22.2713. [DOI] [PubMed] [Google Scholar]
- 15.Tucker MA, Jones PH, Boice JD, Jr, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer: The Late Effects Study Group. Cancer Res. 1991;51:2885–2888. [PubMed] [Google Scholar]
- 16.Ronckers CM, Sigurdson AJ, Stovall M, et al. Thyroid cancer in childhood cancer survivors: A detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166:618–628. doi: 10.1667/RR3605.1. [DOI] [PubMed] [Google Scholar]
- 17.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 18.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): A nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 19.Taylor AJ, Croft AP, Palace AM, et al. Risk of thyroid cancer in survivors of childhood cancer: Results from the British Childhood Cancer Survivor Study. Int J Cancer. 2009;125:2400–2405. doi: 10.1002/ijc.24581. [DOI] [PubMed] [Google Scholar]
- 20.Benichou J, Gail MH. Estimates of absolute cause-specific risk in cohort studies. Biometrics. 1990;46:813–826. [PubMed] [Google Scholar]
- 21.Gail MH. Estimation and interpretation of models of absolute risk from epidemiologic data, including family-based studies. Lifetime Data Anal. 2008;14:18–36. doi: 10.1007/s10985-007-9070-0. [DOI] [PubMed] [Google Scholar]
- 22.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 23.Schrag D, Kuntz KM, Garber JE, et al. Decision analysis: Effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336:1465–1471. doi: 10.1056/NEJM199705153362022. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Pasternak R, Greenland P, et al. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–1492. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 25.Children's Oncology Group: Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, version 3.0, 2008. http://www.survivorshipguidelines.org.
- 26.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Scottish Intercollegiate Guidelines Network: Long Term Follow Up Care for Survivors of Childhood Cancer. Guideline No. 76, January 2004; updated March 2005. http://www.sign.ac.uk/guidelines/fulltext/76/index.html.
- 28.Brander A, Viikinkoski P, Tuuhea J, et al. Clinical versus ultrasound examination of the thyroid gland in common clinical practice. J Clin Ultrasound. 1992;20:37–42. doi: 10.1002/jcu.1870200107. [DOI] [PubMed] [Google Scholar]
- 29.Favus MJ, Schneider AB, Stachura ME, et al. Thyroid cancer occurring as a late consequence of head-and-neck irradiation: Evaluation of 1056 patients. N Engl J Med. 1976;294:1019–1025. doi: 10.1056/NEJM197605062941901. [DOI] [PubMed] [Google Scholar]
- 30.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 31.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svahn-Tapper G, Garwicz S, Anderson H, et al. Radiation dose and relapse are predictors for development of second malignant solid tumors after cancer in childhood and adolescence: A population-based case-control study in the five Nordic countries. Acta Oncol. 2006;45:438–448. doi: 10.1080/02841860600658633. [DOI] [PubMed] [Google Scholar]
- 33.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 34.Diallo I, Lamon A, Shamsaldin A, et al. Estimation of the radiation dose delivered to any point outside the target volume per patient treated with external beam radiotherapy. Radiother Oncol. 1996;38:269–271. doi: 10.1016/0167-8140(96)01713-6. [DOI] [PubMed] [Google Scholar]
- 35.Preston D. Modeling radiation effects on disease incidence. Radiat Res. 1990;124:343–344. [Google Scholar]
- 36.Veiga LH, Lubin JH, Anderson H, et al. A pooled analysis of thyroid cancer incidence following radiotherapy for childhood cancer. Radiat Res. 2012;178:365–376. doi: 10.1667/rr2889.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin DB, Schenker N. Multiple imputation in health-care databases: An overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 38.Rubin DB. Hoboken, NJ: Wiley-Interscience; 2004. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 39.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gail MH. Discriminatory accuracy from single-nucleotide polymorphisms in models to predict breast cancer risk. J Natl Cancer Inst. 2008;100:1037–1041. doi: 10.1093/jnci/djn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graubard BI, Fears TR. Standard errors for attributable risk for simple and complex sample designs. Biometrics. 2005;61:847–855. doi: 10.1111/j.1541-0420.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 43.Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA. 2001;287:1832–1839. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- 44.National Council on Radiation Protection & Measurements (NCRP): Bethesda, MD: NCRP; 2012. Second Primary Cancers and Cardiovascular Disease After Radiotherapy. Scientific Committee, NCRP Report No. 170. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.