Abstract
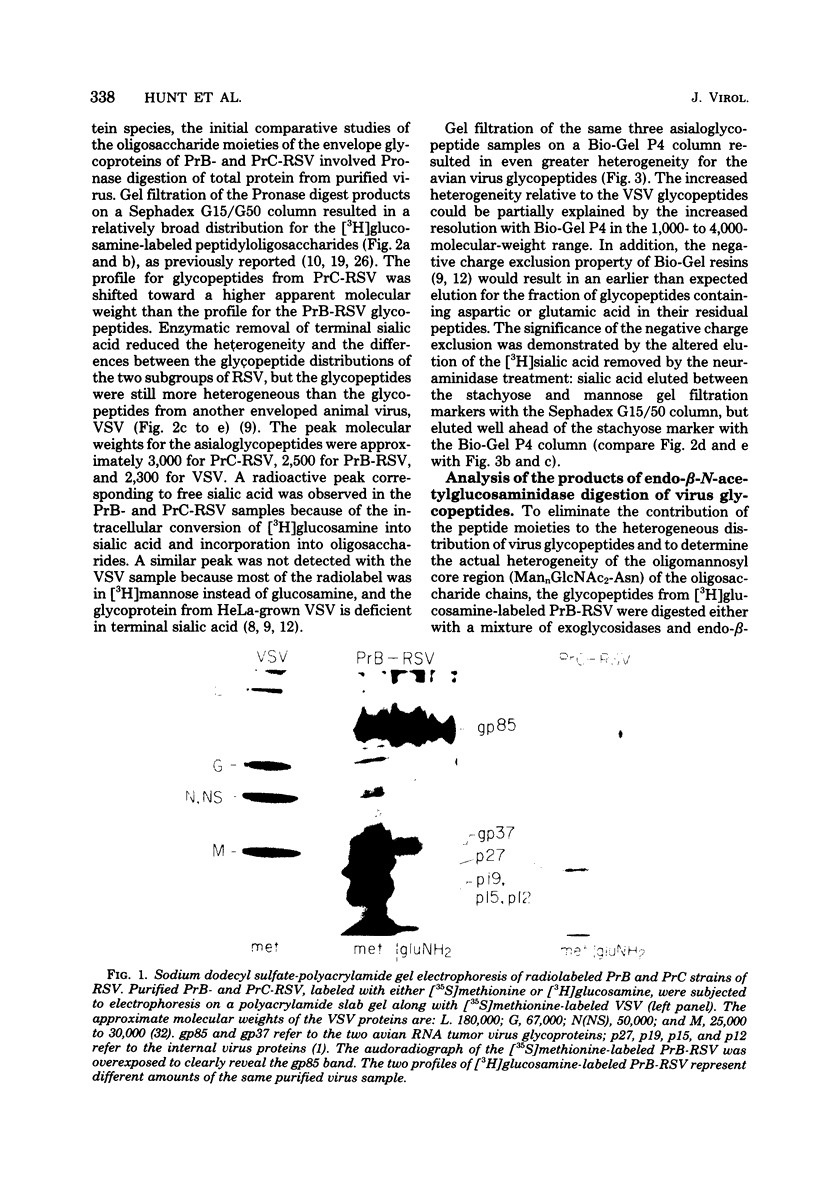
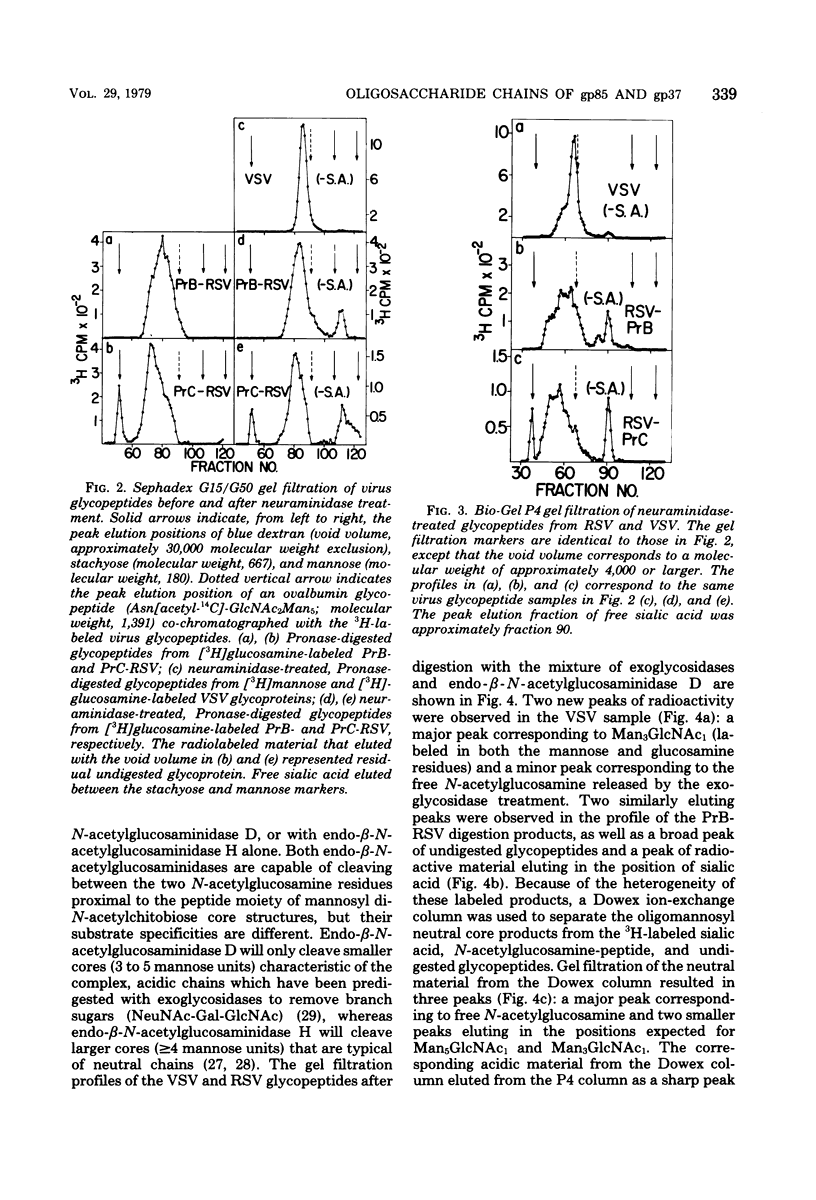
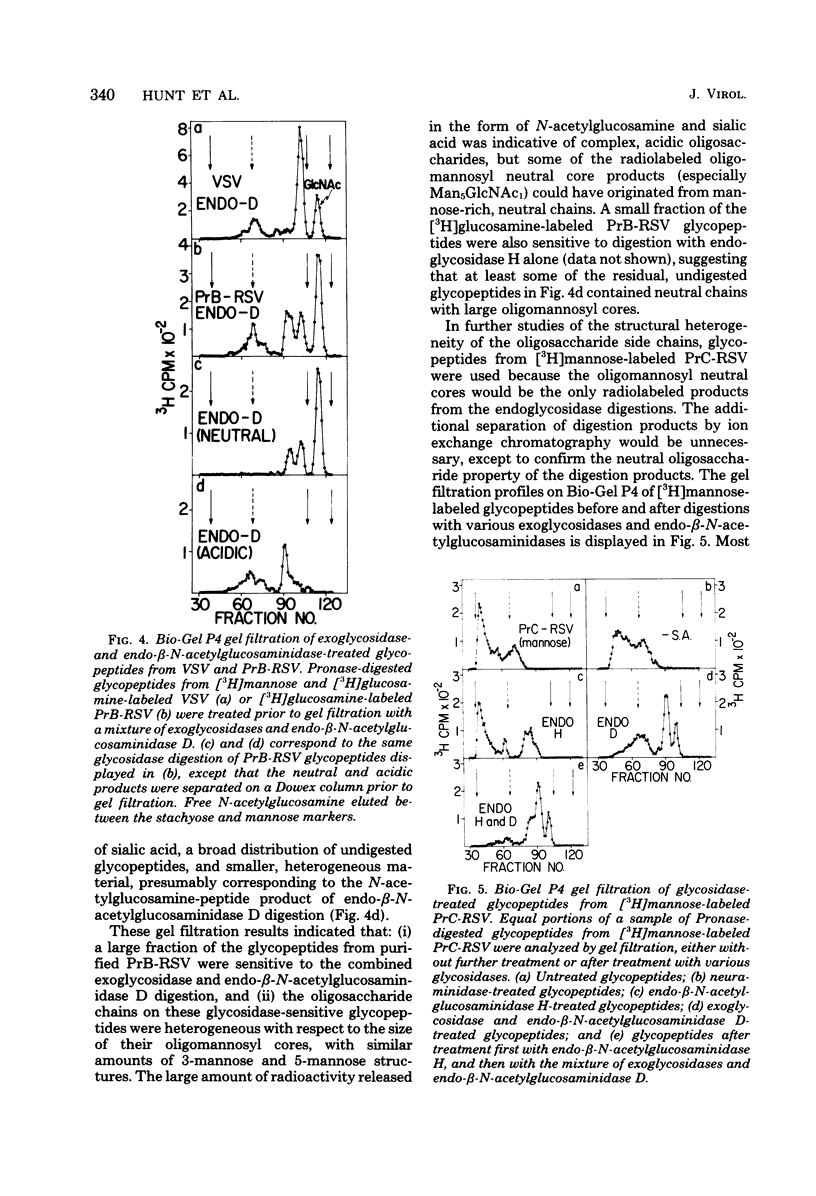
Chicken embryo fibroblasts (C/E phenotype) infected with subgroups B and C of the Prague strain of Rous sarcoma virus were radiolabeled with either [6-3H]-glucosamine or [2-3H]mannose, and virus was purified from the growth medium. The large envelope glycoprotein, gp85, was the only major radiolabeled component of purified virus. Pronase-digested glycopeptides from purified virus were analyzed by a combination of (i) gel filtration with columns of Sephadex G15/G50 and Bio-Gel P4 and (ii) enzymatic digestion of the oligosaccharide chains with specific exoglycosidases and endo-β-N-acetylglucosaminidases. The rather broad molecular weight distribution (approximately 2,000 to 4,000) for glycopeptides in these studies and previous studies in other laboratories was shown to represent actual heterogeneity in the carbohydrate moieties: (i) the glycopeptides contained both mannose-rich, neutral chains and complex, acidic chains with terminal sialic acid; and (ii) both classes of asparagine-linked carbohydrate structures exhibited heterogeneity in the size of the oligomannosyl core (a mixture of approximately 5 to 9 mannose units for the neutral structures, and 3 or 5 mannose units for the acidic structures). With the [2-3H]mannose-labeled glycopeptides from Rous sarcoma virus, Prague strain subgroup C, most of the oligosaccharide chains were high-molecular-weight, acidic structures, with similar numbers of 3-mannose and 5-mannose core structures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H., Gelderblom H., Hüper G. Polypeptides of avian RNA tumor viruses. IV. Components of the viral envelope. Virology. 1972 Mar;47(3):551–566. doi: 10.1016/0042-6822(72)90545-4. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Case K. R., Morgan H. R. Surface biochemical changes accompanying primary infection with Rous sarcoma virus. I. Electrokinetic properties of cells and cell surface glycoprotein:glycosyl transferase activities. Exp Cell Res. 1974 Jan;83(1):15–24. doi: 10.1016/0014-4827(74)90682-x. [DOI] [PubMed] [Google Scholar]
- Burke D. J., Keegstra K. Purification and composition of the proteins from Sindbis virus grown in chick and BHK cells. J Virol. 1976 Dec;20(3):676–686. doi: 10.1128/jvi.20.3.676-686.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- England J. M., Bolognesi D. P., Dietzschold B., Halpern M. S. Evidence that a precursor glycoprotein is cleaved to yield the major glycoprotein of avian tumor virus. J Virol. 1977 Feb;21(2):810–814. doi: 10.1128/jvi.21.2.810-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus grown in four mammalian cell lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4011–4014. doi: 10.1073/pnas.71.10.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Summers D. F. Glycosylation of vesicular stomatitis virus glycoprotein in virus-infected HeLa cells. J Virol. 1976 Dec;20(3):646–657. doi: 10.1128/jvi.20.3.646-657.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K., Sefton B., Burke D. Sindbis virus glycoproteins: effect of the host cell on the oligosaccharides. J Virol. 1975 Sep;16(3):613–620. doi: 10.1128/jvi.16.3.613-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide N., Muramatsu T. Endo-beta-N-acetylglucosaminidase acting on carbohydrate moieties of glycoproteins. Purification and properties of the enzyme from Diplococcus pneumoniae. J Biol Chem. 1974 Aug 10;249(15):4897–4904. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Krantz M. J., Lee Y. C., Hung P. P. Carbohydrate groups in the major glycoprotein of Rous sarcoma virus. Nature. 1974 Apr 19;248(5450):684–686. doi: 10.1038/248684a0. [DOI] [PubMed] [Google Scholar]
- Krantz M. J., Lee Y. C., Hung P. P. Characterization and comparison of the major glycoprotein from three strains of Rous sarcoma virus. Arch Biochem Biophys. 1976 May;174(1):66–73. doi: 10.1016/0003-9861(76)90324-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Differences between the envelope glycoproteins and glycopeptides of avian tumor viruses released from transformed and from nontransformed cells. Virology. 1972 Nov;50(2):359–372. doi: 10.1016/0042-6822(72)90387-x. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Halpern M. S. Subunit structure of the glycoprotein complex of avian tumor virus. J Virol. 1976 Jun;18(3):956–968. doi: 10.1128/jvi.18.3.956-968.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. N., Weiss R. A. Pseudotypes of vesicular stomatitis virus determined by exogenous and endogenous avian RNA tumor viruses. Virology. 1974 Jan;57(1):271–278. doi: 10.1016/0042-6822(74)90127-5. [DOI] [PubMed] [Google Scholar]
- Mosser A. G., Montelaro R. C., Rueckert R. R. Proteins of Rous-associated virus type 61: polypeptide stoichiometry and evidence that glycoprotein gp35 is not a cleavage product of gp85. J Virol. 1977 Jul;23(1):10–19. doi: 10.1128/jvi.23.1.10-19.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Yamaguchi N., Kuchino T., Aoi Y. Alterations in surface glycoproteins and level of sialyltransferase of cells transformed by a temperature-sensitive mutant of simian virus 40. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4090–4094. doi: 10.1073/pnas.73.11.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Etchison J. R., Summers D. F. Glycosylation sites of vesicular stomatitis virus glycoprotein. J Virol. 1976 Sep;19(3):871–878. doi: 10.1128/jvi.19.3.871-878.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. A., Etchison J. R., Robertson J. S., Summers D. F., Stanley P. Specific changes in the oligosaccharide moieties of VSV grown in different lectin-resistnat CHO cells. Cell. 1978 Mar;13(3):515–526. doi: 10.1016/0092-8674(78)90325-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M. Virus-dependent glycosylation. J Virol. 1975 Jan;17(1):85–93. doi: 10.1128/jvi.17.1.85-93.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ito S., Kobata A. Structures of the carbohydrate moiety of ovalbumin glycopeptide III and the difference in specificity of endo-beta-N-acetylglucosaminidases CII and H. J Biol Chem. 1977 Oct 10;252(19):6687–6694. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Kobata A. The substrate specificities of endo-beta-N-acetylglucosaminidases CII and H. Biochem Biophys Res Commun. 1977 Sep 9;78(1):434–441. doi: 10.1016/0006-291x(77)91273-6. [DOI] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]