Abstract
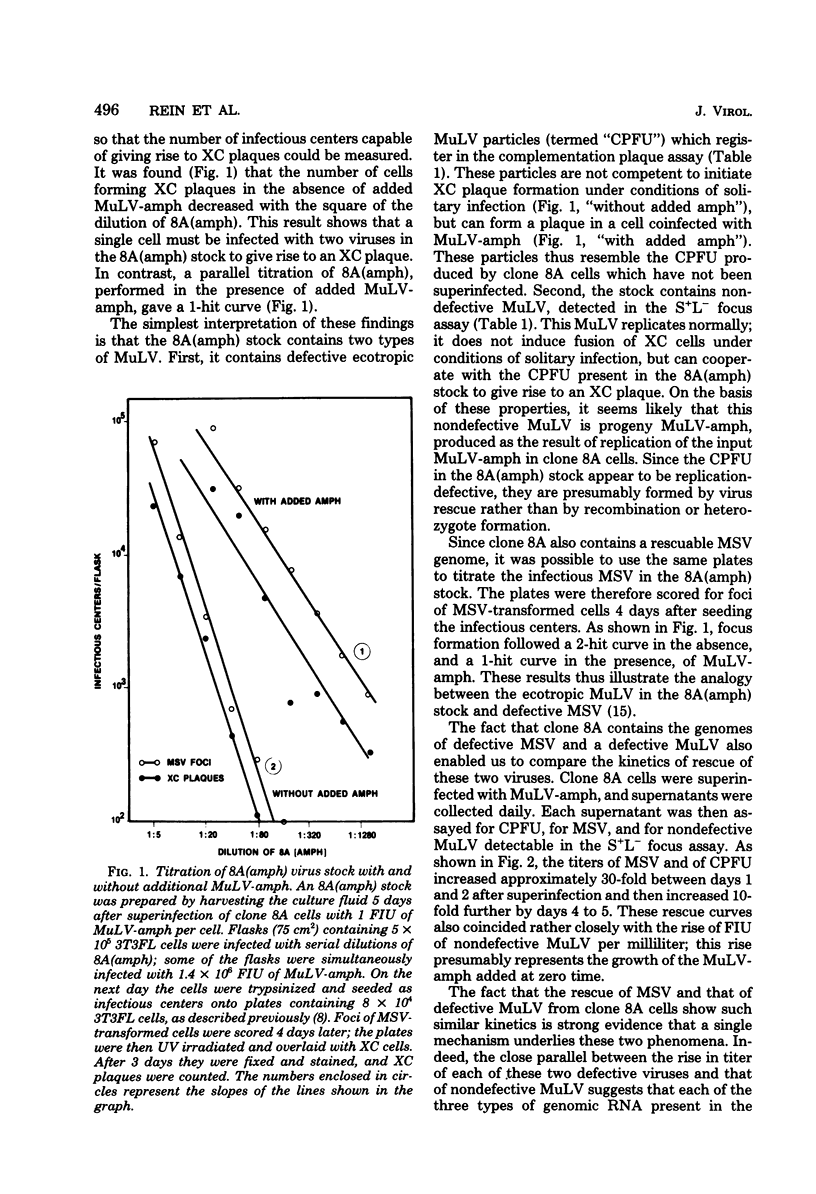
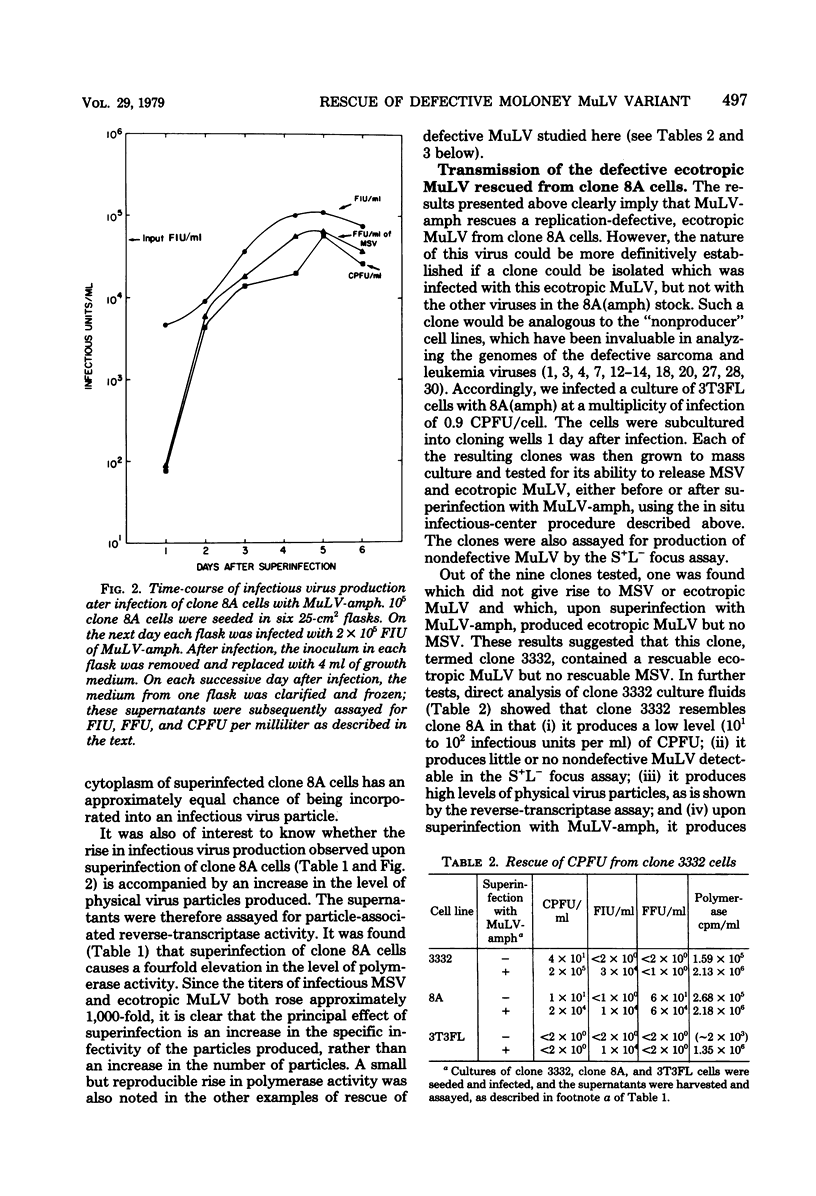
We have described a clone of mouse cells, termed “8A,” which appears to be infected with a replication-defective variant of Moloney murine leukemia virus (MuLV) (Rein et al., J. Virol. 25:146-156, 1978). Clone 8A cells release virus particles which do not form plaques in the standard XC test. However, approximately 102 particles per ml of clone 8A supernatant do form plaques in a modified XC test (the “complementation plaque assay”), in which the assay cells are coinfected with the XC-negative, nondefective amphotropic MuLV as well as the test virus. Superinfection of clone 8A cells themselves with amphotropic MuLV results in the production of ∼105, rather than ∼102, particles per ml which register in the complementation plaque assay. This increase is due to the rescue of replication-defective ecotropic MuLV from clone 8A cells by amphotropic MuLV since (i) this ecotropic MuLV can only form XC plaques in cells which are coinfected with amphotropic MuLV; and (ii) it is possible to transmit this defective variant, rescued from superinfected clone 8A cells, to a fresh clone of normal mouse cells. The time course of production of the rescued MuLV particles by superinfected clone 8A cells is virtually identical to that of rescue from these cells of murine sarcoma virus. Amphotropic MuLV superinfection of “NP-N” cells, which contain a “non-plaque-forming” variant of N-tropic MuLV (Hopkins and Jolicoeur, J. Virol. 16:991-999, 1975), also increases the titer of particles registering in the complementation plaque assay; thus, NP-N cells, like clone 8A cells, contain a rescuable defective variant of ecotropic MuLV.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Biologic characterization of mammalian cells transformed by a primate sarcoma virus. Virology. 1973 Apr;52(2):562–567. doi: 10.1016/0042-6822(73)90351-6. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Jainchill J. L., Todaro G. J. Murine sarcoma virus transformation of BALB-3T3 cells: lack of dependence on murine leukemia virus. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1236–1243. doi: 10.1073/pnas.66.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bryant M. L., Klement V. Clonal heterogeneity of wild mouse leukemia viruses: host ranges and antigenicity. Virology. 1976 Sep;73(2):532–536. doi: 10.1016/0042-6822(76)90415-3. [DOI] [PubMed] [Google Scholar]
- Chan E. W., Schiop-Stansly P. E., O'Connor T. E. Rescue of cell-transforming virus from a non-virus-producing bovine cell culture transformed by feline sarcoma virus. J Natl Cancer Inst. 1974 Feb;52(2):469–472. doi: 10.1093/jnci/52.2.469. [DOI] [PubMed] [Google Scholar]
- Duran-Troise G., Bassin R. H., Rein A., Gerwin B. I. Loss of Fv-1 restriction in Balb/3T3 cells following infection with a single N tropic murine leukemia virus particle. Cell. 1977 Mar;10(3):479–488. doi: 10.1016/0092-8674(77)90035-6. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., O'Connor T. E. Tissue culture assay of helper activity of murine leukemia virus for murine sarcoma virus. J Natl Cancer Inst. 1968 Jun;40(6):1199–1212. [PubMed] [Google Scholar]
- Gardner M. B. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr Top Microbiol Immunol. 1978;79:215–259. doi: 10.1007/978-3-642-66853-1_5. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S., Bassin R. H., Gerwin B. I., Rein A. Dual susceptibility of A 3T3 mouse cell line to infection by N- and B-tropic murine leukemia virus: apparent lack of expression of the FV-1 gene. Int J Cancer. 1974 Jul 15;14(1):106–113. doi: 10.1002/ijc.2910140113. [DOI] [PubMed] [Google Scholar]
- Graf T., Royer-Pokora B., Schubert G. E., Beug H. Evidence for the multiple oncogenic potential of cloned leukemia virus: in vitro and in vitro studies with avian erythroblastosis virus. Virology. 1976 Jun;71(2):423–433. doi: 10.1016/0042-6822(76)90370-6. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Hopkins N., Jolicoeur P. Variants of N-tropic leukemia virus derived from BALB/c mice. J Virol. 1975 Oct;16(4):991–999. doi: 10.1128/jvi.16.4.991-999.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki R., Langlois A. J., Chabot J., Beard J. W. Component of strain MC29 avian leukosis virus with the property of defectiveness. J Virol. 1971 Dec;8(6):821–827. doi: 10.1128/jvi.8.6.821-827.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Hays E. F., Doyle T., Linkhart S., Medeiros E., Pickering R. Oncornaviruses produced by murine leukemia cells in culture. Virology. 1977 Sep;81(2):363–370. doi: 10.1016/0042-6822(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Parkman R., Levy J. A., Ting R. C. Murine sarcoma virus: the question of defectiveness. Science. 1970 Apr 17;168(3929):387–389. doi: 10.1126/science.168.3929.387. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C. Endogenous ecotropic mouse type C viruses deficient in replication and production of XC plaques. J Virol. 1976 May;18(2):411–417. doi: 10.1128/jvi.18.2.411-417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A. L., Gerwin B. I., Bassin R. H., Schwarm L., Schidlovsky G. A replication-defective variant of Moloney murine leukemia virus. I. Biological characterization. J Virol. 1978 Jan;25(1):146–156. doi: 10.1128/jvi.25.1.146-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Bassin R. H. Replication-defective ecotropic murine leukemia viruses: Detection and quantitation of infectivity using helper-dependent XC plaque formation. J Virol. 1978 Nov;28(2):656–660. doi: 10.1128/jvi.28.2.656-660.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P. Isolation and characterization of a primate sarcoma virus: mechanism of rescue. Int J Cancer. 1973 Jul 15;12(1):138–147. doi: 10.1002/ijc.2910120115. [DOI] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Parks W. P., Vass W. C., Scolnick E. M. Isolation of a fibroblast nonproducer cell line containing the Friend strain of the spleen focus-forming virus. Virology. 1977 Feb;76(2):602–615. doi: 10.1016/0042-6822(77)90242-2. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Hirokawa Y. Endogenous C-type virus of a mouse cell line and its defectiveness. J Virol. 1974 Jun;13(6):1319–1325. doi: 10.1128/jvi.13.6.1319-1325.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


