Abstract
Objective
To compare the impact of the prototype prolapse mesh Gynemesh PS to that of two new generation lower stiffness meshes, UltraPro and SmartMesh, on vaginal morphology and structural composition.
Design
A mechanistic study employing a non-human primate (NHP) model.
Setting
Magee-Womens Research Institute at the University of Pittsburgh.
Population
Parous rhesus macaques, with similar age, weight, parity and POP-Q scores.
Methods
Following IACUC approval, 50 rhesus macaques were implanted with Gynemesh PS (n=12), UltraPro with its blue line perpendicular to the longitudinal axis of vagina (n=10), UltraPro with its blue line parallel to the longitudinal axis of vagina (n=8) and SmartMesh (n=8) via sacrocolpopexy following hysterectomy. Sham operated animals (n=12) served as controls.
Main Outcome Measures
The mesh-vagina complex (MVC) was removed after 12 weeks and analyzed for histomorphology, in situ cell apoptosis, total collagen, elastin, glycosaminoglycan content and total collagenase activity. Appropriate statistics and correlation analyses were performed accordingly.
Results
Relative to sham and the two lower stiffness meshes, Gynemesh PS had the greatest negative impact on vaginal histomorphology and composition. Compared to sham, implantation with Gynemesh PS caused substantial thinning of the smooth muscle layer (1557 ± 499μm vs 866 ± 210 μm, P=0.02), increased apoptosis particularly in the area of the mesh fibers (P=0.01), decreased collagen and elastin content (20% (P=0.03) and 43% (P=0.02), respectively) and increased total collagenase activity (135% (P=0.01)). GAG (glycosaminoglycan), a marker of tissue injury, was the highest with Gynemesh PS compared to sham and other meshes (P=0.01).
Conclusion
Mesh implantation with the stiffer mesh Gynemesh PS induced a maladaptive remodeling response consistent with vaginal degeneration.
Keywords: Polypropylene mesh, vagina, morphology, connective tissue, collagenase activity
Introduction
Pelvic organ prolapse (POP) is a common condition in aging women. The annual incidence of surgery for pelvic organ prolapse is approximately 4.9 cases per 1000 women [1–4] with a direct annual cost estimated to be greater than $1 billion [5]. In recent years, synthetic polypropylene meshes have been increasingly used in the surgical repair of prolapse to overcome the high failure rates associated with native tissue repairs [1]. The goal of mesh is to provide mechanical support to what may be structurally compromised tissues [6], to improve anatomical outcomes and reduce recurrence rates [7–11]. However, the use of mesh has been limited by mesh related complications including infection, pain, exposure through the vaginal wall and erosion into adjacent structures, prompting the FDA to issue two public health notices warning against mesh use – the first in 2008 warning of serious complications associated with mesh use and the second in 2011 warning that complications are not rare events [12–16]. Exposure of mesh through the vaginal wall into the vaginal lumen is the most common complication occurring in 4–10% of abdominally [17–20] and 5–20% of vaginally implanted meshes [17–19].
To date, the impact of mesh on the vagina has not been defined and thus, the mechanism by which mesh complications occur remains unknown. Currently, light-weight (< 45 g/m2), large pore (>1000mm2) monofilament polypropylene meshes appear to be best tolerated by the vagina in reconstructive pelvic surgeries. Of these meshes, Gynemesh PS (Ethicon, Sommersville, NJ, USA), the prototype prolapse mesh, is the most widely used [20]. Since the introduction of Gynemesh PS, numerous other lighter weight polypropylene meshes have been brought to market. Virtually all are light weight and wide-pore, making it difficult to discern whether one may be associated with better patient outcomes. In a recent study, however, it was found that most of the newer generation polypropylene meshes are less stiff than Gynemesh PS [21], meaning that they undergo a greater degree of stretch when a force is applied to them. High stiffness meshes, i.e. those resistant to stretch when deformed, have been purported to directly influence the rate of mesh related complications [22, 23].
The concept that the stiffness of a material relative to that of the tissue in which it is implanted correlates with the rate of complications is not novel. Indeed, there is good evidence that the stiffness of a device directly impacts the remodeling response following implantation [24, 25]. Specifically, stiffer devices that shield the implanted tissue from experiencing normal, physiologic levels of stretch have been shown to induce thinning and degradation (degeneration) of the associated tissue due to a breakdown of key structural proteins like collagen and elastin [26, 27] in a process commonly described as “stress shielding” [24, 25]. One could argue that a similar response may occur with prolapse meshes with stiffer materials having a more negative impact on the newly incorporated tissue and underlying vagina; thereby increasing risk for mesh exposure.
To improve our understanding of the impact of synthetic mesh on the vagina and to determine how the stiffness of a mesh influences the host response, we implanted the higher stiffness prototype prolapse mesh (Gynemesh PS, Ethicon, Sommersville, NJ, USA) via sacrocolpopexy after hysterectomy and compared the response of the underlying and associated vagina to that of two lower stiffness meshes UltraPro (Ethicon, Sommersville, NJ, USA) and SmartMesh (Coloplast, Minneapolis, MN). Since the stiffness of UltraPro is highly dependent upon the direction in which it is loaded [28], we implanted it with its blue orientation lines perpendicular (less stiff) and parallel (more stiff) to the longitudinal axis of vagina. We implanted via sacrocolpopexy since it is currently the gold standard prolapse repair surgery that uses mesh. We argued that we must first define the impact of mesh on the vagina using this method prior to studying the more controversial vaginal approach. We chose to implant the mesh in parous animals with minimal prolapse as we aimed to understand the impact of mesh on the “normal” vagina prior to understanding its effect on the structurally compromised prolapsed vagina. It is noteworthy that mesh stiffness has been recently shown to be highly correlated with the mesh textile properties of weight, pore size and porosity [49]. Therefore, these factors will be considered when interpreting results. In the following paper, the histomorphologic and biochemical endpoints are reported.
Materials and Methods
Mesh
Sterile samples of three prolapse meshes [Gynecare PS (Ethicon, Sommersville, NJ, USA), UltraPro (Ethicon, Sommersville, NJ, USA) and SmartMesh (Coloplast, Minneapolis, MN)] were obtained. Based on a previously developed uniaxial tensile testing and ball-burst testing protocols, the ex-vivo stiffness of each mesh was defined (Table 1). The implantation orientation of the meshes on the vagina was according to the axis of uniaxial tensile testing which in turn reflected that which is used clinically. Gynemesh PS, the prototype polypropylene mesh for prolapse repair, was the stiffest mesh (uniaxial 0.29 ± 0.02 N/mm, ball-burst 27.5 ± 2.7 N/mm). For comparison, we used two lower stiffness meshes with (UltraPro, Ethicon) and without (SmartMesh, Coloplast) an absorbable component (poliglecaprone 25 [29]). UltraPro is highly anisotropic, in that it displays dramatically different mechanical properties according to the direction it is loaded. Thus, the orientation of implantation could significantly affect its impact on the vagina. For this reason, UltraPro was implanted with the blue orientation lines perpendicular (UltraPro perpendicular, which has the lowest stiffness, uniaxial 0.01 ± 0.00 N/mm and ball-burst 23.2 ± 2.8 N/mm) and parallel (UltraPro parallel, which has the highest stiffness, uniaxial 0.26 ± 0.09 N/mm and ball-burst 23.2 ± 2.8 N/mm) to the longitudinal axis of the vagina. The stiffness of SmartMesh is 0.18 ± 0.026 N/mm in uniaxial testing and 11.1 ± 0.9 N/mm in ball-burst testing. The weight, stiffness, pore size and thickness of these meshes are compared in Table 1.
Table 1.
Relevant textile and biomechanical properties of the three meshes used in this study [48].
| Mesh | Company | Stiffness (N/mm)
|
Density/ Weight (g/m2) | Porosity (%) | |
|---|---|---|---|---|---|
| Uniaxial test | Ball Burst test | ||||
| Gynemesh PS | Ethicon | 0.29 ± 0.02 | 27.5 ± 2.7 | 44 | 62.1 ± 3.2 |
| UltraPro perpendicular | Ethicon | 0.01 ± 0.00* | 23.2 ± 2.8 | 28 | 66.7 ± 1.5 |
| UltraProparallel | Ethicon | 0.26 ± 0.09** | 23.2 ± 2.8 | 28 | 66.7 ± 1.5 |
| SmartMesh | Coloplast | 0.18 ± 0.03 | 11.1 ± 0.9 | 19 | 77.9 ± 1.4 |
highly anisotropic mesh; stiffness measured in direction perpendicular to the blue lines;
stiffness measured in the direction of blue line.
Animals
The animals used in this study were maintained and treated according to experimental protocols approved by the Institutional Animal Care Use Committee of the University of Pittsburgh (IACUC #1008675) and in adherence to the National Institutes of Health Guidelines for the use of laboratory animals such that a minimum number of rhesus macaques (macacca mulatta) were used to answer our research question. Routine laboratory tests and regular examinations by veterinarians during a quarantine period were used to certify that these experimental animals were pathogen-free and in good physical condition. Animals were maintained in standard cages with ad libitum water and a scheduled monkey diet supplemented with fresh fruit, vegetables, and multiple vitamins daily. A 12-hour light/dark cycle (7 am to 7 pm) was used, and menstrual cycle patterns were recorded daily. Available demographic data of each NHP including age, body mass index (BMI), and parity were collected prior to and after surgery.
Surgical procedures
Following IACUC approval at the University of Pittsburgh, a total of 50 animals were used. Thirty eight middle aged parous rhesus macaques were implanted with Gynemesh PS (n=12), UltraPro perpendicular (n=10), UltraPro parallel (n=8) and SmartMesh (n=8) via sacrocolpopexy after a hysterectomy. Twelve animals underwent the identical surgery (sham) without insertion of mesh (n=12). Briefly, under general anesthesia, an abdominal hysterectomy was performed through a midline incision. The bladder was dissected off the anterior vagina to the level of the trigone and the rectum from the posterior vaginal wall to the level of the perineal body. The surgeon performing the surgery (PM) was blinded to the mesh groups until the dissection affording insertion of the mesh had been completed. At that time, the mesh which had been assigned was disclosed according to a previously determined randomization procedure with only the biotechnician unblinded in advance. If the animal was randomized to sham, no mesh was inserted and the peritoneal closure used to retroperitonealize the mesh was performed. In cases of mesh insertion, two 3 cm wide by 10 cm long straps of mesh were laid flat on the anterior and posterior wall, respectively, and secured in place with delayed absorbable suture (2-0 Biosyn™ Synovis, St. Paul, MN) along each lateral edge. The two straps of mesh were then anchored into the longitudinal ligament overlying the sacral promonotory with two delayed absorbable sutures. Excess mesh was trimmed prior to closing the peritoneum over the mesh. The abdominal muscle layer was closed with a single continuous suture, while multiple interrupted sutures were used to re-approximate the subcutaneous fat followed by a continuous subcutaneous closure (4-0 polysorb). Post-surgical care inclusive of pain medication, oral intake and activity was carried out according to a standard post-operative protocol. Twelve weeks after surgery, the mesh-tissue complex (MTC) was harvested for histomorphology and biochemical composition.
For histomorphology, the vaginal mesh-tissue complex was embedded in O.C.T. compound (Sakura Finetek USA, Inc, Torrance, CA) and cryosectioned at 7μm of thickness. The tissue was oriented with the sectioned aspect cut perpendicular to the vagina. Tissue blocks that did not achieve the correct orientation requisite for quantitative analysis, i.e., not containing full layers of vagina, obliquely sectioned with subepithelium thicker than 1039μm (mean of sham values that were ensured perpendicular sectioning plus 2 standard deviation), were excluded. Three samples from sham, Gynemesh PS and UltraPro perpendicular and two samples from SmartMesh were excluded for this reason. For biochemistry, total collagen, elastin, glycosaminoglycan (GAG) and total collagenase activity were measured from deep frozen tissue (−70°C).
Masson’s trichrome staining
Masson’s trichrome staining was performed following the instructions provided by the manufacturer. For consistency and comparability, staining times were standardized for all of the specimens. In the completed sections, nuclei stained black, collagen blue, cytoplasm and smooth muscle pink-red. The slides were viewed under a 90i Nikon Light-Fluorescence microscope. The NIS-Elements AR3.2 (Nikon) software was used to analyze the images. These images were used for an overall qualitative assessment of the impact of mesh on the vagina.
Immunofluorescent labeling of α-SMA and in situ TUNEL labeling of cell apoptosis
Briefly, tissue sections were fixed and blocked with 2% BSA. After incubating with the primary antibody, mouse anti α-SMA (Sigma), a Texas-red conjugated goat anti-mouse secondary antibody was added, after which the protocol of in situ TUNEL assay (Roche, Branchburg NJ) was followed as instructed by the manufacturer. The cell nuclei were labeled with DAPI. 2% BSA omitting primary antibody was used as a negative control for α-SMA and the enzyme solution was used as a negative control for the TUNEL assay. Positive control for cell apoptosis was set up by using the DNase induced apoptosis of vaginal fibroblast in culture. Full size images were taken under a 90i Nikon Light-Fluorescence microscope at 10x magnification. The NIS-Elements AR3.2 (Nikon) software was used to analyze the images. The smooth muscle layer was identified by red labeling of α-SMA, which gave clear boundaries between the smooth muscle layer and subepithelium and between the smooth muscle layer and adventitia. To quantify the thickness of the subepithelium and smooth muscle layer, the images were first marked with 200μm grids, and the layers measured with an interval of 200μm, perpendicular to the longitudinal axis of the vagina. The thickness of layers in each sample was then calculated by averaging the values derived from the whole image. In addition, the number of apoptotic cells was counted in the subepithelium, smooth muscle and adventitia layers, as well as around the mesh fibers. The counting was completed across the whole image. The cells undergoing apoptosis were distinguished by green FITC-labeling overlapping with blue DAPI-labeling of nuclei. The DAPI labeled nuclei were counted to represent total cell number. The apoptotic cell numbers were expressed as % of total number of cells.
Hydroxyproline assay
Total collagen content was measured using the hydroxyproline assay as previously described [30]. The vaginal tissue was lyophilized, weighed and digested in 50 μg/ml papain solution. Purified hydroxyproline solution (0 – 20μg) was set up as standards and serially diluted purified collagen type I solution (4.7mg/ml, Sigma, St. Louis, MO) was used as an internal control. Briefly, aliquots of standards and test samples were hydrolyzed in 6N HCl at 130°C for 3 hours. Chloramine-T was added to the hydrolytes, and the oxidation was allowed to proceed. Ehrlich’s aldehyde reagent was added to each sample, and the chromophore was developed by incubating the samples at 65°C for 20 min. Absorbance of each sample was read at 550 nm using a spectrophotometer. The collagen content was estimated by assuming that 14% of the amino acid composition of collagen is hydroxyproline. Each animal was sampled three times and the experiment was repeated at least in triplicate.
Desmosine measurement
Mature elastin content was measured via a desmosine crosslink radioimmunoassay (picomoles of crosslinks / mg of total protein) as previously described [31]. The assay was carried out in collaboration with Dr. Barry Starcher.
1,9-Dimethylmethylene Blue assay
The total content of sulfated glycosaminoglycans (GAG) was measured using the 1,9-Dimethylmethylene Blue assay as previously described [32]. GAG standards were used from the Sulfated Gycosaminoglycan Assay kit (Blyscan TM, Northern Ireland). Briefly, the standards and papain digested tissue were prepared in PBS buffer and mixed with the 1, 9-Dimethylmethylene Blue solution. The mixture was centrifuged and the supernatant was read at 595nm using a spectrophotometer. The content of GAG was determined with respect to the tissue dry weight.
Total collagenase activity
After Trizol extraction of mRNA and DNA, the protein in the tissue was dialyzed against 0.1% SDS for 96 hours. The resulting protein was lyophilized and reconstituted in DPBS buffer. A biocinchoninic acid (BCA) protein assay (Thermo Fisher Scientific, Rockford IL) was performed on all samples to determine protein concentration. Total collagenase activity was measured using a collagenase activity assay (ChondrexInc, Redmond WA). The measurement was performed in duplicate following the manufacturer’s protocol except that the procollagenase activator was not added to the tested samples. After the FITC-labeled soluble type 1 collagen substrate was added to samples, fluorescence intensity indicating the enzyme activity was determined at Em/Ex = 520 nm/490 nm with the SpectraMax M2 system (Molecular Devices). Since collagenase activator was not utilized in this assay, the assay determined solely the enzyme activity of endogenous total active collagenases taking into account the activity of their inhibitors present in these samples. The enzyme activity in this assay was expressed as units per mg of protein. One unit of collagenolytic activity is defined as the cleavage of 1mg of collagen per minute.
Statistical analysis
Differences among groups in demographic data, histomorphometric endpoints and biochemical endpoints were evaluated using a one-way analysis of variance (ANOVA). Pairwise comparisons were made using the Bonferroni method. The association of the mesh stiffness, weight, and porosity with histomorphometric and biochemical data was analyzed using Spearman’s correlation coefficiency. Significance was set as P < 0.05.
Results
Demographics of nonhuman primates
As shown in Table 2, animals had similar age, parity, BMI and POP-Q scores. Although some of the primates had increased vaginal laxity consistent with multiparity, none had prolapse beyond the hymen.
Table 2.
Demographics of primates in the study. Age and BMI are expressed as mean ± standard deviation. Parity and POP-Q stage are expressed as median (1st quartile, 3rd quartile).
| Age (years) | Parity | BMI | POPQ Stage | |
|---|---|---|---|---|
| Sham | 12.9 ± 2.7 | 2 (2,4) | 26.3 ± 6.0 | 1 (1, 2) |
| Gynemesh PS | 12.9 ± 2.2 | 4 (2, 5) | 26.6 ± 3.9 | 1 (1, 1) |
| UltraPro perpendicular | 11.8 ± 2.6 | 2 (1, 3) | 23.8 ± 2.3 | 1 (1, 2) |
| UltraPro parallel | 13.0 ± 0.5 | 4 (1, 5) | 18.4 ± 1.7 | 1 (0, 1) |
| SmartMesh | 14.0 ± 2.0 | 5 (3, 5) | 29.9 ± 5.4 | 2 (1, 2) |
| P* | 0.29 | 0.2 | 0.08 | 0.39 |
indicates the overall comparison among the groups
Gross morphology of vagina
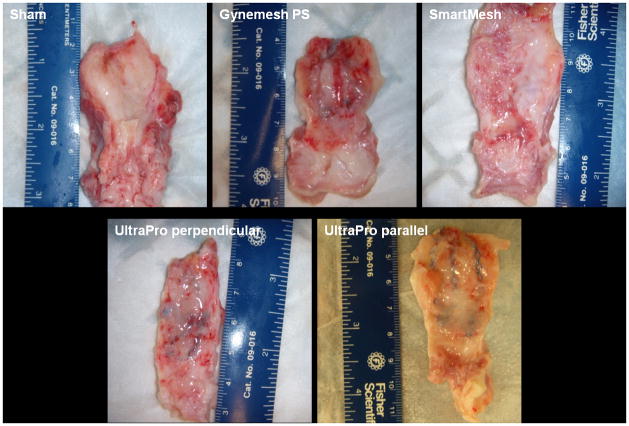
At the time of harvesting of the mesh-vagina complex, mild to moderate intra-abdominal adhesions were observed in all groups. Among the mesh implanted animals, the mesh contour was readily discernible in Gynemesh PS and UltraPro groups on the abluminal vaginal wall, but was barely visible in the SmartMesh group (Figure 1). With transvaginal palpation of the grafted area of the vagina, Gynemesh PS was more easily palpated through the vaginal lumen than UltraPro and SmartMesh.
Figure 1.
Abluminal view of the vaginal walls implanted with synthetic meshes demonstrating that mesh contour following implantation of of Gynemesh PS and UltraPro meshes is more apparent than Smartmesh which is highly integrated into the tissue.
Histology, immunofluorescent labeling of smooth muscle and cell apoptosis
As shown with Masson’s Trichrome staining (Figure 2), the sham group had clearly delineated epithelial, subepithelial connective tissue, muscularis and adventitial layers. The smooth muscle fibers were organized and oriented into inner circular and outer longitudinal sheets. The layers of the vagina were less clearly defined in the mesh implanted groups. The muscularis was most negatively impacted by mesh insertion with disruption of muscle bundles and disorganization of the surrounding dense connective tissue. Cell infiltration, predominantly in the area of the mesh fibers, was increased in the Gynemesh PS group.
Figure 2.
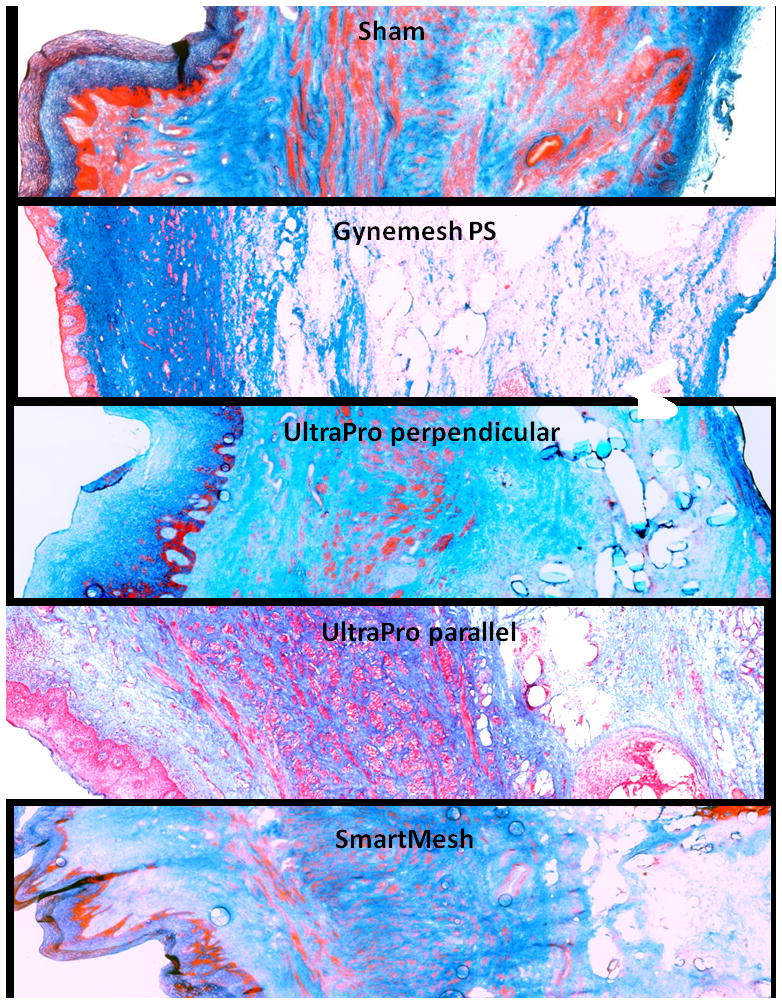
Masson Trichrome staining of (A) sham; (B) Gynemesh PS; (C) UltraPro perpendicular; (D) UltraPro parallel; (E) SmartMesh. Arrows indicate mesh fibers. Compared to the sham group which had clearly delineated layers and organized smooth muscle fibers, the layers of the vagina were less clearly defined in the mesh implanted groups, particularly in the muscularis. Mesh insertion induced disruption of smooth muscle bundles and disorganization of the surrounding dense connective tissue. Magnification: 10 x.
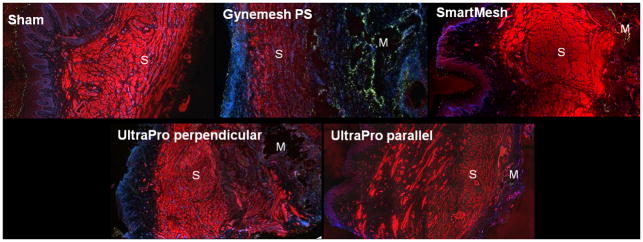
To further define these changes, we used a semi-quantitative histomorphology assessment. To do this, smooth muscle actin, cell nuclei and apoptotic cells were simultaneously labeled and viewed within a single section of full thickness vagina. By this method, all meshes were found to have a substantial negative impact on overall vaginal morphology with the greatest impact again observed in the smooth muscle layer corroborating the results of the trichrome staining (Figure 3). While the smooth muscle was clearly delineated in the sham group, it appeared more disorganized with a loss of integrity of muscle bundles in the mesh implanted groups (Figure 3). Gynemesh PS had the most profound negative impact. In the quantitative analysis, the thickness of the smooth muscle layer was significantly decreased following implantation with Gynemesh PS (866 ± 210 μm, P=0.02) relative to sham (1557 ± 499μm). In the UltraPro perpendicular (1844 ± 1101 μm), UltraPro parallel (1972 ± 660 μm) and SmartMesh (1369 ± 437 μm) groups, inspite of its disorganized appearance, the thickness of the smooth muscle layer was not different from sham and thicker than that of Gynemesh PS (Table 3). Overall, the thickness of subepithelium in five groups was similar (P= 0.8, Table 3). The correlation analysis showed that smooth muscle layer thickness was significantly correlated to the mesh properties of ball burst stiffness (ρ=−0.455, P=0.013), uniaxial stiffness (ρ=−0.491, P=0.007), weight (ρ=−0.455, P=0.013) and porosity (ρ=0.455, P=0.013).
Figure 3.
Immunofluorescent labeling of smooth muscle and in situ cell apoptosis. The red signal represents positive staining of α-SMA; the green signal represents apoptotic cells; the blue signal represents nuclei. S indicates the smooth muscle layer. M indicates the area of mesh fibers. The thickness of smooth muscle layer was significantly reduced in the Gynmesh PS group. In addition, following implantation with Gynemesh PS, the number of apoptotic cells was significantly increased in the subepithelium and adventitia compared to sham and lower stiffness meshes, predominantly surrounding the mesh fibers. For the lower stiffness meshes, apoptotic cells were higher following implantation of UltraPro perpendicular and UltraPro parallel than Smartmesh. Magnification: x10.
Table 3.
Thickness of the subepithelium and smooth muscle layers in mesh implanted vagina as compared to the sham group. Results are expressed as mean ± standard deviation.
| Subepithelium (μm) | Smooth Muscle (μm) | |
|---|---|---|
| Sham | 522 ± 189 | 1557 ± 499 |
| Gynemesh PS | 517 ± 180 | 866 ± 210* |
| UltraPro perpendicular | 700 ± 287 | 1844 ± 1101 |
| UltraPro parallel | 615 ± 127 | 1972 ± 660 |
| SmartMesh | 623 ± 167 | 1369 ± 437 |
p <0.05 as compared to the sham, UltraPro perpendicular, UltraPro parallel and SmartMesh
The number of apoptotic cells was increased in the subepithelial and adventitial areas following mesh implantation (P=0.017, P=0.004, respectively, Table 4, Figure 3). The presence of apoptotic cells in the epithelium was consistent with the high turnover of this layer according to stage of the menstrual cycle while few apoptotic cells were observed in the smooth muscle layer. Following implantation with Gynemesh PS, the number of apoptotic cells was significantly increased in the subepithelium, and adventitia compared to sham (P=0.019, P=0.015, respectively). Although apoptotic cells were present in the subepithelium after implantation with UltraPro perpendicular, UltraPro parallel and SmartMesh, the amount was not different from that of sham animals or between these less stiff products. Yet, in the adventitia there were more apoptotic cells following implantation of UltraPro perpendicular (P=0.003) and UltraPro parallel (P=0.021), but not Smartmesh (P=0.169). In addition, the absolute number of apoptotic cells surrounding the mesh fibers was increased following implantation with Gynemesh PS relative to the lower stiffness meshes (P=0.024). Interestingly, mesh stiffness as measured in the ball-burst test, mesh weight and porosity were moderately to highly correlated with the number of apoptotic cells in the adventitia (ρ =0.635, P<0.001), and peri-mesh area (0.645, P<0.001).
Table 4.
The average percentage of cells undergoing apoptosis in different tissue layers and around the mesh fibers in the vaginal wall in primates. Results are expressed as mean ± standard deviation.
| Subepithelium | Smooth muscle | Adventitia | Peri-Mesh | |
|---|---|---|---|---|
| Sham | 0.43 ± 0.50 | 0.11 ± 0.09 | 1.56 ± 1.42 | |
| GynemeshPS | 7.22 ± 7.76* | 0.84 ± 0.96 | 32.49± 29.14* | 22.34 ± 24.27* |
| UltraPro perpendicular | 2.34 ± 2.78 | 1.56 ± 2.72 | 13.19± 8.23 | 6.87 ± 3.19 |
| UltraPro parallel | 0.66 ± 1.21 | 0.14 ± 0.15 | 14.54± 12.95 | 8.74 ± 8.58 |
| SmartMesh | 2.14 ± 2.81 | 0.72 ± 1.02 | 3.32± 2.77 | 0.63 ± 0.80 |
p < 0.05 as compared to the UltraPro groups, SmartMesh and sham
Total collagen content
As shown in Table 5, overall collagen content was decreased after mesh implantation (P=0.028) compared to the sham (48.9 ± 8.3% of vaginal tissue dry weight). Further analysis showed that collagen was decreased by 20% in the Gynemesh PS group (39.3 ± 5.2%, P=0.003) relative to sham, but not statistically different from sham in the UltraPro perpendicular (48.0 ± 8.8%), UltraPro parallel (49.2 ± 8.0%) and SmartMesh (45.9 ± 9.6%) groups. The amount of collagen in UltraPro perpendicular and UltraPro parallel was not significantly different (P=0.84). The associations between total collagen content and mesh properties were established as collagen content correlated negatively to mesh stiffness as measured via the ballburst test (spearman ρ=−0.368, P=0.023), mesh stiffness measured in a uniaxial load to failure test (spearman ρ= −0.395, P=0.014), and mesh weight (spearman ρ=−0.368, P=0.023) while total collagen content positively correlated with mesh porosity (spearman value 0.368, P=0.023).
Table 5.
Biochemical analysis of the vaginal wall after mesh implantation as compared to sham. Results are expressed as mean ± standard deviation.
| Collagen content (% of dry weight) | Elastin content (pm/mg protein) | GAG content (% of dry weight) | Total collagenase activity (units/mg) | |
|---|---|---|---|---|
| Sham | 48.9 ± 8.3 | 716 ± 449 | 1.37 ± 0.33 | 0.82± 0.56 |
| Gynemesh PS | 39.3 ± 5.2 | 405 ± 106 | 1.71 ± 0.36 | 1.93± 1.07 |
| UltraPro perpendicular | 48.0 ± 8.8 | 386 ± 113 | 1.65± 0.40 | 1.00 ± 0.78 |
| UltraPro parallel | 49.2 ± 8.0 | 362 ± 122 | 1.30± 0.20 | 1.15 ± 0.63 |
| SmartMesh | 45.9± 9.6 | 704 ± 348 | 1.36 ± 0.18 | 1.15 ± 0.89 |
| P* | 0.028 | 0.007 | 0.011 | 0.010 |
indicates the overall comparison among the groups
Mature elastin content
Overall mature elastin decreased following mesh implantation (Table 5, overall P=0.007). Mature elastin was decreased in the Gynemesh PS (405 ± 106 pm/mg), UltraPro perpendicular (386 ± 113 pm/mg) and UltraPro parallel (362 ± 122) groups by 43%, 46% and 49% relative to sham (716 ± 449 pm/mg protein, P=0.034, 0.031 and 0.030 respectively). Implantation with SmartMesh did not result in any significant change in mature elastin content (704 ± 348pm/mg, P=0.74) compared to sham. When comparing UltraPro perpendicular and UltraPro parallel, no significant difference was found (P=1.0). We found no clear correlations between mature elastin content and mesh properties including ball burst stiffness (spearman ρ=0.317; P=0.053), uniaxial stiffness (spearman ρ=−0.127, P=0.448), weight (spearman ρ=−0.317, P=0.053), and porosity (spearman ρ=0.317, P=0.053).
Sulfated GAG content
Consistent with acute tissue injury [33], the sulfated GAG content increased following mesh implantation (overall P=0.011, Table 5). Further analysis showed that the most significant change occurred in the Gynemesh PS group, with a 20% increase in GAGs (1.71 ± 0.36% of dry weight) compared to sham (1.37 ± 0.33%, P=0.035). For UltraPro perpendicular (1.65 ± 0.40%), Ultra parallel (1.30 ± 0.18%) and SmartMesh (1.36 ± 0.18%), the GAG content was not significantly different from sham (P = 0.12, 1.0 and 1.0, respectively). GAG content was correlated positively to the mesh ball burst stiffness (spearman ρ=0.364, P=0.025), and mesh weight (spearman ρ=0.364, P=0.025), and negatively to mesh porosity (spearman ρ=−0.364, P=0.025), but not mesh uniaxial stiffness (spearman ρ= 0.072, P=0.669).
Total collagenase activities
Total collagenase activity, a measure of the combined activity of all of the collagen degrading enzymes in the tissue plus the activity of their endogenous inhibitors, was significantly increased following mesh implantation relative to sham (0.82 ± 0.56 units/mg of protein, overall P=0.01, Table 5). Consistent with the decreases observed in total collagen, the highest collagenase activity (135% higher than that of sham) occurred in the Gynemesh PS group (1.93 ± 1.07 units/mg protein, P=0.005). In contrast, collagenase activity was similar to sham in the UltraPro perpendicular (1.00 ± 0.78 units/mg protein, P = 0.52), UltraPro parallel (1.15 ± 0.63, P=0.62) and SmartMesh groups (1.15 ± 0.89 units/mg protein, P=0.60, respectively). Comparison between the UltraPro perpendicular and UltraPro parallel did not show a statistical difference (P=0.67). The total collagenase activity was correlated significantly to the mesh ball burst stiffness (spearman ρ=0.326, P=0.046), mesh weight (spearman ρ=0.326, P=0.046), and mesh porosity (spearman ρ=−0.326, P=0.046). It was also positively correlated to the mesh uniaxial stiffness (spearman ρ= 0.359, P=0.027).
Discussion
Meshes are widely used in prolapse surgeries to improve anatomical outcomes with little knowledge of the impact on the vagina. In spite of good anatomical outcomes, the use of mesh has been hampered by mesh related complications. While some may be related to the innate host response to a foreign material, others are likely related to the properties of the mesh itself. The goal of the current study was to define the impact of mesh with overall similar textile properties (ie all wide pore, light weight, monofilament polypropylene), but distinct mechanical behavior, on the vagina. Specifically, the impact of three meshes of varying stiffness, i.e. the resistance of a material to deformation when subjected to a load or force, was determined. To this end, we compared the impact of Gynemesh PS to that of two lighter weight and lower stiffness meshes, UltraPro and SmartMesh using histomorphometric and biochemical endpoints. The most significant findings of this study were that the stiffer and heavier mesh Gynemesh PS had a profoundly negative impact on vaginal morphology and the structural proteins that maintain the integrity of the vagina relative to sham operated controls. We also found moderate correlations of mesh stiffness, weight and porosity to vaginal biochemical parameters such as total collagen content and GAG content. The combined findings suggest that the stiffness of a mesh as well as its weight and porosity impact the quantity of key vaginal structural proteins with increased stiffness, increased weight and decreased porosity having a more negative impact.
Our results showed that following implantation with the stiffer mesh, Gynemesh PS, the vagina demonstrated evidence of a maladaptive remodeling response characterized by thinning of the smooth muscle layer, increased cell apoptosis, increased collagenase activity, decreased collagen and elastin content, and increased GAG content. These findings are consistent with our previous study which showed that the tissue mechanical properties of the underlying and associated grafted vagina deteriorated following implantation with the stiffer mesh Gynemesh PS but not the lower stiffness meshes UltraPro and Smartmesh [44]. The cause for the tissue degeneration was found to be in a large part related to mesh stiffness. In a previous study, we showed that the stiffness of a mesh is intrinsically related to its weight, pore size and porosity (Feola et al 2012 BJOG, in press) [34]. Thus, it is likely that under physiologic loading conditions, a heavier weight, less porous, stiffer mesh will have a more negative impact on the underlying and newly incorporated vagina due to a maladaptive remodeling response induced in part by stress-shielding.
Stress shielding, a phenomenon well known to other fields employing biomaterials, refers to a phenomenon that occurs when two solid materials are connected [35, 36]. According to this perspective, following implantation, the stiffer material buffers or “shields” the adjacent tissue from the physiological loads (forces) that it normally experiences. As all tissues throughout the body depend on a certain amount of load (force or stretch) to maintain their structure, with stress shielding, the affected tissue degenerates as a result of the decrease in loading. Similar to the findings in this study, tissue degeneration that occurs in this context has been shown to be due to a loss of key structural proteins such as collagen, elastin and smooth muscle myosin [39] [40, 41].
Our observation that Gynemesh PS induced a tissue degenerative response is in congruence with the stress shielding responses that have been observed in other tissues where biomaterials have been used. For example, following total hip arthroplasty, stress shielding has been observed when the stiffness of the prostheses exceeds that of the femur. As a result of stress shielding, there is substantial bone loss of the proximal femur resulting in osteopenia, osteoporosis and periprosthetic fractures [37, 38]. Similarly, in soft tissue studies, Rumian et al showed that the stress-shielding of the patellar tendon in the rabbit caused significant reduction in its structural and mechanical properties [26, 27]. Also, the absence of stress (a load) on soft tissues such as ligament/tendons has been shown to increase collagenase activity and accelerate collagen turnover consistent with a degenerative response [39] [40, 41]. To further define the role of stress shielding in the pathogenesis of mesh-related degenerative changes in the vagina, mechanistic studies defining the cellular and molecular responses that lead to a loss of key structural proteins are needed. In addition, as we have examined the mesh tissue complex at only a single point in time, it will be important to determine whether the tissue continues to degenerate over time or a partial recovery occurs.
Although the total collagen content decreased in the Gynemesh PS group and was not different from Sham in the low stiffness mesh groups, the mature elastin content was significantly decreased in both the Gynemesh group and UltraPro. As a major extracellular matrix protein, elastin acts to maintain the shape of vagina and confer its biomechanical properties. When elastin is disrupted, its supportive role is compromised. Studies have shown that the risk of developing pelvic organ prolapse is increased when the elastin metabolism is impaired, as demonstrated in genetic diseases or animal knockout models [42, 43]. Therefore, the finding that both Gynemesh PS and Ultrapro meshes were associated with a decrease in elastin indicates that the elastin metabolism was impacted by a property of these meshes that may not be related to stiffness. Since the UltraPro groups contain an absorbable component (poliglecaprone 25), the decrease of elastin may be related to a negative impact from this component. In addition, due to the complex local (on the level of a single pore) geometric changes that these meshes undergo when a force is applied, their impact on the vagina may be better characterized by describing local mechanical changes rather than gross overall stiffness measurements. The mechanism will be further investigated in future studies.
The GAG content increased significantly following implantation with the stiffest mesh Gynemesh PS but not following the lower stiffness meshes, also supporting that stiffer meshes induce a greater maladaptive tissue response. The sulfated GAG including chondroitin sulfate, heparin sulfate and dermatan sulfate are important macromolecules in the extracellular matrix reacting quickly to tissue injury, tissue growth and changes of mechanical stimulation. GAG content increases in healing tissues [33], in tissue under constant compressive force [44] and in response to stress-shielding [45]. The data in this study support these findings.
Our finding that the number of apoptotic cells was significantly increased in the area around mesh fibers and in the adventitia following implantation with Gynemesh PS, suggests active robust inflammatory response with a high turnover of inflammatory cells likely occurs following implantation. We are currently performing studies aimed at phenotyping the cell population and the type of immune response in the area of the mesh fibers.
Gynemesh PS, the stiffest mesh, significantly negatively impacted the smooth muscle layer resulting in a decreased volume and disrupted muscle bundles. In an analogous study, we demonstrated using an organ bath assay that the decrease in vaginal smooth muscle volume following implantation with Gynemesh PS is associated with a loss of smooth muscle function; specifically, contractility [44]. To date, the role of vaginal smooth muscle in the vagina has been poorly studied but is thought to contribute to the maintenance of vaginal tone and sexual function. As some studies have suggested a loss of smooth muscle volume in women with prolapse [46], additional loss with a prolapse repair likely should be avoided. Interestingly in our study, minimal cell apoptosis was observed in the degenerated smooth muscle layer, which indicates that the cell loss is either triggered earlier after surgery or is due to necrosis rather than apopotosis. We are currently investigating the mechanism by which loss of smooth muscle volume and function occurs following mesh implantation [46, 47].
The response to UltraPro is noteworthy because of its interesting mechanical behavior. Indeed, when tested in a uniaxial load to failure test in a direction perpendicular to the blue orientation lines (UltraPro perpendicular), the stiffness of UltraPro is an order of magnitude lower than that when it is tested parallel to the blue lines (UltraPro parallel). To determine whether these distinct stiffness values influenced the host response, we implanted UltraPro in two perpendicular orientations. Interestingly, we found that the direction of implantation had very limited impact on vaginal morphology and biochemical endpoints. This finding is consistent with the results of our biomechanical study, which showed that the passive biomechanical properties of the vaginal tissue underlying and newly incorporated into the graft were not significantly different despite the different orientation of mesh implantation (Feola et al 2012 BJOG, in press). In contrast, the direction of implantation significantly impacted smooth muscle contractility as measured via active mechanical assays with contractility virtually abolished when implantation occurred in the stiffer (parallel) direction. Thus, while the impact of mesh stiffness on smooth muscle function is consistent with a stress shielding response, the impact on the extracellular matrix proteins (collagen, elastin, etc.) suggest additional mechanisms are contributing to the host response or that the mechanics on the level of a single pore are similar despite altered loading direction. Possible mechanisms include the innate immune response and chronic microinjury from mesh micromotion. Finally, it is possible that absorbable component in the UltraPro groups is an important contributing factor in the host response. The precise of role of this component will be determined in future studies.
The primary limitation of the study is that we were able to analyze only a limited number of meshes. In addition, the sample size in each group has been reduced to the lowest number possible needed to achieve significance. However, the results strongly suggest that the trend in clinical practice toward the use of lower stiffness and lighter weight materials in reconstructive prolapse procedures is justified. Although we did not observe failure of the lower stiffness materials in our study, further clinical trials comparing anatomical outcomes with low vs. high stiffness mesh are warranted. In addition, we observed few complications related to our different materials per se, but rather thinning of the overlying and associated vagina. Again, whether lower stiffness materials are actually associated with fewer complications in women is a question that can be addressed in a clinical trial.
Conclusion
Vaginal morphology and connective tissue remodeling were adversely affected by mesh implantation. Implantation with a stiffer mesh resulted in increased collagenase activity, decreased collagen and elastin content, and increased GAG content, indicating a negative impact on the structural integrity of vagina. Future studies investigating the functional impact of mesh on the vagina as well as the molecular mechanisms resulting in the degenerative changes are needed to further corroborate these findings.
Acknowledgments
NIH funding support R01 HD061811-01. Assistance of Dr. Barry Starcher from University of Texas Health Science Center at Tyler, Department of Biochemistry, in examining total mature elastin content. Assistance of Ms. Leslie Meyn from Magee-Womens Research Institute, University of Pittsburgh, in statistical analyses.
Funding: Financial support provided by the National Institutes of Health (NIH) R01 HD061811-01
Footnotes
Disclosure of Interests: No disclosures.
Contribution to Authorship: RL – conception, surgery, study design and carrying out experiments for histology and biochemical endpoints, analyzing data and writing manuscript; SA – conception, surgery and writing manuscript; KK, AN – carrying out experiments for biochemical endpoints, analyzing data; AF, SP and SS – planning, surgery; PM – supervise, conception, surgery, study design, analyzing data and writing manuscript.
Details of Ethical Approval: This study was conducted with the approval of the University of Pittsburgh’s Institutional Animal Care Use Committee (IACUC #1008675) and in adherence to the National Institutes of Health Guidelines (approval date August 6th, 2010)
References
- 1.Olsen AL, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188(1):108–15. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 3.Kenton K, Mueller ER. The global burden of female pelvic floor disorders. BJU Int. 2006;98(Suppl 1):1–5. doi: 10.1111/j.1464-410X.2006.06299.x. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 4.Wu JM, et al. Predicting the number of women who will undergo incontinence and prolapse surgery, 2010 to 2050. Am J Obstet Gynecol. 2011;205(3):230 e1–5. doi: 10.1016/j.ajog.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subak LL, et al. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98(4):646–51. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 6.Feola A, et al. Parity negatively impacts vaginal mechanical properties and collagen structure in rhesus macaques. Am J Obstet Gynecol. 2010;203(6):595 e1–8. doi: 10.1016/j.ajog.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birch C, Fynes MM. The role of synthetic and biological prostheses in reconstructive pelvic floor surgery. Curr Opin Obstet Gynecol. 2002;14(5):527–35. doi: 10.1097/00001703-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Cervigni M, Natale F. The use of synthetics in the treatment of pelvic organ prolapse. Curr Opin Urol. 2001;11(4):429–35. doi: 10.1097/00042307-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Cosson M, et al. Mechanical properties of biological or synthetic implants used to treat genital prolapse and stress incontinence in women : what is the ideal material? J Gynecol Obstet Biol Reprod (Paris) 2003;32(4):321–8. [PubMed] [Google Scholar]
- 10.Debodinance P, et al. Development of better tolerated prosthetic materials: applications in gynecological surgery. J Gynecol Obstet Biol Reprod (Paris) 2002;31(6):527–40. [PubMed] [Google Scholar]
- 11.Iglesia CB, Fenner DE, Brubaker L. The use of mesh in gynecologic surgery. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(2):105–15. doi: 10.1007/BF02764826. [DOI] [PubMed] [Google Scholar]
- 12.Bader G, et al. Cystocele repair by vaginal approach with a tension-free transversal polypropylene mesh. Technique and results. Gynecol Obstet Fertil. 2004;32(4):280–4. doi: 10.1016/j.gyobfe.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 13.de Tayrac R, et al. Long-term anatomical and functional assessment of trans-vaginal cystocele repair using a tension-free polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(5):483–8. doi: 10.1007/s00192-005-0046-x. [DOI] [PubMed] [Google Scholar]
- 14.de Tayrac R, et al. Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med. 2005;50(2):75–80. [PubMed] [Google Scholar]
- 15.Deffieux X, et al. Vaginal mesh erosion after transvaginal repair of cystocele using Gynemesh or Gynemesh-Soft in 138 women: a comparative study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(1):73–9. doi: 10.1007/s0192-005-0041-2. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer PL, O’Reilly BA. Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG. 2004;111(8):831–6. doi: 10.1111/j.1471-0528.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 17.Committee Opinion no. 513: vaginal placement of synthetic mesh for pelvic organ prolapse. Obstet Gynecol. 2011;118(6):1459–64. doi: 10.1097/AOG.0b013e31823ed1d9. [DOI] [PubMed] [Google Scholar]
- 18.Nieminen K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol. 2010;203(3):235 e1–8. doi: 10.1016/j.ajog.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Bako A, Dhar R. Review of synthetic mesh-related complications in pelvic floor reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(1):103–11. doi: 10.1007/s00192-008-0717-5. [DOI] [PubMed] [Google Scholar]
- 20.Jones KA, et al. Tensile properties of commonly used prolapse meshes. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(7):847–53. doi: 10.1007/s00192-008-0781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones KA, et al. Trends in inpatient prolapse procedures in the United States, 1979–2006. Am J Obstet Gynecol. 2010;202(5):501 e1–7. doi: 10.1016/j.ajog.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mistrangelo E, et al. Rising use of synthetic mesh in transvaginal pelvic reconstructive surgery: a review of the risk of vaginal erosion. J Minim Invasive Gynecol. 2007;14(5):564–9. doi: 10.1016/j.jmig.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Kohli N, et al. Mesh erosion after abdominal sacrocolpopexy. Obstet Gynecol. 1998;92(6):999–1004. doi: 10.1016/s0029-7844(98)00330-5. [DOI] [PubMed] [Google Scholar]
- 24.Goel VK, et al. Effects of rigidity of an internal fixation device. A comprehensive biomechanical investigation. Spine (Phila Pa 1976) 1991;16(3 Suppl):S155–61. doi: 10.1097/00007632-199103001-00023. [DOI] [PubMed] [Google Scholar]
- 25.Huiskes R, et al. Adaptive bone-remodeling theory applied to prosthetic-design analysis. J Biomech. 1987;20(11–12):1135–50. doi: 10.1016/0021-9290(87)90030-3. [DOI] [PubMed] [Google Scholar]
- 26.Rumian AP, et al. The influence of the mechanical environment on remodelling of the patellar tendon. J Bone Joint Surg Br. 2009;91(4):557–64. doi: 10.1302/0301-620X.91B4.21580. [DOI] [PubMed] [Google Scholar]
- 27.Maeda E, et al. Effects of stress shielding and subsequent restressing on mechanical properties of regenerated and residual tissues in rabbit patellar tendon after resection of its central one-third. J Biomech. 2009;42(11):1592–7. doi: 10.1016/j.jbiomech.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Ozog Y, et al. Persistence of polypropylene mesh anisotropy after implantation: an experimental study. BJOG. 2011;118(10):1180–5. doi: 10.1111/j.1471-0528.2011.03018.x. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd J, Feola AJ, Stein S, Moalli P, Abramowitch SD. Uniaxial Biomechanical Properties of 7 Different Vaginally Implanted Meshes for Pelvic Organ Prolapse. International Urogynecology Journal. 2011 doi: 10.1007/s00192-011-1616-8. In-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blumenkrantz N, Asboe-Hansen G. An automated procedure for quantitative determination of hydroxyproline. Clin Biochem. 1974;7(3):251–7. doi: 10.1016/s0009-9120(74)92204-8. [DOI] [PubMed] [Google Scholar]
- 31.Starcher B, Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res. 1995;31(2):133–40. doi: 10.3109/03008209509028401. [DOI] [PubMed] [Google Scholar]
- 32.Muller G, Hanschke M. Quantitative and qualitative analyses of proteoglycans in cartilage extracts by precipitation with 1,9-dimethylmethylene blue. Connect Tissue Res. 1996;33(4):243–8. doi: 10.3109/03008209609028881. [DOI] [PubMed] [Google Scholar]
- 33.Plaas AH, et al. Proteoglycan metabolism during repair of the ruptured medial collateral ligament in skeletally mature rabbits. Arch Biochem Biophys. 2000;374(1):35–41. doi: 10.1006/abbi.1999.1630. [DOI] [PubMed] [Google Scholar]
- 34.Rosch R, et al. Mesh implants in hernia repair. Inflammatory cell response in a rat model. Eur Surg Res. 2003;35(3):161–6. doi: 10.1159/000070045. [DOI] [PubMed] [Google Scholar]
- 35.Nagels J, Stokdijk M, Rozing PM. Stress shielding and bone resorption in shoulder arthroplasty. J Shoulder Elbow Surg. 2003;12(1):35–9. doi: 10.1067/mse.2003.22. [DOI] [PubMed] [Google Scholar]
- 36.Majima T, et al. Stress shielding of patellar tendon: effect on small-diameter collagen fibrils in a rabbit model. J Orthop Sci. 2003;8(6):836–41. doi: 10.1007/s00776-003-0707-x. [DOI] [PubMed] [Google Scholar]
- 37.Rubash HE, et al. Pathogenesis of bone loss after total hip arthroplasty. Orthop Clin North Am. 1998;29(2):173–86. doi: 10.1016/s0030-5898(05)70316-3. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs JJ, Sumner DR, Galante JO. Mechanisms of bone loss associated with total hip replacement. Orthop Clin North Am. 1993;24(4):583–90. [PubMed] [Google Scholar]
- 39.Majima T, et al. In-vitro cyclic tensile loading of an immobilized and mobilized ligament autograft selectively inhibits mRNA levels for collagenase (MMP-1) J Orthop Sci. 2000;5(5):503–10. doi: 10.1007/s007760070030. [DOI] [PubMed] [Google Scholar]
- 40.Gamble JG, Edwards CC, Max SR. Enzymatic adaptation in ligaments during immobilization. Am J Sports Med. 1984;12(3):221–8. doi: 10.1177/036354658401200311. [DOI] [PubMed] [Google Scholar]
- 41.Amiel D, et al. The effect of immobilization on collagen turnover in connective tissue: a biochemical-biomechanical correlation. Acta Orthop Scand. 1982;53(3):325–32. doi: 10.3109/17453678208992224. [DOI] [PubMed] [Google Scholar]
- 42.Paladini D, et al. Association of cutis laxa and genital prolapse: a case report. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1367–70. doi: 10.1007/s00192-007-0362-4. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, et al. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol. 2006;168(2):519–28. doi: 10.2353/ajpath.2006.050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogel KG, et al. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. J Orthop Res. 1993;11(1):68–77. doi: 10.1002/jor.1100110109. [DOI] [PubMed] [Google Scholar]
- 45.Muellner T, et al. Light and electron microscopic study of stress-shielding effects on rat patellar tendon. Arch Orthop Trauma Surg. 2001;121(10):561–5. doi: 10.1007/s004020100281. [DOI] [PubMed] [Google Scholar]
- 46.Boreham MK, et al. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(1):56–63. doi: 10.1067/mob.2002.124843. [DOI] [PubMed] [Google Scholar]
- 47.Northington GM, et al. Contractile response of human anterior vaginal muscularis in women with and without pelvic organ prolapse. Reprod Sci. 2011;18(3):296–303. doi: 10.1177/1933719110392054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shepherd JP, et al. Uniaxial biomechanical properties of seven different vaginally implanted meshes for pelvic organ prolapse. Int Urogynecol J. 2011 doi: 10.1007/s00192-011-1616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feola A, Barone W, Moalli P, Abramowitch S. Characterizing the ex vivo textile and structural properties of synthetic prolapse mesh products. Int Urogynecol J. 2012 Aug 11; doi: 10.1007/s00192-012-1901-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]