Background: The tumor suppressor p53 is frequently mutated in cancer.
Results: The p53 homolog in Drosophila is sensitive to mutations associated with human cancer, affecting its stability and target gene specificity.
Conclusion: The response to mutation reflects structural and functional similarities of human and Drosophila p53.
Significance: Drosophila p53 is an attractive model system for studying p53 function and mutant rescue.
Keywords: Cancer, Drosophila, p53, Protein Misfolding, Protein Stability, Conformational
Abstract
The transcription factor p53 is a key tumor suppressor protein. In about half of human cancers, p53 is inactivated directly through mutation in its sequence-specific DNA-binding domain. Drosophila p53 (Dmp53) has similar apoptotic functions as its human homolog and is therefore an attractive model system for studying cancer pathways. To probe the structure and function of Dmp53, we studied the effect of point mutations, corresponding to cancer hot spot mutations in human p53 (Hp53), on the stability and DNA binding affinity of the full-length protein. Despite low sequence conservation, the Hp53 and Dmp53 proteins had a similar melting temperature and generally showed a similar energetic and functional response to cancer-associated mutations. We also found a correlation between the thermodynamic stability of the mutant proteins and their rate of aggregation. The effects of the mutations were rationalized based on homology modeling of the Dmp53 DNA-binding domain, suggesting that the drastically different effects of a cancer mutation in the loop-sheet-helix motif (R282W in Hp53 and R268W in Dmp53) on stability and DNA binding affinity of the two proteins are related to conformational differences in the L1 loop adjacent to the mutation site. On the basis of these data, we discuss the advantages and limitations of using Dmp53 as a model system for studying p53 function and testing p53 rescue drugs.
Introduction
The tumor suppressor p53 protein is a key transcription factor that regulates the cell cycle and maintains the integrity of the human genome (1, 2). Human p53 (Hp53)4 has a complex structural organization, consisting of a structured DNA-binding domain (DBD) and tetramerization domain, connected by a flexible linker, and intrinsically disordered N- and C-terminal regions (3). In about half of all human tumors, p53 is inactivated as a result of a single missense point mutation. Most cancer-associated p53 mutations are found in the sequence-specific DBD of the protein, with residues Arg-175, Gly-245, Arg-248, Arg-249, Arg-273, and Arg-282 having the highest mutation frequency in human cancer (4).
Common p53 cancer mutations have been divided into two broad categories: DNA contact mutations and structural mutations. DNA contact mutations impair the function of the protein through the loss of a key DNA contact residue (e.g. R248Q and R273H). In contrast, structural mutations perturb the structure of the protein, resulting in different degrees of thermodynamic stability loss. Although some structural mutations have only a moderate effect on stability (e.g. G245S and R249S), others, such as R175H, Y220C, and R282W, reduce the stability of the DNA-binding domain by up to 4 kcal/mol (5–8). This destabilization has a major impact on the folding state of the mutant proteins in cancer cells. The wild-type DBD is only marginally stable and has a melting temperature only slightly above body temperature. Highly destabilized mutants are therefore largely unfolded under physiological conditions, thus losing their transcriptional activity (8). Moreover, they exert a dominant-negative effect on wild-type p53 and the paralogs p63 and p73 (9, 10).
The Drosophila genome contains a single p53 family member (hereafter Dmp53). Despite the large evolutionary distance, Dmp53 appears to be structurally and functionally similar to its human homolog, Hp53, and plays an important role in apoptosis induction (11–13). However unlike Hp53, Dmp53 has no role in cell-cycle arrest (14). In response to radiation-induced DNA damage, Dmp53 activates the transcription of reaper to initiate apoptosis (13). The consensus DNA target sequence of Dmp53 is similar to that of its mammalian counterpart (12, 15). Dmp53 homozygous null mutant flies are viable and fertile and have no obvious developmental defects (16). Ectopic expression of Dmp53 in Drosophila eye discs causes cell death and leads to overt eye defect. Moreover, the Dmp53 R155H and K259H mutant transgenes, like their Hp53 counterpart mutants (R175H and R273H, respectively), exert a dominant-negative effect on transcriptional transactivation by the endogenous wild-type (WT) Dmp53 (12, 13).
There is a growing interest in Drosophila as a model organism for elucidating the processes and networks involved in human diseases, in particular cancer (17), as well as for identifying targets for therapeutic intervention and for drug screening (18–21). Unraveling the structure of Dmp53 and the effect of cancer-associated mutations should contribute toward this effort. Structurally, only the tetramerization domain of Dmp53 has been fully characterized. Like its human counterpart, the Dmp53 tetramer consists of a dimer of dimers but has additional stabilizing structural elements and a fundamentally different tetrameric interface (22). The DBDs of Dmp53 and Hp53 appear to share their overall structural features, and a number of important residues involved in zinc coordination and DNA binding are conserved (13).
Here, we probed the structure and function of Dmp53 by studying the effect of point mutations, corresponding to cancer hot spot mutations in Hp53, on the stability and DNA binding properties of the full-length protein. On the basis of these data, we discuss the use of Dmp53 as a model system for studying p53 function and for testing p53 rescue drug candidates.
EXPERIMENTAL PROCEDURES
Homology Modeling
Homology models of the Drosophila DBD (residues 77–277) were generated using the Swiss-Model server (23) with structures of the human DBD as a template. The quality of the models both globally and locally was assessed based on the scoring functions provided by the Swiss-Model server and on the comparison of the fluctuations in models generated using different Hp53 crystal structures as a template. Another important selection criterion was retention of tetrahedral zinc coordination.
Cloning
The coding sequence of the gene encoding for Drosophila p53 (National Center for Biotechnology Information (NCBI) gi7211766) was amplified using the primers 5′-CTAGGATCCATGTATATATCACAGCCAATGTCG-3′ and 5′-TCTGACCTCGAGTCATGGCAGCTCGTAGGCACG-3′. The Dmp53 mutants were generated by PCR site-directed mutagenesis using primers: 5′-GATTTTGACAGtGGACCACGGGA-3′ for constructing the R155H Dmp53 mutant; 5′-AACTCGTGTATCaGcCGAAAAGAAAC-3′ for G233S; 5′-GTATCGGGCGAAgcGAAACTTCCTT-3′ for K235S; 5′-ATACATGTTcAcATATGTACGTGC-3′ for K259H, and 5′-CAAGCGGGATtGgATCCAAGACGA-3′ for R268W (small letters indicate the substitution made to produce the mutation). The PCR products where digested with BamHI and XhoI, purified, and cloned into a modified pET24a+ expression vector. The resulting plasmids coded for fusion proteins with an N-terminal His6 tag, the lipoyl domain of the dihydrolipoamide acetyltransferase from Bacillus stearothermophilus, and a tobacco etch virus protease cleavage site followed by Dmp53.
Protein Expression and Purification
The appropriate vector was transformed into Escherichia coli BL21 tuner cells (Novagen) for overexpression. Expression cultures were incubated at 37 °C at 220 rpm until A600 nm reached 0.7–0.8. The medium was supplemented with 0.1 mm ZnSO4, and expression was induced with 0.5 mm isopropyl-1-thio-β-d-galactopyranoside at 18 °C. Cells were harvested 14 h later by centrifugation. The cell pellet from 8–10 liters of culture was suspended in 50 mm Tris (pH 7.4), 200 mm NaCl, 0.8% Triton, 10% glycerol, 5 mm imidazole, 10 mm β-mercaptoethanol, and 50× tablets of EDTA-free Complete protease inhibitor (Roche Diagnostics). Cells were sonicated on ice for a total time of 8 min, with 90 s in between 30-s pulses. The soluble fraction was loaded onto an Amersham Biosciences 26/20 column packed with Ni-Sepharose (GE Healthcare). The His-tagged fusion protein was eluted using a 0–500 mm imidazole gradient over 10 column volumes. The pooled fractions from the Ni-Sepharose column were digested with tobacco etch virus protease and dialyzed against low salt buffer containing 50 mm Tris (pH 7.4), 50 mm NaCl, 10% glycerol, and 10 mm β-mercaptoethanol. The dialyzed sample was purified further on a HiPrep heparin 16/10 FF column (GE Healthcare). Elution was done using a 0–500 mm NaCl gradient over 10 column volumes. The pooled fractions were concentrated using an Amicon Ultra-15 30-kDa filter (Millipore) and buffer-exchanged to 25 mm sodium phosphate buffer (pH 7.4), 10% glycerol, 300 mm NaCl, and 1 mm TCEP. Protein samples were flash-frozen and stored in liquid nitrogen for further use. The purified p53 variants were analyzed by liquid chromatography-mass spectrometry on an Orbitrap (Thermo) mass spectrometer and identified by Sequest 3.31 software. Dmp53 variants were further verified by Western blot analysis using a Dmp53 antibody (antibody Dmp53 7A4, Developmental Studies Hybridoma bank). Quadruple mutant of human p53 (QMHp53) was verified using an Hp53 antibody (polyclonal antibody 240, Abcam).
Differential Scanning Fluorometry (DSF)
Experiments were performed using SYPRO Orange (Invitrogen) as the fluorescent probe, which binds quantitatively to the hydrophobic protein patches exposed upon thermal denaturation. Real-time melting analysis was performed using a Corbett Rotor-Gene 6000 real-time quantitative PCR thermocycler. The excitation (λex) and emission (λem) wavelengths used were 460 and 510 nm, respectively. Heating from 28 to 70 °C, a constant heating rate of 250 °C/h was applied. 10 μm of the protein was briefly mixed with SYPRO orange (10×) in 25 mm sodium phosphate buffer (pH 7.4), 300 mm NaCl, 10% glycerol, 10% dimethyl sulfoxide (DMSO), and 1 mm TCEP buffer. The apparent melting temperature (Tm) of the protein was determined from the inflection point of the melting curve.
Differential Scanning Calorimetry (DSC)
Experiments were performed using a MicroCal VP-Capillary DSC instrument (MicroCal, Amherst, MA) with an active cell volume of 0.4 ml. Temperatures from 10 to 90 °C were scanned at a rate of 250 °C/h. 20 μm samples of Dmp53 (and up to 80 μm samples of Dmp53 R155H mutant) were analyzed in 25 mm sodium phosphate buffer (pH 7.4), 300 mm NaCl, 10% glycerol, and 1 mm TCEP buffer, which was also used for base-line scans. Data analysis was performed using MicroCal Origin software.
Light Scattering
We monitored protein aggregation by measuring light scattering at 37 °C at 500 nm as excitation and emission wavelengths (excitation slit width 0.8 nm, emission slit width 2 nm) using a Horiba (Kyoto, Japan) FluoroMax-3 spectrophotometer. Experiments were performed at a p53 protein concentration of 3 μm in 25 mm sodium phosphate buffer (pH 7.4), 300 mm NaCl, 10% glycerol, 5% DMSO, and 1 mm TCEP buffer that was pre-equilibrated for 30 min at 37 °C. The samples were constantly stirred. Data were analyzed using KaleidaGraphTM software (Synergy).
Thioflavin-T Assays
We measured the thioflavin-T fluorescence at 482 nm upon excitation at 450 nm to detect aggregates (excitation slit width 3 nm, emission slit width 4 nm) using a Horiba FluoroMax-3 spectrofluorometer. Time-resolved fluorescence was recorded immediately after adding 3 μm p53 protein to pre-equilibrated buffer (25 mm sodium phosphate buffer (pH 7.4), 300 mm NaCl, 10% glycerol, 5% DMSO, 1 mm TCEP, and 10 μm thioflavin-T).
Fluorescence Anisotropy DNA Binding Measurements
All oligonucleotides for DNA binding studies by fluorescence anisotropy were synthesized by Sigma and HPLC-purified prior to use. They were labeled only on the 5′ end of the forward strand with fluorescein to avoid energy transfer between fluorophores as described before (24). The oligonucleotides were annealed in 10 mm sodium phosphate buffer (pH 7.4), 100 mm NaCl, and 1 mm EDTA by incubating the DNA at 100 °C for 5 min and then allowing the reaction to slowly cool down to room temperature. The tested oligonucleotides represented the gadd45 promoter recognition element (aka RE) and nonspecific DNA (random DNA) as described previously (25). Fluorescence anisotropy measurements were recorded on a PerkinElmer Life Sciences LS55 luminescence spectrometer equipped with a Hamilton MICROLAB titrator and controlled by laboratory software. The excitation and emission wavelengths used were 480 and 530 nm, respectively, and the slit widths for excitation and emission were 20 nm. The photomultiplier voltage used was 700 V with an integration time of 5 s for each measurement. The protein concentration was 0.4–1 μm (monomers), and the initial DNA concentration was 20 nm. Experiments were performed at room temperature in 0.2 mg/ml BSA, 25 mm sodium phosphate buffer (pH 7.4), 213.4 mm NaCl, 10% glycerol, 1 mm TCEP, with a total ionic strength of 225 mm, as described previously to minimize artifacts caused by nonspecific binding effects (24). p53 variants were titrated into a cuvette containing fluorescein-labeled DNA, and the solution was stirred for 30 s. After 60 s, the fluorescence polarization values were measured, using an integration time of 5 s. Data analysis was performed as described previously (24).
RESULTS
Comparison of the DNA-binding Domains of Human and Drosophila p53
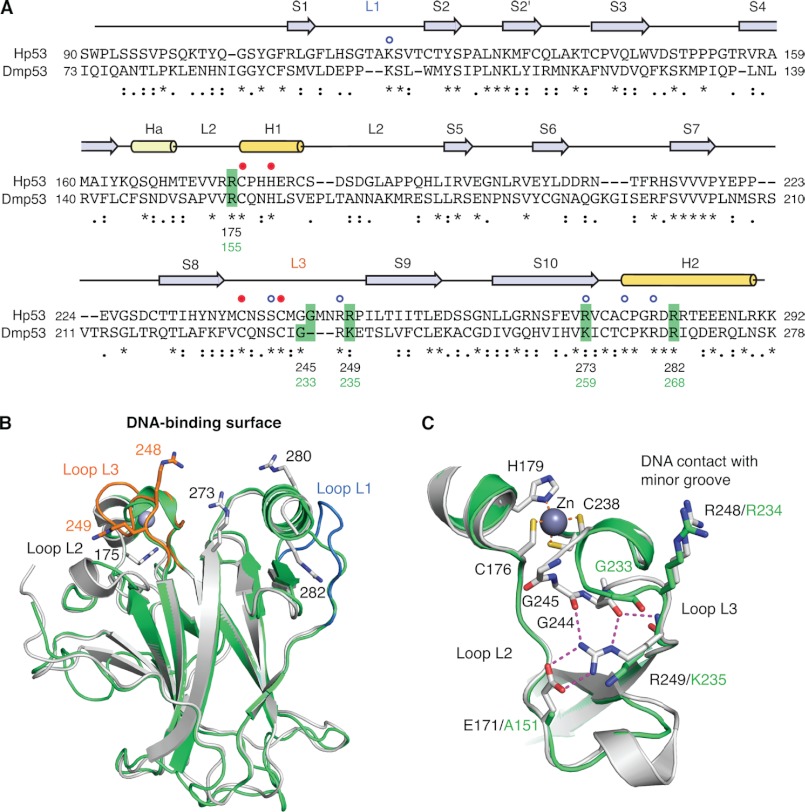
We compared the DBD of human and Drosophila p53 based on sequence alignment and homology modeling (Fig. 1). The structured region of the Hp53 DBD (residues 91–292) shares 25% sequence identity and 43% similarity with the corresponding region of Dmp53. The overall structural features of the human DBD are conserved in Drosophila. The DBD of both proteins consists of a central β-sandwich that forms the basic scaffold for the DNA-binding surface, which is composed of two large loops (L2 and L3) and a loop-sheet-helix motif. The L2 and L3 loops are held together via coordination of a zinc ion by one histidine and three cysteine residues that are conserved in Hp53 and Dmp53. There are a number of insertions and deletions in loop regions of the Hp53 DBD when compared with Dmp53. Most significantly, all three loops forming the DNA-binding surface have small regions that are absent from the fly protein, including the L1 loop that is involved in binding to the DNA major groove via Lys-120 in Hp53. Key DNA contact residues are conserved, except for the DNA backbone contact Arg-273, which is replaced by a lysine that may play a similar role in DNA binding in Dmp53. The L3 loop of Hp53 has a dual role in DNA binding by making direct DNA contacts with the minor groove of p53 response element via Arg-248 and by forming part of the symmetrical DBD-DBD interface between two DBDs upon binding to a DNA half-site. The hairpin conformation of the L3 loop in Hp53 is stabilized via the guanidinium group of Arg-249, which forms the center of an extended polar interaction network that is disrupted in the cancer mutant R249S (7, 26). This loop is shorter in Dmp53 and has a lysine instead of Arg-249 in Hp53 (Fig. 1, B and C). To probe the structure and function of the Dmp53 DBD, we studied the effects of five Dmp53 mutations, corresponding to cancer hot spot mutations in Hp53, on protein stability and DNA binding. These cancer-associated mutations comprise four structural mutations located in different DNA-binding motifs and the DNA contact mutation R273H (Table 1).
FIGURE 1.
Comparison of the DNA-binding domains of human and Drosophila p53. A, structure-based sequence alignment of Hp53 and Dmp53 DBD. Sequence conservation is indicated below the alignment: * denotes conserved residues, : denotes similar residues, and · denotes somewhat similar residues as classified by CLUSTALW2 (44). Secondary structure elements of Hp53 (S, β-strand; H1/2, α-helix; Ha, 310-helix; L, Loop) are shown above the alignment based on Protein Data Bank (PDB) entry 2XWR (45). The blue open circles denote Hp53 DNA contact residues, and the closed red circles indicate the residues coordinating the zinc ion. Dmp53 residues that were mutated in this study and the corresponding cancer hot spot sites in Hp53 are highlighted in green. B, superposition of the crystal structure of Hp53 DBD (gray ribbon diagram; PDB entry 2XWR) (45) and a homology model of the Dmp53 DBD (green ribbon diagram). Loops L1 and L3 of Hp53 are highlighted in blue and orange, respectively. The side chains of a number of structurally and functionally important arginine residues of Hp53 are shown as stick models. C, superposition of the L2/L3 region in Hp53 (gray) and Dmp53 (green) showing large differences in the L3 loop conformation. The extensive polar interaction network of Arg-249 in Hp53 that stabilizes the hairpin conformation of L3 is shown by broken lines.
TABLE 1.
p53 cancer hot spot mutations
| Cancer mutation in Hp53 | Corresponding mutation in Dmp53 | Classification of the mutation in Hp53 | Location in the structure |
|---|---|---|---|
| R175H | R155H | Structural mutation | L2 loop |
| G245S | G233S | Structural mutation | L3 loop |
| R249S | K235S | Structural mutation | L3 loop |
| R273H | K259H | DNA contact mutation | β-Strand 10 |
| R282W | R268W | Structural mutation | Loop-sheet-helix motif |
Thermodynamic Stability of WT Dmp53 and Its Mutants
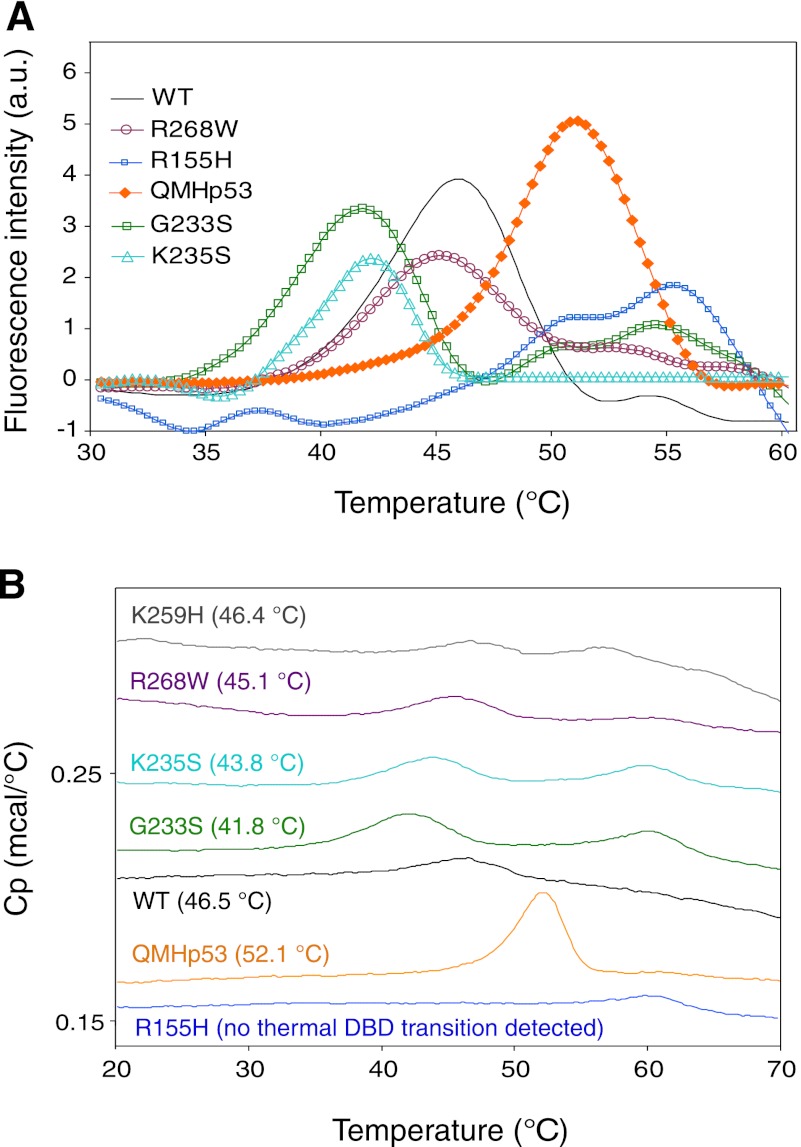
Protein stability plays a major role in regulating cellular p53 levels. The relatively low thermodynamic stability of Hp53, which exhibits an apparent Tm of about 45 °C (25), makes it prone to destabilization by single point mutations. Various oncogenic point mutations in the DBD of Hp53 destabilize the protein thermodynamically (8), rendering it less functional, and as a consequence, the cell has a greater propensity for tumorigenesis (27, 28). We measured the effects of amino acid substitutions in Dmp53, corresponding to cancer hot spot mutations in Hp53 (Table 1), on the stability of Dmp53 by both DSF and DSC. The observed Tm is not the true melting temperature but an apparent one because Hp53 does not denature reversibly with increasing temperature (29). The results (Fig. 2, Table 2), albeit qualitative, reflect the relative destabilizing effects of the mutations. The apparent Tm values derived from the DSF and DSC assays are in close agreement. For example, the apparent Tm obtained for WT Dmp53 by DSF (45.8 °C) is only slightly lower than the Tm measured using DSC (46.5 °C) (Fig. 2, A and B). As such, the Tm of full-length Dmp53 was very similar (only slightly higher) to that of full-length wild-type Hp53 (25). The highest apparent Tm recorded (52.1 °C) was for a super-stable quadruple mutant of human p53 (QMHp53) with a stabilized DBD (30, 31). The apparent Tm values obtained for the Dmp53 mutants K259H and R268W were similar to the WT Dmp53 (46.4, 45.1, and 46.5 °C, respectively, measured by DSC; Fig. 2B), whereas the Dmp53 mutants G233S and K235S displayed a reduced apparent Tm of 41.8 and 43.8 °C, respectively (DSC results; Fig. 2B). Interestingly, the R155H Dmp53 mutant (corresponding to the R175H mutant of Hp53) showed no evidence of any structural transition using DSC, even at high protein concentrations. The results of DSF analysis of these Dmp53 variants are essentially similar to those obtained by DSC (Fig. 2, A and B).
FIGURE 2.
Thermodynamic stability measurements of WT Dmp53 and its mutants. A, melting curves for WT Dmp53 and its mutants obtained by DSF. a. u., arbitrary units. B, DSC. Average apparent Tm values are given in parentheses. Representative DSC melting curves are offset for clarity. The thermodynamics measured by both techniques are in close agreement. Cp, heat capacity.
TABLE 2.
Stability and functional characterization of Dmp53 variants
| p53 variant | Tm (measured by DSF) | Tm (measured by DSC) | Rate of denaturation (relative to WT) | Kd (DNA)a |
|---|---|---|---|---|
| °C | °C | nm | ||
| WT Dmp53 | 45.8 | 46.5 | Same | 180 ± 10 |
| R155H | —b | —b | —b | NDc |
| G233S | 41.8 | 41.8 | Fast | ND |
| K235S | 42.2 | 43.8 | Fast | 310 ± 20 |
| R268W | 45.2 | 45.1 | Same | ND |
| K259H | Not measured | 46.4 | Same | ND |
| QMHp53 | 51.2 | 52.1 | Slow | 150 ± 5 |
a Binding affinity to the gadd45 response element measured by fluorescence anisotropy at a total ionic strength of 225 mm.
b — indicates not detectable.
c ND = No detectable binding in μm range.
These results are very similar to those previously demonstrated for the corresponding human p53 mutants (6, 8, 25). For example, the Hp53 contact mutant R273H (corresponding to the K259H mutant in Dmp53) had a similar apparent Tm as the WT Hp53 protein, whereas the R175H mutation (corresponding to R155H in Dmp53), which is located close to the zinc coordination sphere, highly destabilizes the Hp53 structure. Moderate destabilization of the DBD structure was reported for the G245S and R249S mutants of Hp53 (corresponding to the G233S and K235S mutants in Dmp53). These mutations reduce the thermodynamic stability of human p53 by ∼2.5–5 °C (6, 8, 25). Taken together, the DSC and DSF data indicate a strong resemblance between the thermodynamic stability of WT Hp53 and Dmp53. The Hp53 and Dmp53 mutations, except for Dmp53 R268W, have a similar destabilizing effect on the two proteins.
The different effects of the R268W mutation in Dmp53 and the corresponding R282W mutation in Hp53 on protein stability are most likely the result of differences in the conformation of the adjacent L1 loop of the two proteins. In Dmp53, this loop adopts a more recessed conformation. Hence, the R268W mutation is likely to cause a smaller structural perturbation than the R282W mutation in Hp53 (see Discussion).
Kinetic Stability of Wild-type Dmp53 and Its Mutants
Native and unfolded Hp53 are in equilibrium, but unfolded Hp53 can irreversibly denature and form small, soluble aggregates that can then grow and form larger aggregates that precipitate. Moreover, thermodynamically destabilized Hp53 DBD mutants are also kinetically unstable and unfold, aggregate, and precipitate more rapidly than the wild type at body temperature (32, 33).
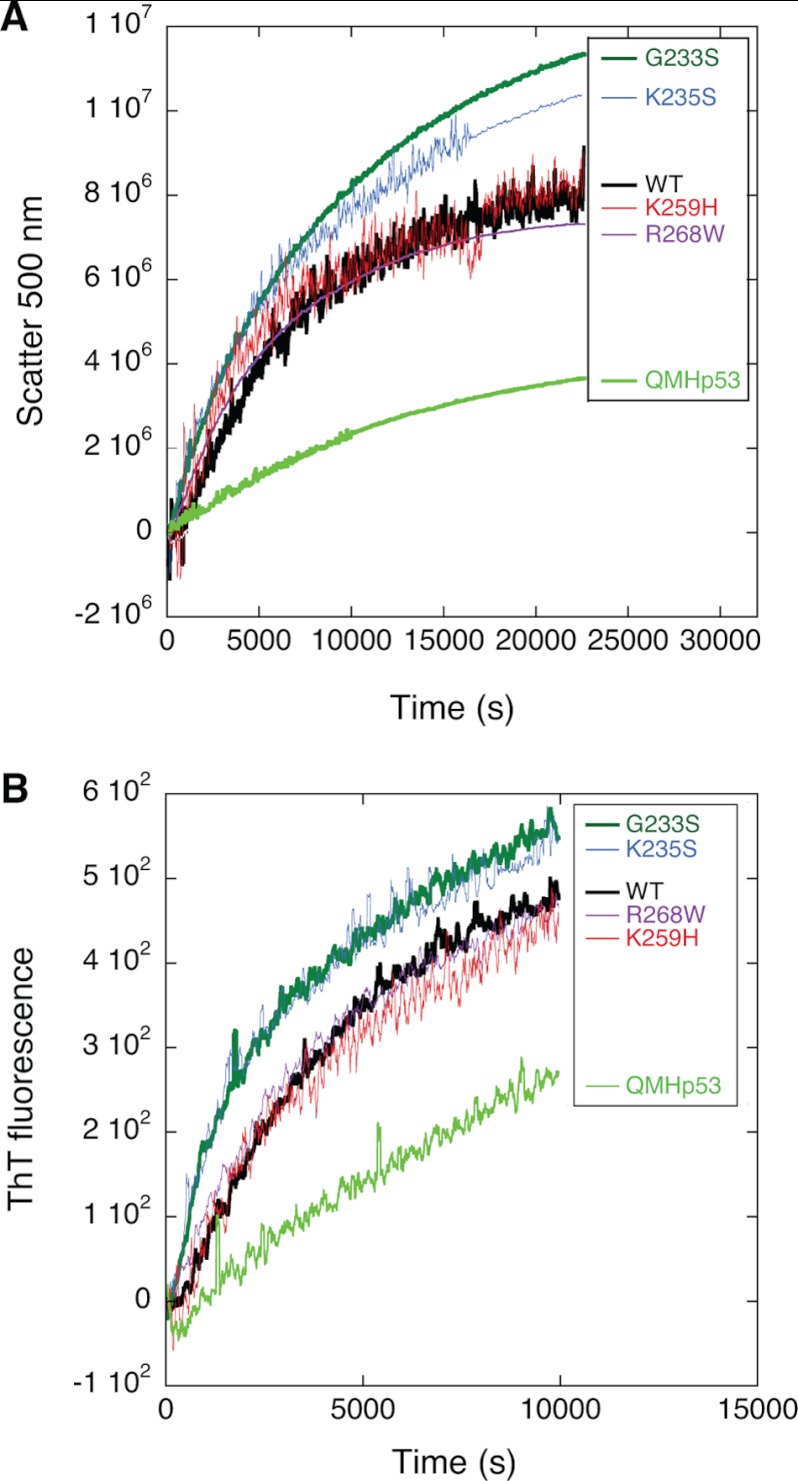
As indicated by our DSF and DSC studies, Hp53 and its hot spot mutants share a high degree of similar thermodynamic stability with the corresponding Dmp53 variants. Therefore, we examined the kinetics of unfolding of Dmp53 variants at 37 °C to gain a deeper understanding of the basis for their inactivation. We measured thioflavin-T fluorescence (λex = 450 nm, λem = 482 nm) and light scattering (λ = 500 nm), which increase greatly upon aggregation (34), to monitor the transition between the native and aggregated forms of the Dmp53 variants (Fig. 3). The slowest aggregation rate was recorded for the Hp53 super-stable mutant (QMHp53). The Dmp53 mutants K259H and R268W exhibited similar rates of aggregation as WT Dmp53. The thermodynamically destabilized Dmp53 mutants, G233S and K235S, displayed faster aggregation rates than the wild type (Table 2).
FIGURE 3.
Kinetic stability measurement of WT Dmp53 and its mutants. Aggregation rate was monitored at 37 °C using thioflavin T (ThT) fluorescence (λex = 450 nm, λem = 482 nm) (A) and static light scattering (λ = 500 nm) (B). The kinetics measured by both techniques are in close agreement.
Taken together, the results from the thermodynamic stability measurements and qualitative analysis of the aggregation data indicate that the more severely thermodynamically destabilized mutants (G233S and K235S) also aggregate faster, whereas mutants that have a thermodynamic stability similar to that of the WT Dmp53 (K259H and R268W) aggregate at a similar rate as the WT Dmp53.
Functional Characterization of Wild-type Dmp53 and Its Mutants
Wild-type Hp53 binds to a double-stranded DNA consensus site containing two copies of the decameric half-site motif RRRC(A/T)|(T/A)GYYY, separated by up to 13 bp. In this sequence, R and Y represent purines and pyrimidines, respectively, and the vertical bar indicates the center of symmetry within the half-site (35). Despite the differences in key DNA-binding motifs between Hp53 and Dmp53, they were shown to have similar DNA binding specificity (15). We analyzed the DNA binding affinity of different Dmp53 variants using fluorescence anisotropy at the relatively high ionic strength of 225 mm to reduce nonspecific electrostatic interactions at the DNA binding interface (36). Two types of 5′-fluorescein-labeled 30-mer double-stranded DNA were tested: one containing the specific recognition element from the gadd45 promoter (RE) and the other containing a random 30-bp sequence as used in previous studies (random DNA) (25).
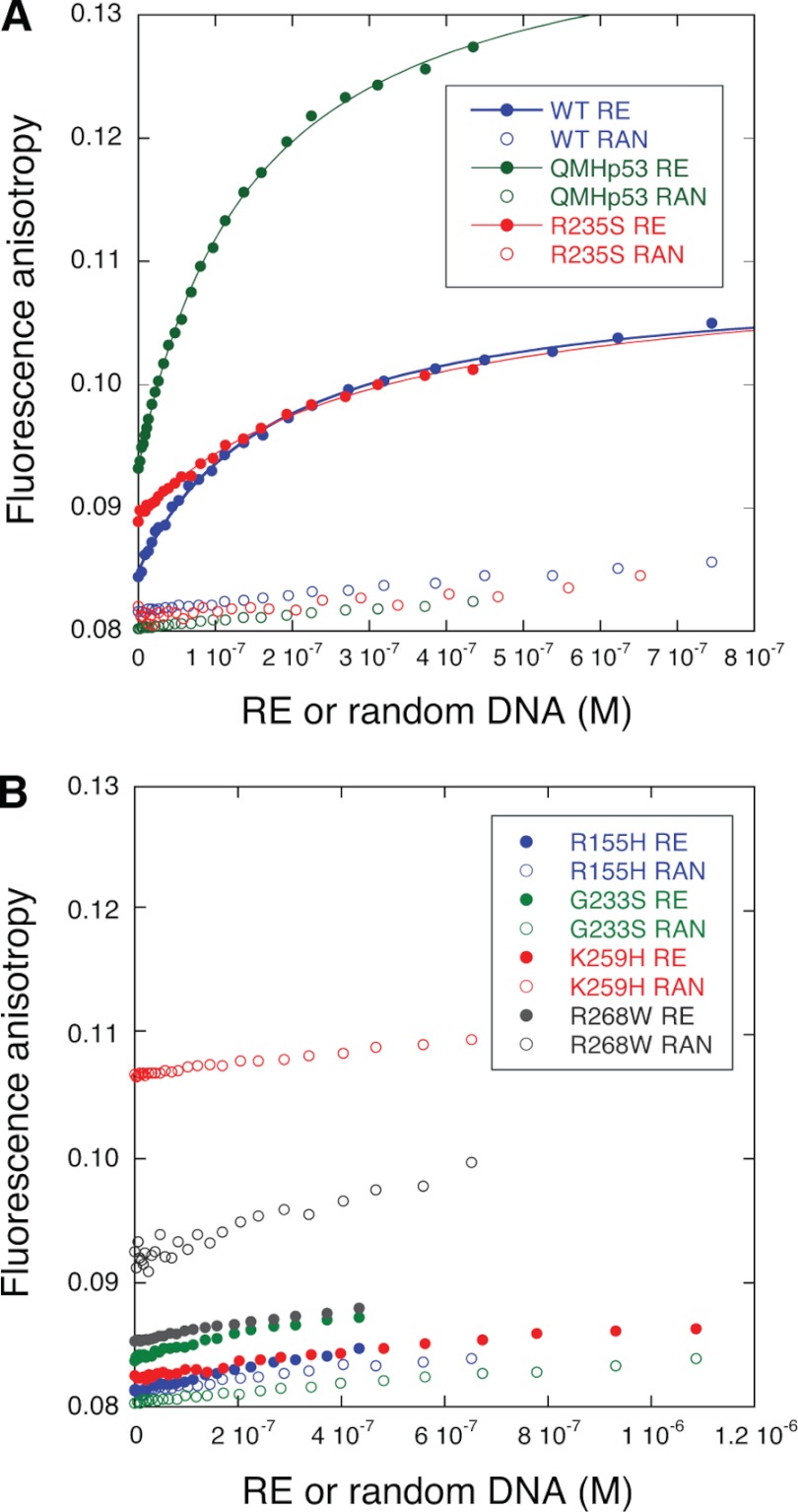
QMHp53 and WT Dmp53 bound the RE with similar affinity. The dissociation constant, KD, defined as the concentration at which 50% of the target DNA is bound to the protein, was 150 ± 5 and 180 ± 10 nm, respectively. Both proteins bound random DNA about 10 times less tightly (Fig. 4A). The mutant K235S, which moderately reduced Dmp53 stability (Figs. 2 and 3), lowered Dmp53 binding affinity to the RE 1.7-fold (KD = 310 ± 20 nm). In contrast, the Dmp53 mutation K259H, which corresponds to the human contact mutant R273H, almost completely abrogated the affinity of the protein to either the RE or the random DNA. The negligible binding of this mutant to the RE can be attributed to nonspecific binding by the C terminus of the protein as shown for human p53 (25). The highly destabilized R155H mutant, the moderately destabilized G233S mutant, and the stable R268W mutant all abrogated binding to the RE like the nonfunctional mutant K259H (Table 2).
FIGURE 4.
Fluorescence anisotropy measurements of WT Dmp53 and its mutants. DNA binding to 5-fluorescein-labeled double-stranded 30-mers containing either the recognition element from the gadd45 promoter (RE, closed circles) or a randomly generated sequence of 30 bases (RAN, open circles) was measured. A, p53 variants that bind the RE with high affinity. Green, QMHp53; blue, WT Dmp53; red, Dmp53 R235S mutant. B, p53 variants that abrogated the affinity to the RE. Blue, Dmp53 R155H; green, G233S; red, K259H; black, R268W.
DISCUSSION
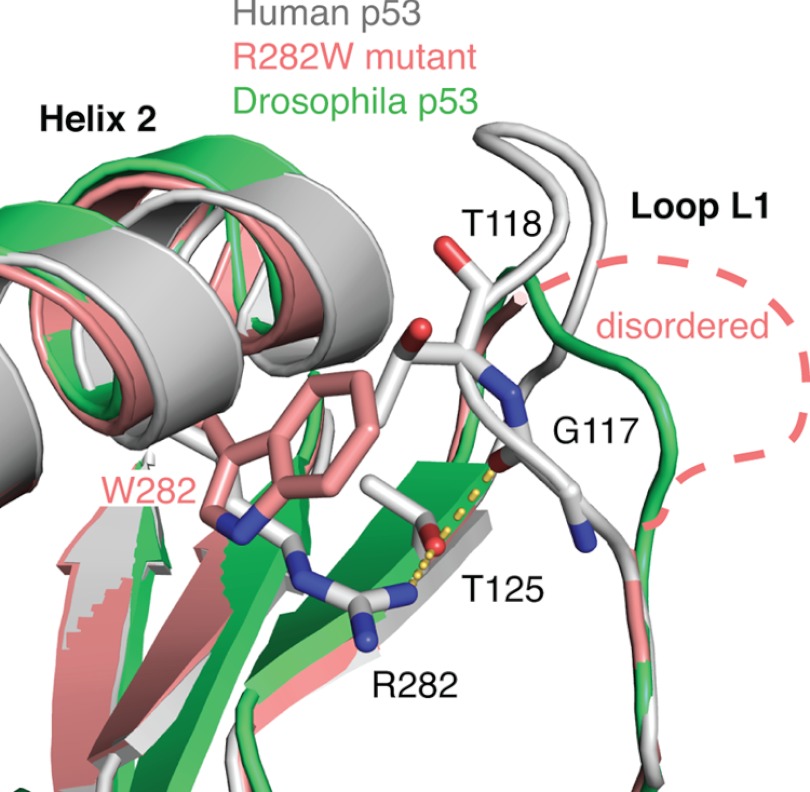
Our stability measurements showed that the Hp53 and Dmp53 proteins have a similar thermodynamic stability despite the relatively low sequence conservation. The structural similarity of the DBDs is reflected by the effects of cancer-related point mutations on protein stability. The only mutation that had a drastically different effect on the stability of the two proteins was the R268W mutation in Dmp53 and its corresponding cancer-associated R282W mutation in Hp53. This diverse effect of the mutation in the fly and the human protein can be rationalized on the basis of the available crystal structures and homology modeling (Fig. 5). Arg-282 in Hp53 is located in helix H2 of the loop-sheet-helix motif, and its guanidinium group forms a number of polar interactions, stabilizing the loop-sheet-helix motif that includes the L1 loop. Upon mutation to Trp, interactions with the L1 loop are broken because of steric clashes, and parts of the L1 loop are disordered in the crystal structure of the mutant (6). This structural perturbation results in a significant loss of protein stability. The L1 loop is three residues shorter in Dmp53 and, consequently, it adopts a more recessed conformation. Mutation of Arg-268 to Trp is therefore likely to be associated with a smaller structural perturbation, and hence smaller stability loss, than the R282W mutation in Hp53.
FIGURE 5.
Loop-sheet-helix motif in Dmp53 and Hp53. Superposition of the structures of wild-type Hp53 (gray; PDB entry 2XWR) (45), the cancer mutant R282W (pink; PDB entry 2J21) (6), and a homology model of Drosophila p53 (green) shows that the L1 loop, which is perturbed upon R282W mutation in Hp53, adopts a recessed conformation in the Drosophila homolog.
The DNA binding properties of most tested Dmp53 mutants paralleled those of the corresponding cancer hot spot mutations in Hp53, yet intriguingly, as observed with the stability measurements, the DNA binding properties of the R268W mutant did not follow the effects of the corresponding mutation in Hp53. Although the R282W mutant of Hp53 retains sequence-specific DNA binding at low temperature (8) and shows significant transcriptional activity in yeast-based assays at 30 °C (37), the R268W mutant of Dmp53 did not bind the specific gadd45 target DNA, suggesting that the introduction of the tryptophan disrupts the DNA binding interface. Loss of DNA binding affinity for the putative DNA contact mutant K259H and the structural mutant R155H is in agreement with the effects of the corresponding mutations in Hp53. The Hp53 contact mutant R273H has lost binding specificity but retains weak DNA binding that was attributed mainly to nonspecific binding of DNA via the C terminus (25). The R175H mutation results in loss of zinc binding and perturbation of the L2/L3 loop region that binds to the minor groove of DNA response elements via Arg-248 (28, 38). Given the difference in length, and hence conformation of the L3 loop in both p53 variants (Dmp53 has a 3-residue deletion), it is not surprising that the effects on DNA binding of L3 mutations, G233S and K235S in Dmp53 and their human counterparts G245S and R249S, do not exactly mirror each other. In both Hp53 and Dmp53, however, mutations that potentially affect the conformation of the L3 loop by substituting glycine residues that provide flexibility and allow unique backbone conformations, or residues that form stabilizing interactions, result in impaired DNA binding, albeit to a different extent. This suggests that retaining the wild-type conformation of the L3 loop is essential for correct positioning of the DNA contact residue Arg-234 in Dmp53 (and potentially also for mediating DBD-DBD interactions upon binding to target DNA as a tetramer, as reported for Hp53 (39)).
In recent years, Drosophila has attracted increased interest as a model organism for studying the evolution of tumor suppressor pathways and their deregulation in cancer (18, 40). Despite the low sequence conservation between Dmp53 and Hp53, many structural and functional features, in particular the DNA binding specificity, are conserved. Dmp53 is therefore an attractive model system for studying different aspects of p53 function, especially because the complexity of the mammalian p53 interactome with its multiple feedback loops complicates the analysis of cell-based studies or animal models. Another advantage of the Drosophila p53 system is the possibility to easily evaluate its function in vivo. Ectopic overexpression of WT Dmp53 in the fly eyes causes overt eye phenotype, whereas expression of a nonfunctional Dmp53 mutant results in normal eyes (13).
One of the emerging fields of cancer research is the development of mutant p53 rescue drugs. About one-third of p53 cancer mutations simply destabilize the protein, making such mutants amenable to rescue by small-molecule stabilizers (41). Such stabilization restores transcriptional activation and inhibits aggregation of p53 mutants (42, 43). Given the comparable thermodynamic stability of Hp53 and Dmp53 and their broadly similar energetic and functional response to cancer-associated mutations, Dmp53-based studies, such as the eye defect mentioned above, could be used for identifying and testing p53 stabilizers, e.g. chemical chaperones. Such assays in the intact organism could complement studies with the human variants. Dmp53 would generally not be suitable, however, for developing or testing drugs that are mechanistically linked to specific surface properties that may differ in the two proteins, for example, by binding to a mutation-induced surface crevice in the cancer mutant Y220C that is unique to the Hp53 variant (42).
Overall, our stability and DNA binding measurements suggest similar structural and functional features of the Hp53 and Dmp53 proteins, making Dmp53 an interesting model system for developing protein-refolding small molecules to rescue the function of conformational p53 mutants. Drosophila should be useful for large-scale screening for such drug candidates in the whole animal. The unique characteristics of the DBD in Dmp53 have to be taken into account when using Dmp53 models.
Acknowledgments
We thank Christopher Johnson and Rainer Wilcken for technical advice.
This research was supported in part by grants from the Israel Science Foundation and United States Department of Defense (Congressionally Directed Medical Research Programs (CDMRP)) and by MRC Programme Grant G0901534.
- Hp53
- human p53
- Dmp53
- Drosophila p53
- RE
- gadd45 response element
- DSF
- differential scanning fluorometry
- DSC
- differential scanning calorimetry
- DBD
- DNA-binding domain
- TCEP
- tris(2-carboxyethyl)phosphine
- DMSO
- dimethyl sulfoxide.
REFERENCES
- 1. Vousden K. H., Prives C. (2009) Blinded by the light: the growing complexity of p53. Cell 137, 413–431 [DOI] [PubMed] [Google Scholar]
- 2. Lane D., Levine A. (2010) p53 research: the past thirty years and the next thirty years. Cold Spring Harb. Perspect. Biol. 2, a000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joerger A. C., Fersht A. R. (2008) Structural biology of the tumor suppressor p53. Annu. Rev. Biochem. 77, 557–582 [DOI] [PubMed] [Google Scholar]
- 4. Petitjean A., Mathe E., Kato S., Ishioka C., Tavtigian S. V., Hainaut P., Olivier M. (2007) Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum. Mutat. 28, 622–629 [DOI] [PubMed] [Google Scholar]
- 5. Cho Y., Gorina S., Jeffrey P. D., Pavletich N. P. (1994) Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science 265, 346–355 [DOI] [PubMed] [Google Scholar]
- 6. Joerger A. C., Ang H. C., Fersht A. R. (2006) Structural basis for understanding oncogenic p53 mutations and designing rescue drugs. Proc. Natl. Acad. Sci. U.S.A. 103, 15056–15061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joerger A. C., Ang H. C., Veprintsev D. B., Blair C. M., Fersht A. R. (2005) Structures of p53 cancer mutants and mechanism of rescue by second-site suppressor mutations. J. Biol. Chem. 280, 16030–16037 [DOI] [PubMed] [Google Scholar]
- 8. Bullock A. N., Henckel J., Fersht A. R. (2000) Quantitative analysis of residual folding and DNA binding in mutant p53 core domain: definition of mutant states for rescue in cancer therapy. Oncogene 19, 1245–1256 [DOI] [PubMed] [Google Scholar]
- 9. Li Y., Prives C. (2007) Are interactions with p63 and p73 involved in mutant p53 gain of oncogenic function? Oncogene 26, 2220–2225 [DOI] [PubMed] [Google Scholar]
- 10. Xu J., Reumers J., Couceiro J. R., De Smet F., Gallardo R., Rudyak S., Cornelis A., Rozenski J., Zwolinska A., Marine J. C., Lambrechts D., Suh Y. A., Rousseau F., Schymkowitz J. (2011) Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 7, 285–295 [DOI] [PubMed] [Google Scholar]
- 11. Jin S., Martinek S., Joo W. S., Wortman J. R., Mirkovic N., Sali A., Yandell M. D., Pavletich N. P., Young M. W., Levine A. J. (2000) Identification and characterization of a p53 homologue in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 97, 7301–7306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ollmann M., Young L. M., Di Como C. J., Karim F., Belvin M., Robertson S., Whittaker K., Demsky M., Fisher W. W., Buchman A., Duyk G., Friedman L., Prives C., Kopczynski C. (2000) Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell 101, 91–101 [DOI] [PubMed] [Google Scholar]
- 13. Brodsky M. H., Nordstrom W., Tsang G., Kwan E., Rubin G. M., Abrams J. M. (2000) Drosophila p53 binds a damage response element at the reaper locus. Cell 101, 103–113 [DOI] [PubMed] [Google Scholar]
- 14. Brodsky M. H., Weinert B. T., Tsang G., Rong Y. S., McGinnis N. M., Golic K. G., Rio D. C., Rubin G. M. (2004) Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol. Cell Biol. 24, 1219–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brandt T., Petrovich M., Joerger A. C., Veprintsev D. B. (2009) Conservation of DNA-binding specificity and oligomerisation properties within the p53 family. BMC Genomics 10, 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rong Y. S., Titen S. W., Xie H. B., Golic M. M., Bastiani M., Bandyopadhyay P., Olivera B. M., Brodsky M., Rubin G. M., Golic K. G. (2002) Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev. 16, 1568–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vidal M., Cagan R. L. (2006) Drosophila models for cancer research. Curr. Opin. Genet. Dev. 16, 10–16 [DOI] [PubMed] [Google Scholar]
- 18. Rudrapatna V. A., Cagan R. L., Das T. K. (2012) Drosophila cancer models. Dev Dyn. 241, 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giacomotto J., Ségalat L. (2010) High-throughput screening and small animal models, where are we? Br. J. Pharmacol. 160, 204–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaltiel-Karyo R., Frenkel-Pinter M., Egoz-Matia N., Frydman-Marom A., Shalev D. E., Segal D., Gazit E. (2010) Inhibiting α-synuclein oligomerization by stable cell-penetrating β-synuclein fragments recovers phenotype of Parkinson's disease model flies. PLoS One 5, e13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scherzer-Attali R., Pellarin R., Convertino M., Frydman-Marom A., Egoz-Matia N., Peled S., Levy-Sakin M., Shalev D. E., Caflisch A., Gazit E., Segal D. (2010) Complete phenotypic recovery of an Alzheimer's disease model by a quinone-tryptophan hybrid aggregation inhibitor. PLoS One 5, e11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ou H. D., Löhr F., Vogel V., Mäntele W., Dötsch V. (2007) Structural evolution of C-terminal domains in the p53 family. EMBO J. 26, 3463–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnold K., Bordoli L., Kopp J., Schwede T. (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics 22, 195–201 [DOI] [PubMed] [Google Scholar]
- 24. Weinberg R. L., Freund S. M., Veprintsev D. B., Bycroft M., Fersht A. R. (2004) Regulation of DNA binding of p53 by its C-terminal domain. J. Mol. Biol. 342, 801–811 [DOI] [PubMed] [Google Scholar]
- 25. Ang H. C., Joerger A. C., Mayer S., Fersht A. R. (2006) Effects of common cancer mutations on stability and DNA binding of full-length p53 compared with isolated core domains. J. Biol. Chem. 281, 21934–21941 [DOI] [PubMed] [Google Scholar]
- 26. Suad O., Rozenberg H., Brosh R., Diskin-Posner Y., Kessler N., Shimon L. J., Frolow F., Liran A., Rotter V., Shakked Z. (2009) Structural basis of restoring sequence-specific DNA binding and transactivation to mutant p53 by suppressor mutations. J. Mol. Biol. 385, 249–265 [DOI] [PubMed] [Google Scholar]
- 27. Hamroun D., Kato S., Ishioka C., Claustres M., Béroud C., Soussi T. (2006) The UMD TP53 database and website: update and revisions. Hum. Mutat. 27, 14–20 [DOI] [PubMed] [Google Scholar]
- 28. Joerger A. C., Fersht A. R. (2007) Structure-function-rescue: the diverse nature of common p53 cancer mutants. Oncogene 26, 2226–2242 [DOI] [PubMed] [Google Scholar]
- 29. Bullock A. N., Henckel J., DeDecker B. S., Johnson C. M., Nikolova P. V., Proctor M. R., Lane D. P., Fersht A. R. (1997) Thermodynamic stability of wild-type and mutant p53 core domain. Proc. Natl. Acad. Sci. U.S.A. 94, 14338–14342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nikolova P. V., Henckel J., Lane D. P., Fersht A. R. (1998) Semirational design of active tumor suppressor p53 DNA binding domain with enhanced stability. Proc. Natl. Acad. Sci. U.S.A. 95, 14675–14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joerger A. C., Allen M. D., Fersht A. R. (2004) Crystal structure of a superstable mutant of human p53 core domain: insights into the mechanism of rescuing oncogenic mutations. J. Biol. Chem. 279, 1291–1296 [DOI] [PubMed] [Google Scholar]
- 32. Friedler A., Veprintsev D. B., Hansson L. O., Fersht A. R. (2003) Kinetic instability of p53 core domain mutants: implications for rescue by small molecules. J. Biol. Chem. 278, 24108–24112 [DOI] [PubMed] [Google Scholar]
- 33. Wang G., Fersht A. R. (2012) First-order rate-determining aggregation mechanism of p53 and its implications. Proc. Natl. Acad. Sci. U.S.A. 109, 13590–13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parker T. G., Dalgleish D. G. (1977) The use of light-scattering and turbidity measurements to study the kinetics of extensively aggregating proteins: αs-casein. Biopolymers 16, 2533–2547 [DOI] [PubMed] [Google Scholar]
- 35. el-Deiry W. S., Kern S. E., Pietenpol J. A., Kinzler K. W., Vogelstein B. (1992) Definition of a consensus binding site for p53. Nat. Genet. 1, 45–49 [DOI] [PubMed] [Google Scholar]
- 36. Weinberg R. L., Veprintsev D. B., Fersht A. R. (2004) Cooperative binding of tetrameric p53 to DNA. J. Mol. Biol. 341, 1145–1159 [DOI] [PubMed] [Google Scholar]
- 37. Dearth L. R., Qian H., Wang T., Baroni T. E., Zeng J., Chen S. W., Yi S. Y., Brachmann R. K. (2007) Inactive full-length p53 mutants lacking dominant wild-type p53 inhibition highlight loss of heterozygosity as an important aspect of p53 status in human cancers. Carcinogenesis 28, 289–298 [DOI] [PubMed] [Google Scholar]
- 38. Paleček E., Ostatná V., Černocká H., Joerger A. C., Fersht A. R. (2011) Electrocatalytic monitoring of metal binding and mutation-induced conformational changes in p53 at picomole level. J. Am. Chem. Soc. 133, 7190–7196 [DOI] [PubMed] [Google Scholar]
- 39. Kitayner M., Rozenberg H., Kessler N., Rabinovich D., Shaulov L., Haran T. E., Shakked Z. (2006) Structural basis of DNA recognition by p53 tetramers. Mol Cell 22, 741–753 [DOI] [PubMed] [Google Scholar]
- 40. Rutkowski R., Hofmann K., Gartner A. (2010) Phylogeny and function of the invertebrate p53 superfamily. Cold Spring Harb. Perspect. Biol. 2, a001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joerger A. C., Fersht A. R. (2010) The tumor suppressor p53: from structures to drug discovery. Cold Spring Harb. Perspect. Biol. 2, a000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilcken R., Liu X., Zimmermann M. O., Rutherford T. J., Fersht A. R., Joerger A. C., Boeckler F. M. (2012) Halogen-enriched fragment libraries as leads for drug rescue of mutant p53. J. Am. Chem. Soc. 134, 6810–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilcken R., Wang G., Boeckler F. M., Fersht A. R. (2012) Kinetic mechanism of p53 oncogenic mutant aggregation and its inhibition. Proc. Natl. Acad. Sci. U.S.A. 109, 13584–13589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 45. Natan E., Baloglu C., Pagel K., Freund S. M., Morgner N., Robinson C. V., Fersht A. R., Joerger A. C. (2011) Interaction of the p53 DNA-binding domain with its N-terminal extension modulates the stability of the p53 tetramer. J. Mol. Biol. 409, 358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]