In UV-B–induced photomorphogenesis in Arabidopsis, COP1 is a UV-B–inducible gene and FHY3 and HY5 directly activate COP1, dependent on UV-B, by binding to the COP1 promoter to ensure photomorphogenic UV-B signaling. The working mode of FHY3 and HY5 in UV-B–specific signaling is distinct from that in far-red light and circadian conditions.
Abstract
As sessile organisms, higher plants have evolved the capacity to sense and interpret diverse light signals to modulate their development. In Arabidopsis thaliana, low-intensity and long-wavelength UV-B light is perceived as an informational signal to mediate UV-B–induced photomorphogenesis. Here, we report that the multifunctional E3 ubiquitin ligase, CONSTITUTIVE PHOTOMORPHOGENESIS1 (COP1), a known key player in UV-B photomorphogenic responses, is also a UV-B–inducible gene. Two transcription factors, FAR-RED ELONGATED HYPOCOTYL3 (FHY3) and ELONGATED HYPOCOTYL5 (HY5), directly bind to distinct regulatory elements within the COP1 promoter, which are essential for the induction of the COP1 gene mediated by photomorphogenic UV-B signaling. Absence of FHY3 results in impaired UV-B–induced hypocotyl growth and reduced tolerance against damaging UV-B. Thus, FHY3 positively regulates UV-B–induced photomorphogenesis by directly activating COP1 transcription, while HY5 promotes COP1 expression via a positive feedback loop. Furthermore, FHY3 and HY5 physically interact with each other, and this interaction is diminished by UV-B. Together, our findings reveal that COP1 gene expression in response to photomorphogenic UV-B is controlled by a combinatorial regulation of FHY3 and HY5, and this UV-B–specific working mode of FHY3 and HY5 is distinct from that in far-red light and circadian conditions.
INTRODUCTION
Though a minor component of sunlight, UV-B light (280 to 315 nm) that reaches the earth’s surface exerts strong influences on plant growth and development. Plants have evolved the capacity to sense UV-B and perceive it not only as a damaging stimulus but also as an informational signal. In general, there are two broad categories of plant responses to UV-B, nonspecific and specific pathways, featuring UV-B–induced damage and photomorphogenic responses, respectively. In response to high-fluence and short-wavelength UV-B light, plants experience stress-related physiological processes, including DNA damage, generation of reactive oxygen species, and inhibition of photosynthesis (Brosché et al., 2002; Frohnmeyer and Staiger, 2003). By contrast, low-fluence and long-wavelength UV-B acts as a positive signal that promotes plant photomorphogenic development, which is characterized by hypocotyl growth inhibition (Kim et al., 1998), flavonoid accumulation (Christie and Jenkins, 1996), and acclimation to UV-B stress (Kliebenstein et al., 2002). Such acclimation renders plants a certain level of tolerance against harmful UV-B (Frohnmeyer and Staiger, 2003; Ulm and Nagy, 2005; Hectors et al., 2007; Jenkins, 2009).
Molecularly, photomorphogenic UV-B is effective in triggering differential gene expression in Arabidopsis thaliana. Based on genetic and transcriptional analyses, several positive regulators involved in UV-B–specific responses have been isolated. They include the bZIP transcription factor ELONGATED HYPOCOTYL5 (HY5) (Ulm et al., 2004), the UV RESISTANCE LOCUS8 (UVR8) (Brown et al., 2005), and the multifunctional E3 ubiquitin ligase CONSTITUTIVE PHOTOMORPHOGENESIS1 (COP1) (Oravecz et al., 2006). Mutants for each of these three genes suffer from decreased activation of UV-B–inducible genes, leading to reduced inhibition of hypocotyl elongation, impaired anthocyanin accumulation, and defective acclimation under UV-B (Brown et al., 2005; Oravecz et al., 2006; Favory et al., 2009).
HY5, an extensively studied bZIP transcription factor, functions downstream of multiple photoreceptors and plays key roles in promoting photomorphogenesis under diverse light conditions (Oyama et al., 1997; Ang et al., 1998; Osterlund et al., 2000; Ulm et al., 2004). HY5 targets a large number of light-responsive genes in vivo by directly binding to the ACGT-containing elements (ACEs) of their promoters (Oyama et al., 1997; Ang et al., 1998; Lee et al., 2007; Zhang et al., 2011). It also mediates crosstalk between light and hormone signaling, such as abscisic acid, gibberellins, and auxins (Oyama et al., 1997; Cluis et al., 2004; Lau and Deng, 2010), and integrates light and stress responses, such as low temperature (Catalá et al., 2011). In the UV-B–specific signaling pathway, HY5 serves as a hub that is required for the accumulation of transcripts of a subset of UV-B–responsive genes. HY5’s own UV-B–induced expression largely depends on UVR8 and COP1 (Ulm et al., 2004; Oravecz et al., 2006; Favory et al., 2009). However, the specific cis-element that is responsible for mediating low-fluence and long-wavelength UV-B–responsive gene expression remains undefined.
Recently, the molecular mechanism for UVR8-mediated UV-B perception was structurally described (Christie et al., 2012; Wu et al., 2012). Unlike the other photoreceptors, UVR8 possesses an internal chromophore shaped by the Trp residues Trp-233 and Trp-285. Without UV-B, UVR8 forms a symmetric homodimer that is stabilized by intra- and intermolecular interactions, principally through the Arg residues Arg-286 and Arg-338 and surrounding Trp residues. Exposure to UV-B leads to a conformational switch from dimeric to monomeric UVR8 within seconds. The monomeric UVR8 then triggers downstream signaling pathways (Rizzini et al., 2011; Wu et al., 2011; Christie et al., 2012; Wu et al., 2012). Upon UV-B irradiation, UVR8 rapidly accumulates in the nucleus and interacts with COP1 (Kaiserli and Jenkins, 2007). Interestingly, there appears to be residual nuclear UVR8, which constitutively associates with chromatin regions of several UV-B–activated genes, including HY5, regardless of the presence or absence of UV-B (Brown et al., 2005; Cloix and Jenkins, 2008).
In far-red and visible light–induced photomorphogenesis, which will be designated as traditional photomorphogenesis hereafter, COP1 is a central repressor that targets photomorphogenesis-promoting transcription factors, including HY5 for 26S proteasome–mediated degradation. The function of COP1 is modulated primarily via nucleocytoplasmic translocation and interaction with regulatory factors (Yi and Deng, 2005). By contrast, in UV-B–induced photomorphogenesis, COP1 is a positive regulator for HY5, triggering downstream UV-B–specific responses via an unknown mechanism (Favory et al., 2009).
Here, we show that COP1 gene expression is induced by photomorphogenic UV-B. FAR-RED ELONGATED HYPOCOTYL3 (FHY3) and HY5 activate COP1 expression by respectively targeting their distinct motifs in the COP1 promoter region, contributing to UV-B–induced photomorphogenesis and tolerance against damaging UV-B. The genetic and molecular evidence presented in this study demonstrates that in the UV-B–specific responses, FHY3 is a positive regulator upstream of COP1, while HY5 forms a positive feedback loop on COP1. Our findings uncover the elaborate control of photomorphogenic UV-B on COP1 by dual transcriptional regulation to ensure efficient early signaling.
RESULTS
COP1 Expression Is Induced by Photomorphogenic UV-B
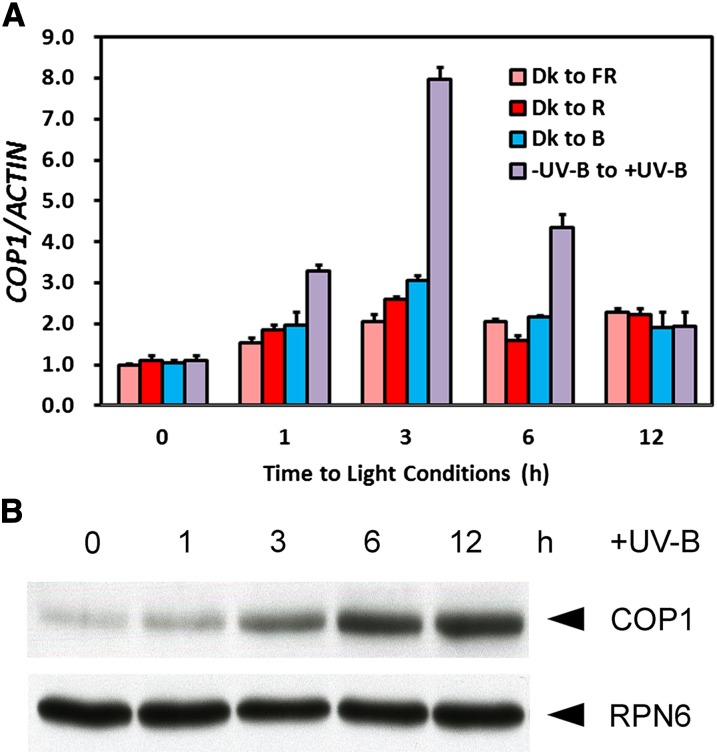
In response to low-fluence and long-wavelength UV-B, a set of genes was found to be activated in a temporal manner (Oravecz et al., 2006). To determine how COP1 is regulated by photomorphogenic UV-B, we examined the expression pattern of COP1 in 4-d-old seedlings grown under no UV-B light (−UV-B) and then transferred to UV-B light (+UV-B). The accumulation of COP1 transcripts rises within 1 h and reaches a peak of eightfold induction after 3 h of exposure to +UV-B and then decreases (Figure 1A). However, when using 4-d-old seedlings grown in darkness and then transferred to far-red, red, or blue light conditions, we found the expression of COP1 was only slightly affected. These results suggest that, compared with monochromatic far-red and visible light signals, photomorphogenic UV-B preferably induces COP1 expression. Besides, COP1 protein abundance continues to increase over 12 h of photomorphogenic UV-B irradiation (Figure 1B). These results show that photomorphogenic UV-B induces COP1 expression at both mRNA and protein levels.
Figure 1.
COP1 Is Induced by UV-B at the mRNA and Protein Levels.
(A) Changes in COP1 transcript levels in 4-d-old wild-type (Col) seedlings transferred from darkness (Dk) to far-red (FR), red (R), or blue (B) light conditions or from −UV-B to +UV-B and harvested at indicated time points. The transcript level at 0 h was set as 1. Error bars represent standard deviation (SD) of three biological replicates.
(B) Changes in COP1 protein levels in 4-d-old wild-type (Col) seedlings transferred from −UV-B to +UV-B and harvested at indicated time points. Anti-RPN6 was used as a loading control.
FHY3 Binds to the COP1 Promoter via an FHY3 Binding Site Motif in Vitro and in Vivo
The induction of COP1 by photomorphogenic UV-B prompted us to explore transcription factors involved in the regulation of COP1 expression. Examination of the COP1 promoter identified a putative FHY3 binding site (FBS; Lin et al., 2007) ∼190 bp upstream of the COP1 start codon (Figure 2A). A 1143-bp fragment of the COP1 promoter, which was reported to be sufficient for COP1 promoter activity (Kang et al., 2009) was cloned and used for yeast one-hybrid assays. We found that the transcriptional activation domain fused FHY3 (AD-FHY3), but not the AD control, induced LacZ reporter gene expression driven by the COP1 promoter (Figures 2A and 2B). Site-directed mutagenesis of this putative FBS abolished AD-FHY3 binding to the COP1 promoter. Subsequently, an electrophoretic mobility shift assay (EMSA) was performed to confirm the direct binding of FHY3 to the FBS on the COP1 promoter. The first 200 amino acids of FHY3, C-terminally fused to glutathione S-transferase, (GST-FHY3N) was previously shown to harbor DNA binding activity (Lin et al., 2007; Li et al., 2010). In our assay, addition of GST-FHY3N, but not GST, resulted in slower migration of the FBS probe, indicating that FHY3 is able to bind to the COP1 promoter. When assayed with a probe with the FBS site mutated, GST-FHY3N was not able to bind to the COP1 promoter fragment (Figure 2D).
Figure 2.
FHY3 and HY5 Bind to the COP1 Promoter in Vitro and in Vivo.
(A) Diagram of fragments of the COP1 promoter. The adenine of the translational start codon (ATG) is designated as position +1. Orange and blue blocks indicate putative FBS and ACEs, respectively. Arrows indicate the fragments amplified in ChIP-PCR assays. The wild-type and mutated sites of the COP1 promoter subfragments are shown in uppercase and lowercase letters, respectively.
(B) and (C) FHY3 (B) and HY5 (C) bind to the COP1 promoter in yeast one-hybrid assays. Empty vector expressing the AD domain alone is the negative control. WT, the wild type.
(D) and (E) GST-FHY3N (D) and GST-HY5 (E) specifically bind to COP1p-FBS and COP1p-ACE3 probes respectively in EMSA assays. FP, free probes.
(F) Representative results of ChIP-PCR assays. Chromatin fragments prepared from 4-d-old wild-type (Col) seedlings grown under −UV-B and +UV-B were immunoprecipitated by the polyclonal anti-FHY3 and anti-HY5 antibodies, respectively. Input, PCR reactions using the samples before immunoprecipitation. Ab, antibody. a and b correspond to the DNA fragments shown in (A).
(G) FHY3 and HY5 protein levels of 4-d-old wild-type (Col) seedlings grown under −UV-B and +UV-B. Anti-RPN6 was used as a loading control.
(H) qPCR analyses of the FBS-containing fragment (COP1pro-FBS) and the ACE3-containing fragment (COP1pro-ACE3) in the COP1 promoter in anti-FHY3 and anti-HY5 ChIP assays, respectively, using 4-d-old wild-type (Col) seedlings grown under −UV-B and +UV-B. The ChIP values were normalized to their respective DNA inputs. Error bars represent sd of three biological replicates.
To investigate whether FHY3 protein associates with the COP1 promoter in vivo, a chromatin immunoprecipitation (ChIP) assay was performed followed by regular PCR and quantitative PCR (qPCR) analyses. In 4-d-old −UV-B– and +UV-B–grown seedlings, the “a” fragment, which contains the FBS motif of the COP1 promoter, was found to be enriched by antibodies against native or transgenic FHY3. However, the “b” fragment, which is further upstream of the COP1 promoter, was used as a negative control and was not enriched (Figure 2F; see Supplemental Figure 1A online). The reduced enrichment in +UV-B–grown seedlings can probably be ascribed to the lower protein level of FHY3 under +UV-B (Figures 2G and 2H; see Supplemental Figures 1B and 1C online). Taken together, these results indicate that FHY3 protein directly binds to the COP1 promoter through the FBS motif under both −UV-B and +UV-B.
HY5 Binds to the COP1 Promoter via an ACE Element in Vitro and in Vivo
In addition to the FBS motif, three putative ACEs, typical HY5 binding sites, are present in the COP1 promoter region. Yeast one-hybrid assays were again used to identify the ACE(s) that is important for HY5 binding. AD-HY5 was able to bind the wild-type COP1 promoter, whereas mutation in ACE3 resulted in little affinity of AD-HY5 to the COP1 promoter, demonstrating that ACE3 is the dominant, if not the only, site for HY5 binding (Figure 2C). The ACE3 is located ∼80 bp upstream of the COP1 start codon and 100 bp downstream of the FBS motif (Figure 2A). HY5-HOMOLOG (HYH) overlaps in function with HY5 in the stimulation of some gene expression by UV-B (Brown and Jenkins, 2008), but no obvious signal for HYH binding to the COP1 promoter was detected (see Supplemental Figure 2 online). In an EMSA test, the wild-type but not the mutated ACE3 probe was bound by the recombinant GST-HY5, as evidenced by the presence of bands with retarded mobility (Figure 2E).
Furthermore, ChIP-PCR analysis validated the in vivo association of HY5 protein with the COP1 promoter in −UV-B– and +UV-B–grown wild-type seedlings. Anti-HY5 antibodies were found to enrich the ACE3-containing “a” fragment, but not the control “b” fragment (Figure 2F). ChIP-qPCR assays confirmed that the COP1 promoter can be immunoprecipitated by antibodies against HY5 under both −UV-B and +UV-B (Figures 2G and 2H). These data illustrate that through the ACE3 site, HY5 is also able to bind to the COP1 promoter regardless of photomorphogenic UV-B treatment.
FHY3 and HY5 Are Capable of Mediating COP1 Activation in Response to UV-B
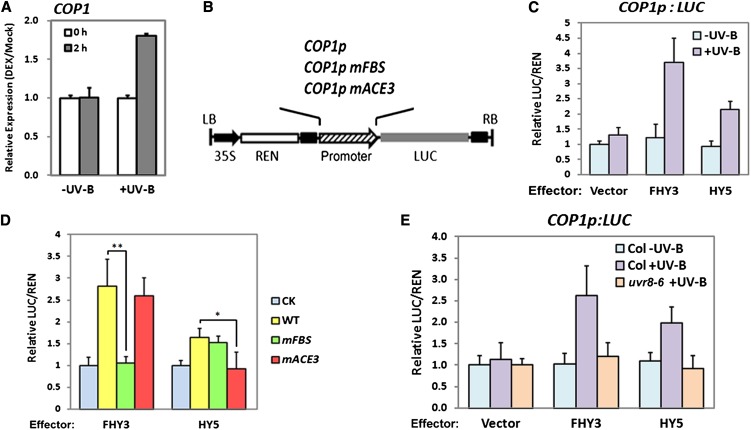
Since FHY3 and HY5 exhibit direct binding to the COP1 promoter via FBS and ACE3, respectively, these two transcription factors might contribute to the regulation of COP1 expression. To test this hypothesis, we first took advantage of the transgenic line FHY3p:FHY3-GR/fhy3-4 expressing an FHY3-glucocorticoid receptor (GR) fusion protein driven by the FHY3 native promoter (Lin et al., 2007). The fusion protein will translocate from the cytoplasm into the nucleus after treatment with dexamethasone (DEX) (Lloyd et al., 1994). The seedlings were grown under −UV-B for 4 d and then treated with DEX before transferred to −UV-B and +UV-B, respectively. The expression of COP1 is induced within 2 h under +UV-B but not −UV-B (Figure 3A). Nevertheless, FHY1, an identified target of FHY3 in far-red light signaling (Lin et al., 2007), is induced under −UV-B instead of +UV-B (see Supplemental Figure 3A online), consistent with no change in the FHY1 mRNA level in wild-type seedlings grown under −UV-B and then transferred to +UV-B (see Supplemental Figure 3B online).
Figure 3.
FHY3 and HY5 Target COP1 to UV-B–Dependent Transcriptional Activation.
(A) The transcript levels of COP1 in 4-d-old FHY3p:FHY3-GR/fhy3-4 seedlings grown under −UV-B and then treated with 10 μM DEX before being transferred to −UV-B and +UV-B. Relative expression levels were normalized to the Mock (equal volume of ethanol) treatment. Error bars represent sd of three biological replicates.
(B) Structure of the dual-luciferase reporter construct in which the firefly LUC reporter gene is driven by the wild type, FBS-mutated (mFBS), or ACE-mutated (mACE3) COP1 promoter. The REN luciferase reporter gene is controlled by the constitutive 35S promoter. The T-DNA left border and right border are indicated as LB and RB, respectively.
(C) The relative LUC activity normalized to the REN activity (LUC/REN) in tobacco leave cells transiently transformed with the indicated effectors and wild-type reporter construct COP1p:LUC under −UV-B and +UV-B. Empty effector vectors were included as negative controls.
(D) The LUC/REN values in tobacco leave cells transiently transformed with the indicated effectors and wild-type (WT) or mutated reporter constructs under +UV-B. Empty reporter vectors were included as negative controls. *P < 0.05 and **P < 0.01 by Student’s t test. CK, empty reporter vector. Error bars represent sd of four biological replicates.
(E) The relative LUC activity normalized to the REN activity (LUC/REN) in wild-type (Col) and mutant (uvr8-6) Arabidopsis leave cells transiently transformed with the indicated effectors and wild-type reporter construct COP1p:LUC under −UV-B and +UV-B. Empty effector vectors were included as negative controls.
Then, a dual luciferase assay was conducted in Nicotiana benthamiana to check the effects of FHY3 and HY5 on COP1 transcription. Recombinant reporter constructs were generated to allow the wild-type or mutated COP1 promoter to drive expression of the firefly luciferase (LUC) reporter gene (Figure 3B). Under photomorphogenic UV-B, the overexpression of FHY3 leads to a 3.7-fold increase in the activity of the LUC reporter under the COP1 promoter (Figure 3C). By contrast, it had no effect when either UV-B irradiation was absent (Figure 3C) or the FBS motif in the COP1 promoter was disrupted (Figure 3D). ACE3 mutation still led to the induction of LUC expression by FHY3 (Figure 3D). In a parallel analysis, HY5 also activated the COP1 promoter-driven LUC expression in a UV-B–dependent manner, though with reduced effectiveness compared with FHY3 (Figure 3C). With the mutated ACE3 construct, HY5 was no longer able to promote LUC expression (Figure 3D). The FBS mutation exerted no influence on the induction of LUC expression by HY5 (Figure 3D). Altogether, these results demonstrate that upon UV-B irradiation, FHY3 and HY5 can promote transcription of COP1 in plant cells.
Furthermore, to get more insight how FHY3 and HY5 activate COP1, the dual luciferase assay was perform in Arabidopsis. Under photomorphogenic UV-B, in wild-type plants, the transiently overexpressed FHY3 and HY5 led to 2.6- and 2.0-fold increases, respectively, in the activity of the LUC reporter under the COP1 promoter. However, little reporter activity was detected when uvr8-6 plants were infiltrated (Figure 3E), indicating that the loss of UVR8 function impinges on FHY3 and HY5 in COP1 activation. Together with the fact that UVR8 is a UV-B receptor (Rizzini et al., 2011; Wu et al., 2011; Christie et al., 2012; Wu et al., 2012), we conclude that the activation of COP1 by FHY3 and HY5 is dependent on UVR8 initiated UV-B signaling.
FHY3 Is Critical for UV-B–Induced Photomorphogenesis at the Seedling Stage
As shown above, FHY3 is able to bind to the COP1 promoter and activate its transcription under UV-B. COP1 is a positive regulator of UV-B–induced photomorphogenesis. Therefore, it is possible that FHY3 may be critical in this pathway.
As inhibition of hypocotyl elongation is a characteristic response to photomorphogenic UV-B (Favory et al., 2009; Gardner et al., 2009), the relative hypocotyl length, a percentage defined as the hypocotyl length under +UV-B relative to the length under −UV-B, was measured using 4-d-old seedlings grown in typical photomorphogenic UV-B experimental conditions (see Supplemental Figure 4 online). Consistent with earlier reports (Oravecz et al., 2006; Favory et al., 2009; Gardner et al., 2009), a UV-B photoreceptor mutant uvr8-6 and a key promoter mutant cop1-4 showed much longer relative hypocotyl length than the wild type, but such a defect was not found in the other photoreceptor mutants phyAB, phyABDE, and cry1 cyr2 or the DNA repair mutant uvr1-1. These observations confirmed that the condition used is specific to UV-B–induced photomorphogenesis instead of UV-B stress.
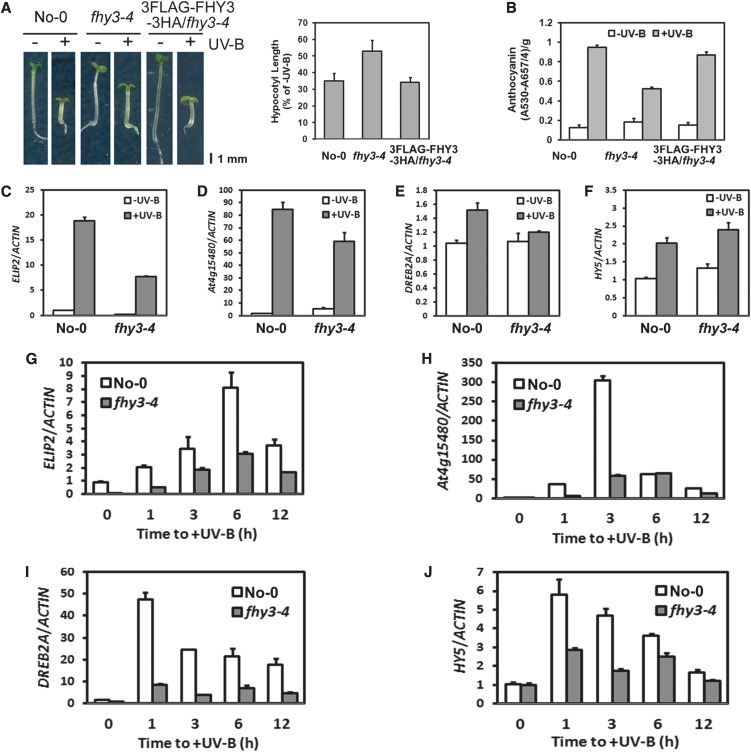
Interestingly, we found that fhy3-4 mutants displayed a dramatically reduced ability to inhibit hypocotyl elongation with UV-B irradiation compared with wild-type Nossen-0 (No-0) (Figure 4A). The relative hypocotyl length in No-0 seedlings was around 35% but was 53% in fhy3-4 mutants. This defect was also observed in four additional fhy3 mutant alleles, fhy3-1, fhy3-3, fhy3-5, and fhy3-10 (see Supplemental Figures 5A and 5B online). Photomorphogenic UV-B accelerates biosynthesis of sunscreen flavonoids (Oravecz et al., 2006). Quantitatively, we found that there was less anthocyanin content in fhy3-4 than in No-0 (Figures 4B).
Figure 4.
FHY3 Is Involved in UV-B–Induced Photomorphogenesis at the Seedling Stage.
(A) Phenotypes of 4-d-old wild-type (No-0), fhy3-4, and 3FLAG-FHY3-3HA/fhy3-4 seedlings grown under −UV-B and +UV-B. Quantitative analysis of hypocotyl length is presented as percentage of −UV-B. Error bars represent sd of three biological replicates. Bar = 1 mm.
(B) Quantitative analysis of anthocyanin accumulation of the seedlings in (A). Error bars represent sd of three biological replicates.
(C) to (F) The transcript levels of UV-B–induced marker genes ELIP2 (C), At4g15480 (D), DREB2A (E), and HY5 (F) in 4-d-old wild-type (No-0) and fhy3-4 seedlings grown under −UV-B to +UV-B. The transcript level in No-0 under −UV-B was set as 1. Error bars represent sd of three biological replicates.
(G) to (J) The transcript levels of UV-B–induced marker genes ELIP2 (G), At4g15480 (H), DREB2A (I), and HY5 (J) in 4-d-old wild-type (No-0) and fhy3-4 seedlings transferred from −UV-B to +UV-B and harvested at indicated time points. The transcript level at 0 h in No-0 was set as 1. Error bars represent sd of three biological replicates.
[See online article for color version of this figure.]
Next, we examined the expression of several marker genes responsive to photomorphogenic UV-B in 4-d-old seedlings grown under −UV-B and +UV-B. We found that the photomorphogenic UV-B–mediated transcript accumulation of EARLY LIGHT-INDUCIBLE PROTEIN2 (ELIP2) (Figure 4C), At4g15480 (Figure 4D), and DEHYDRATION-RESPONSIVE ELEMENT BINDING PROTEIN2A (DREB2A) (Figure 4E) was diminished in fhy3-4, but HY5 (Figure 4F) accumulated slightly more transcripts. Next, we continued to analyze the temporal expression of these genes using 4-d-old seedlings grown under −UV-B and then transferred to +UV-B for various time periods. The temporal induction of all these genes by photomorphogenic UV-B was much weaker in fhy3-4 than in No-0 (Figures 4G to 4J). These results demonstrate that FHY3 positively contributes to the transcriptional response to photomorphogenic UV-B, particularly at an early stage.
Furthermore, introduction of a 35S promoter–driven 3FLAG-FHY3-3HA construct in fhy3-4 (see Supplemental Figure 5C online) rescues the defects in its hypocotyl growth (Figure 4A) and anthocyanin accumulation (Figure 4B), supporting that FHY3 is involved in UV-B–induced photomorphogenesis at the seedling stage.
FAR-RED IMPAIRED RESPONSE1 (FAR1) is homologous to FHY3 and shares functional redundancy with FHY3 in phytochrome A (phyA) signaling, circadian clock, chloroplast development, and chlorophyll biosynthesis (Wang and Deng, 2002; Hiltbrunner et al., 2005; Allen et al., 2006; Lin et al., 2007; Genoud et al., 2008; Li et al., 2010; Li et al., 2011; Ouyang et al., 2011; Tang et al., 2012). Thus, we are interested if FAR1 plays a role in UV-B–induced photomorphogenesis as well. Surprisingly, far1 mutants showed no apparent difference from wild-type seedlings in UV-B–promoted hypocotyl growth (see Supplemental Figure 6 online). In addition, the fhy3 far1 double mutants displayed the same extent of hypocotyl growth in the presence of UV-B as that of fhy3 single mutant (see Supplemental Figure 6 online). These results suggest that FAR1 is not essential in photomorphogenic UV-B response.
A T-DNA Insertion hy5 Null Mutant Exhibits Impaired UV-B–Induced Photomorphogenesis
Previous studies have reported reduced UV-B–mediated gene activation in hy5-1 and hy5-215 mutants and impaired UV-B tolerance in hy5-1, suggesting that HY5 is a positive regulator in mediating gene expression and protective survival under UV-B (Ulm et al., 2004; Oravecz et al., 2006; Gruber et al., 2010; Stracke et al., 2010). Here, we found that hy5-ks50, a T-DNA insertion hy5 null mutant, is also impaired in UV-B-induced photomorphogenesis. The relative hypocotyl length of the mutant seedlings was around 74% (see Supplemental Figure 7A online), and there was much less anthocyanin content in hy5-ks50 than that in Wassilewskija (Ws) (see Supplemental Figure 7B online). These defects were restored through the overexpression of 35S:HY5 (see Supplemental Figure 7 online). This indicates that the hy5-ks50 null mutant, similar to the other hy5 alleles reported, is defective in UV-B–induced photomorphogenesis and thus was used for further investigation in our analysis.
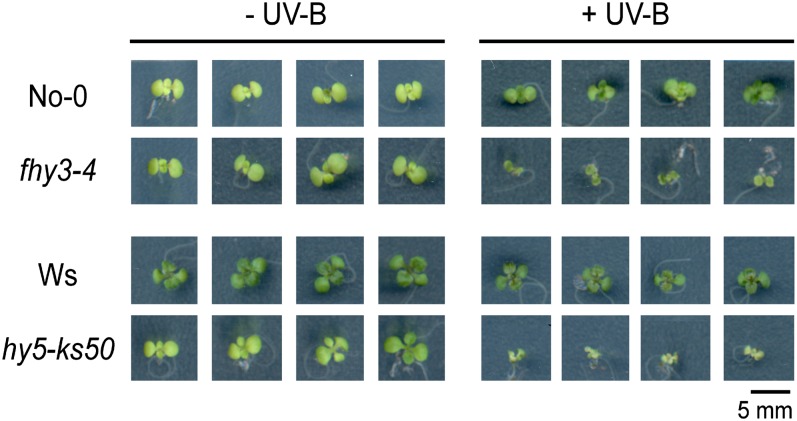
Both fhy3 and hy5 Mutants Are Hypersensitive to Damaging UV-B
Earlier studies reported that UV-B–induced photomorphogenesis is a fundamental event for plants to acquire tolerance against damaging UV-B (Favory et al., 2009). Based on the observations of abnormal UV-B–induced photomorphogenesis in our fhy3 and hy5 mutants, we attempted to investigate if they suffer from UV-B–induced damage. Wild-type seedlings were apparently resistant to the UV-B stress. By contrast, the leaves of fhy3-4 mutants shriveled, and hy5-ks50 mutants displayed even more severe defects with shrunken and pale leaves (Figure 5). The hypersensitivity to UV-B stress in fhy3-4 and hy5-ks50 mutants might be ascribed to their failure to properly respond to photomorphogenic UV-B, further demonstrating the involvement of FHY3 and HY5 in UV-B–induced photomorphogenesis and acclimation.
Figure 5.
FHY3 and HY5 Are Required for Tolerance to Damaging UV-B.
Wild-type (No-0 and Ws), fhy3-4, and hy5-ks50 seedlings were grown under white light supplemented with photomorphogenic UV-B for 12 d and then were irradiated without (−UV-B) or with (+UV-B) damaging broadband UV-B for 12 h. Bar = 5 mm.
Absence of FHY3 or HY5 Results in Abnormal Accumulation of COP1 Transcript and Protein under Photomorphogenic UV-B
To further explore the roles of FHY3 and HY5 in UV-B–specific signaling, especially in regulating COP1 expression in vivo, temporal expression of UV-B responsive genes was analyzed using 4-d-old seedlings grown under -UV-B and then transferred to +UV-B for various time periods.
CHALCONE SYNTHASE (CHS), which encodes a key enzyme at the first committed step in anthocyanin biosynthesis, is another UV-B–induced marker gene (Oravecz et al., 2006). In No-0, CHS was rapidly activated with a peak of 64-fold induction after 3 h of UV-B treatment and starts to fall afterwards. In fhy3-4, the CHS transcript rose with a diminished and retarded peak of 39-fold increase after 6 h of UV-B treatment (Figure 6A). COP1 transcript peaked with 6.3- and 3.9-fold increases in No-0 and fhy3-4, respectively, after 6 h of UV-B treatment (Figure 6B). At the protein level, compared with No-0, less COP1 protein accumulated in fhy3-4 over 12 h of photomorphogenic UV-B irradiation (Figure 6C). In parallel assays, the UV-B–induced transcription of CHS was found to be almost completely lost in hy5-ks50 (Figure 6D). COP1 transcript peaked with 6.7- and 3.6-fold increase in Ws and hy5-ks50, respectively (Figure 6E). The increase in COP1 protein mediated by photomorphogenic UV-B was largely abolished in hy5-ks50 (Figure 6F). These results indicate that both FHY3 and HY5 are required for the full activation of COP1 and COP1 protein accumulation mediated by photomorphogenic UV-B.
Figure 6.
FHY3 and HY5 Are Required for Accumulation of COP1 Transcripts and Proteins under Photomorphogenic UV-B.
(A) and (B) Changes in CHS (A) and COP1 (B) transcript levels in 4-d-old wild-type (No-0) and fhy3-4 seedlings transferred from −UV-B to +UV-B and harvested at indicated time points. The transcript level at 0 h in No-0 was set as 1. Error bars represent sd of three biological replicates.
(C) COP1 protein levels in 4-d-old wild-type (No-0) and fhy3-4 seedlings transferred from −UV-B to +UV-B and harvested at indicated time points. Anti-RPN6 was used as a loading control.
(D) and (E) CHS (D) and COP1 (E) transcript levels in 4-d-old wild-type (Ws) and hy5-ks50 seedlings transferred from −UV-B to +UV-B and harvested at the indicated time points. The transcript level at 0 h in Ws was set as 1. Error bars represent sd of three biological replicates.
(F) COP1 protein levels in 4-d-old wild-type (Ws) and hy5-ks50 seedlings transferred from −UV-B to +UV-B and harvested at indicated time points. Anti-RPN6 was used as a loading control.
COP1 and HY5 Genetically Interact with FHY3 in Mediating UV-B Photomorphogenic Responses
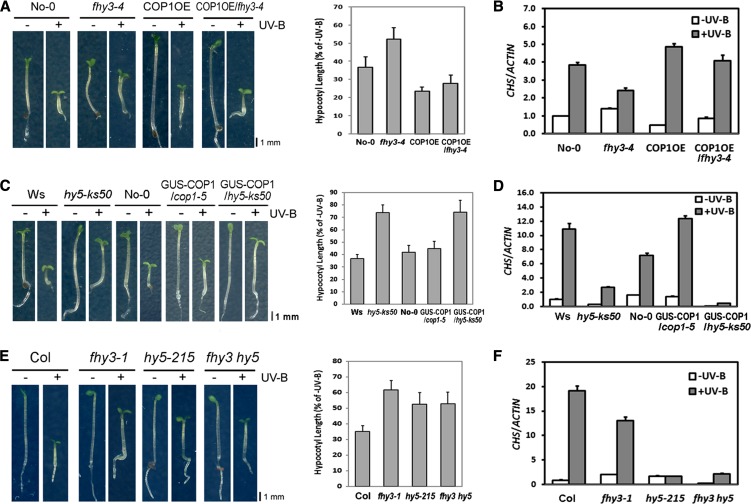
In order to further define the genetic role of FHY3 in the photomorphogenic UV-B signaling pathway, we examined the possible genetic interaction between COP1, FHY3, and HY5. The seedlings of a previously described COP1 overexpression line, COP1OE (see Supplemental Figure 8 online), displayed a stronger inhibition of hypocotyl elongation compared with the wild-type, confirming COP1’s positive role in UV-B signaling (Figure 7A). When introduced into fhy3-4, the COP1 overexpression completely suppressed the reduced inhibition of hypocotyl elongation found in fhy3-4, to a level that is similar to COP1OE (Figure 7A). Quantitative analysis of the CHS transcripts confirmed that COP1OE/fhy3-4 phenocopies COP1OE (Figure 7B). Therefore, we conclude that COP1 acts downstream of FHY3.
Figure 7.
COP1, FHY3, and HY5 Genetically Interact with Each Other under Photomorphogenic UV-B.
(A) and (B) Phenotypes (A) and CHS transcript levels (B) of 4-d-old wild-type (No-0), fhy3-4, COP1OE, and COP1OE/fhy3-4 seedlings grown under −UV-B and +UV-B.
(C) and (D) Phenotypes (C) and the CHS transcript levels (D) of 4-d-old wild-type (Ws and No-0), hy5-ks50, GUS-COP1/cop1-5, and GUS-COP1/hy5-ks50 seedlings grown under −UV-B and +UV-B.
(E) and (F) Phenotypes (E) and the CHS transcript levels (F) of 4-d-old wild-type (Col), fhy3-1, hy5-215, and fhy3-1 hy5-215 seedlings grown under −UV-B and +UV-B. Quantitative analysis of hypocotyl length is presented as percentage of −UV-B. The transcript level in the wild type under -UV-B was set as 1. Error bars represent sd of three biological replicates. Bars = 1 mm.
[See online article for color version of this figure.]
GUS-COP1/cop1-5 was previously found to rescue the constitutive photomorphogenesis of cop1-5 in darkness by overexpressing COP1 (von Arnim et al., 1997; see Supplemental Figure 8 online). Under photomorphogenic UV-B, GUS-COP1/cop1-5 exhibited comparable hypocotyl growth to No-0. When crossed with GUS-COP1/cop1-5, hy5-ks50 showed no recovery of hypocotyl shortening (Figure 7C). Besides, in response to photomorphogenic UV-B, GUS-COP1/cop1-5 displayed stronger induction of CHS than No-0, but GUS-COP1/hy5-ks50 failed to induce CHS like hy5-ks50 (Figure 7D). In the absence of HY5, COP1 overexpression was not sufficient to regain the regular response to photomorphogenic UV-B, suggesting epistasis of hy5-ks50 mutation over COP1 overexpression. According to the phenotypes of COP1OE/fhy3-4 and GUS-COP1/hy5-ks50, we deduce that the main function of HY5 is downstream of FHY3 and COP1.
Next, we crossed fhy3-1 with hy5-215, which was reported to have a reduced inhibition of hypocotyl elongation under +UV-B (Jiang et al., 2012). We observed that fhy3-1 hy5-215 double mutant seedlings closely resembled the hy5-215 single mutants in hypocotyl growth upon UV-B treatment (Figure 7E). Consistently, fhy3-1 hy5-215 mimicked hy5-215 in its photomorphogenic UV-B–mediated CHS induction (Figure 8F). These results also agree with the notion that HY5 acts as a main downstream transcription factor of COP1 in the central pathway in UV-B–mediated photomorphogenesis. It is likely that participating in COP1 gene activation is one of the regulatory roles of HY5.
Figure 8.
Photomorphogenic UV-B Affects the Functional Interaction between FHY3 and HY5.
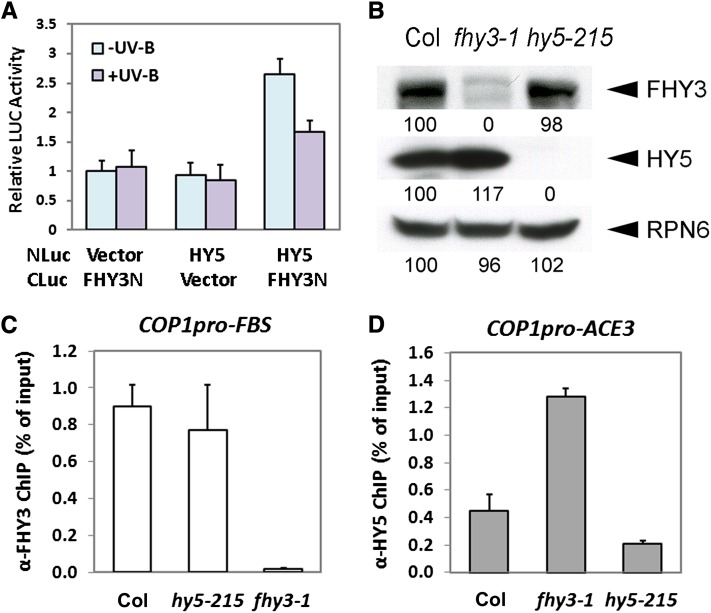
(A) The interaction between FHY3 and HY5 under −UV-B and +UV-B in LCI assays. Error bars represent sd of four biological replicates.
(B) FHY3 and HY5 protein levels in 4-d-old wild-type (Col), fhy3-1, and hy5-215 seedlings grown under +UV-B. Anti-RPN6 was used as a loading control. Band intensities of FHY3, HY5, and RPN6 in Col seedlings were set to 100. Relative band intensities were then calculated and are indicated by numbers below blots.
(C) and (D) qPCR analysis of the FBS-containing fragment (COP1pro-FBS) in the COP1 promoter in anti-FHY3 ChIP assays (C) and the ACE3-containing fragment (COP1pro-ACE3) in the COP1 promoter in anti-HY5 ChIP assays (D) using 4-d-old wild-type (Col), hy5-215, and fhy3-1 seedlings grown under +UV-B. The ChIP values were normalized to their respective DNA inputs. Error bars represent sd of three biological replicates.
[See online article for color version of this figure.]
Photomorphogenic UV-B Affects the Functional Interaction between FHY3 and HY5
It was previously noted that the antagonistic roles of FHY3 and HY5 in phyA signaling and their cooperative functions in the circadian clock both rely on their physical interaction (Li et al., 2010; Li et al., 2011). It is therefore of interest to examine whether FHY3 and HY5 functionally interact in UV-B responses. We first conducted firefly luciferase complementation imaging (LCI) assays (Chen et al., 2008) by transiently overexpressing CLuc and NLuc fusion proteins in N. benthamiana leaf cells. Obvious LUC activity was observed when CLuc-FHY3N and HY5-NLuc were coexpressed under −UV-B, whereas much less was observed under +UV-B (Figure 8A). This result suggests that UV-B attenuates the association between FHY3 and HY5 in plant cells.
In addition, the presence of both the FBS motif and the ACE element in the COP1 promoter also hints at a possible functional interaction between FHY3 and HY5. Thus, we examined whether the loss of HY5 or FHY3 affected the other with regard to their protein abundance and their affinity to the COP1 promoter. In hy5-215, the protein level of FHY3 is comparable to that of the wild type (Figure 8B). ChIP of FHY3 also precipitated the FBS motif–containing promoter fragment of COP1 at a similar efficiency with the wild type (Figure 8C). However, in fhy3-1, more HY5 proteins accumulate (Figure 8B), and antibodies against HY5 enriched twofold more DNA fragments containing the ACE3 in the fhy3-1 mutant than that in the wild type (Figure 8D). These observations reveal that FHY3 or HY5 are each able to associate with the COP1 promoter in the absence of the other.
DISCUSSION
The E3 ubiquitin ligase COP1 is a well-known repressor in traditional photomorphogenesis, primarily via mediating the degradation of photomorphogenesis-promoting transcription factors (Yi and Deng, 2005). By contrast, COP1 plays a positive role in UV-B–induced photomorphogenesis, but how photomorphogenic UV-B regulates COP1 is unknown. Our analyses reveal that COP1 is a UV-B–inducible gene, whose full activation requires transcription factors FHY3 and HY5. Furthermore, FHY3 and HY5 are engaged in UV-B–induced photomorphogenesis and acclimation and show a genetic interaction with COP1 (Figure 9). Thus, this work uncovers a functional mode of FHY3 and HY5 beyond phyA signaling and circadian regulation.
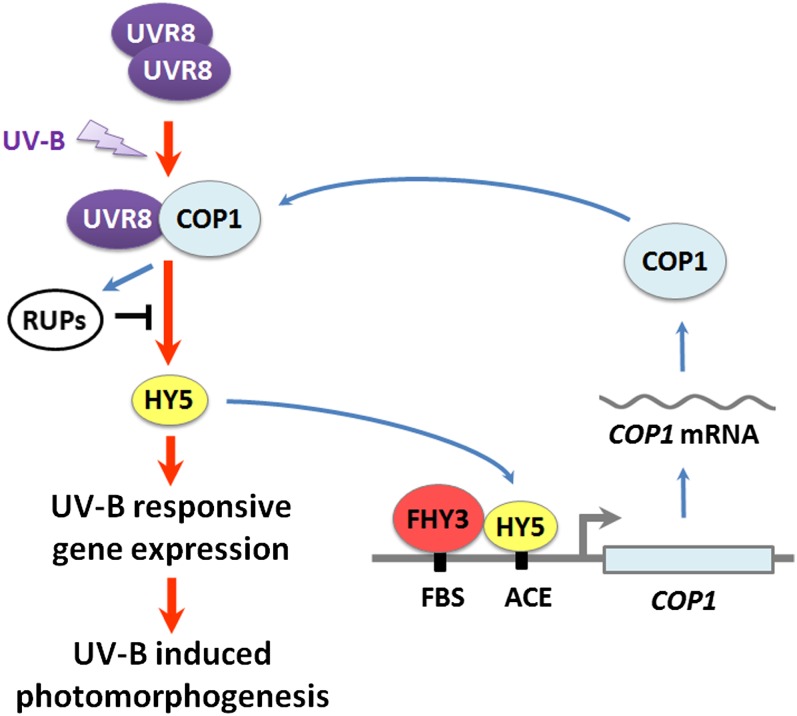
Figure 9.
A Model for the Functional Interaction of COP1, FHY3, and HY5 in UV-B–Induced Photomorphogenesis.
Under photomorphogenic UV-B, UVR8 monomerizes and interacts with COP1, triggering downstream signaling, including HY5-regulated gene expression, which is repressed by RUPs. COP1 is a UV-B–inducible gene. FHY3 and HY5 bind to the FBS motif and the ACE element in the COP1 promoter, respectively, and activate COP1 transcription. This transcriptional modulation ensures more active UVR8-COP1-HY5 core pathway. Red arrows, positive regulation in the core pathway; blue arrows, positive regulation in UV-B–mediated gene induction; black bar, negative regulation.
Photomorphogenic UV-B Regulates COP1 Expression via FHY3 and HY5 Binding LREs
During the last decades, the reprogramming of the plant transcriptome by light has been extensively examined. Transcriptional reprogramming requires light-responsive cis-elements (LREs), which are commonly present in the promoter regions of light-regulated genes. Combinations of LREs confer distinct transcriptional responses to various light signals and further depict a sophisticated light regulated transcriptional network (Puente et al., 1996; Jiao et al., 2007). Such transcriptional regulation is also coupled with UV-B–specific signaling. Photomorphogenic UV-B promotes differential gene expression (Ulm et al., 2004; Brown et al., 2005; Oravecz et al., 2006; Favory et al., 2009), histone modification, and chromatin remodeling (Casati et al., 2008). In Arabidopsis, although the UVBox, a promoter motif, is required for the induction of ARABIDOPSIS THALIANA NAC DOMAIN PROTEIN13 by shorter-wavelength and high-intensity UV-B, this element is not regulated by longer-wavelength UV-B (Safrany et al., 2008). In our study, COP1 was induced upon treatment with photomorphogenic UV-B (Figure 1). The FBS motif and the ACE element in the COP1 promoter serve as LREs to positively mediate COP1 expression in this transcriptional response to photomorphogenic UV-B (Figures 2 and 3).
As a multifunctional E3 ligase, COP1 is subjected to changes in activity in response to diverse stimuli. For example, the cryptochromes rapidly inactivate COP1 through their direct association (Wang et al., 2001; Yang et al., 2001), four functional redundant SUPPRESSOR OF PHYA (SPA) proteins act as regulatory subunits of COP1-SPA heterogeneous complexes (Saijo et al., 2003; Zhu et al., 2008), and at a relatively slower rate, light inhibits its E3 ligase activity partially by excluding it from the nucleus (von Arnim and Deng, 1994). Here, we establish how COP1 is controlled at the transcriptional level in Arabidopsis. Specifically, the accurate composition of the LREs for FHY3 and HY5 in the COP1 promoter (Figure 2) defines its ability to positively respond to the stimulus of photomorphogenic UV-B (Figure 3).
FHY3 Is Associated with UV-B–Induced Photomorphogenesis
UV-B–induced photomorphogenesis was first observed in Arabidopsis in the late 1990s and expanded light control of plant development beyond the traditional photomorphogenesis by far-red and visible light wavelengths (Kim et al., 1998). To date, this response has been shown in several plant species, such as maize (Zea mays) (Casati and Walbot, 2003; Casati et al., 2008), cucumber (Cucumis sativus) (Shinkle et al., 2010), and moss (Physcomitrella patens) (Wolf et al., 2011). In Arabidopsis, besides HY5, UVR8, and COP1, there are a growing number of factors found to be involved in the responses to photomorphogenic UV-B, including two potential Damaged DNA Binding Protein1 (DDB1) binding WD40 proteins, REPRESSOR OF UV-B PHOTOMORPHOGENESIS1 (RUP1) and RUP2. They are UVR8-interacting proteins and act downstream of UVR8 and COP1 in a negative feedback loop (Gruber et al., 2010).
Recently, the identification of genome-wide binding sites of FHY3 profoundly extended our understanding of the diversity of FHY3 function, especially in environmental adaptation (Ouyang et al., 2011; Stirnberg et al., 2012; Tang et al., 2012). In response to photomorphogenic UV-B, FHY3 functions primarily via activating COP1 expression. Also, it was found that photomorphogenic UV-B entrains the plant clock. Without UV-B pulses, amplified circadian rhythms are eliminated in clock genes like CIRCADIAN CLOCK ASSOCIATED1, GIGANTEA, and EARLY FLOWERING4 (ELF4) (Fehér et al., 2011). Among these genes, ELF4 loses its rhythmic expression in fhy3 mutants in visible light and it needs FHY3 for transcriptional activation (Li et al., 2011). Thus, FHY3 might be a link between UV-B–induced photomorphogenesis and the circadian clock. Interestingly, FAR1 does not seem to function in early photomorphogenic UV-B responses (see Supplemental Figure 6 online). Based on previous promoter-swapping experiments, FHY3 may undertake a divergent role from FAR1 through protein subfunctionalization (Lin et al., 2008).
The Expression and Activity of FHY3 Is Regulated by Photomorphogenic UV-B
Time-course microarray-based expression profiling of light-responsive genes has documented the light regulation of transcription factors (Tepperman et al., 2001, 2004; Jiao et al., 2003). For example, HY5 protein accumulates under various light conditions (Oyama et al., 1997; Hardtke et al., 2000; Osterlund et al., 2000). FHY3 is repressed by far-red light (Lin et al., 2007) but is activated by photomorphogenic UV-B (see Supplemental Figures 3B and 9A online). This differential regulation shows that FHY3 can be specifically controlled by distinct wavelengths of light. Meanwhile, FHY1, a target of FHY3 under far-red light, shows no transcriptional response to UV-B (see Supplemental Figure 3 online). Such distinct transcriptional behaviors of FHY3 and FHY1 indicate a fine-tuning of light signals that differentiates the far-red light pathway from the UV-B pathway. Additionally, though FHY3 mRNA level is rapidly upregulated by photomorphogenic UV-B, its protein level is eventually downregulated (Figure 2G; see Supplemental Figures 1B, 9B, and 9C online). This observation indicates that FHY3 might be regulated by photomorphogenic UV-B in a posttranscriptional manner, which awaits further investigation.
FHY3 contains an N-terminal C2H2 zinc finger domain, a central putative core transposase domain, and a C-terminal SWIM zinc finger domain (named after SWI2/SNF and MuDR transposases) (Makarova et al., 2002). The C2H2 zinc finger domain mediates its DNA binding activity, and the transposase catalytic domain and SWIM motif are important for its transcriptional activation activity (Lin et al., 2008). FHY3 shows high affinity to the COP1 promoter (Figure 2), whose FBS-containing region is on the list of global FBSs (Ouyang et al., 2011). A series of ChIP assays collectively suggests that FHY3 binds to the COP1 promoter in far-red, dark, −UV-B, and +UV-B conditions (Ouyang et al., 2011; Figure 2; see Supplemental Figure 1 online). However, our earlier microarray data did not detect altered COP1 mRNA levels in response to FHY3 translocation into the nucleus in far-red light or dark conditions (Ouyang et al., 2011). The transcriptional activation activity of FHY3 toward the COP1 promoter is strictly induced by the UV-B signaling (Figures 3C and 3E).
COP1 and HY5 Form a Positive Feedback Loop in UV-B–Induced Photomorphogenesis
As one of the core transcription factors in light control of plant development, HY5 is induced by light and its protein accumulation is promoted by multiple photoreceptors under distinct wavelengths of light (Oyama et al., 1997; Hardtke et al., 2000; Osterlund et al., 2000). The SPA-COP1 complexes are substrate receptors for CULLIN4-DDB1 E3 ligases, which ubiquitinate HY5 for selective degradation by the 26S proteasome (Osterlund et al., 2000; Saijo et al., 2003; Chen et al., 2006, 2010; Zhu et al., 2008). In UV-B–induced photomorphogenesis, HY5 acts downstream of UVR8 and COP1 (Ulm et al., 2004; Oravecz et al., 2006; Favory et al., 2009). Meanwhile, HY5 directly associates with the COP1 promoter via the ACE element independent of UV-B irradiation (Figure 2) and induces COP1 expression specifically dependent on UV-B signaling (Figures 3C and 3E). Thus, COP1 and HY5 form a positive feedback loop in UV-B photomorphogenic responses.
Upon UV-B treatment, three-fourths of the early UV-B–activated genes lose induction in cop1-4, but more than a half remain unchanged in hy5-1 (Oravecz et al., 2006), suggesting there is a group of COP1-dependent genes that are regulated independent of HY5. Besides, there may no longer be a COP1-mediated degradation of HY5 under UV-B (Favory et al., 2009). In this case, the mutual transcriptional regulation between COP1 and HY5 might stand as one of their interaction modes. Still, the positive feedback is important, together with FHY3-induced COP1 expression, to produce adequate early signal transducers to maintain UVR8-COP1–mediated signaling. Downstream of UVR8-COP1, RUP1 and RUP2 rapidly accumulate their transcripts and proteins and result in a negative feedback loop to repress UV-B–induced gene expression. Meanwhile, RUP1 and RUP2 are likely to sequester UVR8 from COP1. In these two ways, RUP1 and RUP2 prevent an exaggerated UV-B photomorphogenesis response and further balance UV-B acclimation and plant development (Gruber et al., 2010; Figure 9). A recent study on another negative regulator, SALT TOLERANCE, that might antagonize HY5 transcriptional activity (Jiang et al., 2012) also supports coordination of positive and negative feedback processes as crucial for appropriate UV-B photomorphogenic responses in planta.
The Combinatorial Functional Interaction of the FHY3-HY5 Pair Is Distinct in UV-B–Specific Response from That in Far-Red Light Signaling and Circadian Regulation
Two contrasting working models between FHY3 and HY5 were hypothesized in recent years. In far-red light responses, FHY3 and FAR1 directly activate FHY1 and FHL, which produce chaperone proteins to facilitate the phyA-FHY1/FHL nuclear import complex for downstream signaling, while they are downregulated by phyA in a negative feedback loop (Lin and Wang, 2004; Hiltbrunner et al., 2005; Genoud et al., 2008). HY5 acts antagonistically to FHY3 and suppresses FHY1/FHL expression by interfering with FHY3/FAR1 binding to the FHY1/FHL promoters (Li et al., 2010). However, FHY3 and HY5 work in concert in circadian expression of ELF4. FHY3 is tied to circadian regulation in the daytime phase, relied on its role in gating red light signaling to the circadian clock and in maintaining the rhythmic expression of ELF4. FHY3/FAR1 and HY5 directly activate ELF4 expression during the day (Allen et al., 2006; Li et al., 2011).
In UV-B photomorphogenic responses, FHY3 and HY5 share some similarity in their temporal mRNA accumulation upon UV-B treatment (see Supplemental Figure 9 online), their mutant phenotypes and their effects on COP1 and CHS expression. Our data clearly defined a set of two cis-elements, the FBS motif and the ACE element, that act in the FHY3- and HY5-mediated transcriptional modulation of COP1 in response to UV-B. In the COP1 promoter, there is probably little steric hindrance for FHY3 and HY5 binding since the cis-elements bound by these two proteins are ∼100 bp away (Figure 2A). This distance is even greater than that in the ELF4 promoter (30 bp). Additionally, photomorphogenic UV-B diminishes the physical contact of FHY3 and HY5 (Figure 8A), possibly due to the reduced FHY3 protein abundance (Figure 2G; see Supplemental Figures 1B, 9B, and 9C online). Either FHY3 or HY5 alone can maintain a low level of UV-B–mediated COP1 upregulation (Figures 6B and 6E), to show affinity to the COP1 promoter (Figures 8C and 8D), and to accomplish limited photomorphogenic responses to UV-B when the other one is absent (Figure 4; see Supplemental Figure 7 online). These data suggest that FHY3 and HY5 work in a noncompetitive manner. FHY3 acts as a positive regulator of UV-B–induced photomorphogenesis through directly activating COP1 transcription, while HY5 forms a positive feedback on COP1. Such dual transcriptional regulation is required for the full activation of UV-B–induced COP1 gene expression. To some extent, HY5 might compensate for fhy3 mutation by elevating its own mRNA and protein levels (Figures 4F and 8B). Compared with far-red light and circadian conditions, photomorphogenic UV-B endows the same set of signaling intermediates, FHY3 and HY5, a distinct working mode.
Taken together, our data support a refined UV-B signaling model (Figure 9). In UV-B photomorphogenic responses, a core UV-B signaling pathway is established by UVR8-COP1-HY5. Upon UV-B treatment, UVR8 undergoes a rapid conformational switch from homodimer to monomer, which enables its interaction with COP1. The UVR8-COP1 complex triggers downstream signaling events, including gene expression regulated by HY5, which is repressed by a negative feedback formed by RUPs. In UV-B regulation of gene expression, COP1 is one of the UV-B–inducible genes. FHY3 and HY5, whose physical interaction is altered by UV-B, directly bind to the FBS motif and the ACE element in the COP1 promoter respectively and activate the transcription of COP1. This combinatorial transcriptional modulation helps produce abundant COP1 and thus ensures a more active UVR8-COP1-HY5 UV-B core signaling pathway.
METHODS
Plant Materials and Growth Conditions
The wild-type Arabidopsis thaliana used in this study was of the Columbia (Col), Ws, No-0, and Landsberg erecta ecotypes. Some of the mutants and transgenic lines used in this study were described previously: fhy3-1 and far1-2 (Whitelam et al., 1993; Kang et al., 2009), fhy3-3, fhy3-4, fhy3-5, and fhy3-10 (Wang and Deng, 2002), fhy3-4 far1-2 and FHY3p:FHY3-GR/fhy3-4 (Lin et al., 2007), 3FLAG-FHY3-3HA/fhy3-4 (Li et al., 2011), hy5-215, and hy5-ks50 (Oyama et al., 1997), 35S-HY5/hy5-ks50 (Hardtke et al., 2000), COP1OE, and cop1-4 (McNellis et al., 1994), GUS-COP1/cop1-5 (von Arnim et al., 1997), phyAB (Reed et al., 1994), phyABDE (Franklin et al., 2003), cry1 cry2 (Mao et al., 2005), uvr8-6 (Favory et al., 2009), and uvh1-1 (Liu et al., 2001).
The Arabidopsis seeds were surface-sterilized and sown on solid 1% Murashige and Skoog medium supplemented with 1% Suc for biochemical assays or with 0.3% Suc for phenotypic analysis and cold treated at 4°C for 4 d. Then, for photomorphogenic UV-B treatment, seedlings were grown under continuous white light (3 μmol·m−2·s−1, measured by LI-250 Light Meter; LI-COR Biosciences) supplemented with Philips TL20W/01RS narrowband UV-B tubes (1.5 μmol·m−2·s−1, measured by a TN-340 UV-B light meter) under a 350-nm cutoff (half-maximal transmission at 350 nm) filter ZUL0350 (−UV-B; Asahi Spectra) or a 300-nm cutoff (half-maximal transmission at 300 nm) filter ZUL0300 (+UV-B; Asahi Spectra). For damaging UV-B treatment, seedlings were grown under continuous white light (30 μmol·m−2·s−1) supplemented with narrowband UV-B (1.5 μmol·m−2·s−1) for 12 d and then were irradiated by Philips TL40W/12RS broadband UV-B tubes (20 μmol·m−2·s−1) with ZUL0350 (−UV-B) or ZUL0300 (+UV-B) filters for 12 h. The UV-B light conditions are essentially identical to those reported recently (Oravecz et al., 2006; Favory et al., 2009). For monochromatic light treatment, cold-treated seeds were grown in darkness for 4 d and then transferred to far-red light (0.5 μmol·m−2·s−1), red light (10 μmol·m−2·s−1), or blue light (4 μmol·m−2·s−1).
Site-Directed Mutagenesis and Plasmid Construction
For yeast one-hybrid assays, to generate the COP1p:LacZ reporter construct, a reported COP1 promoter region (Kang et al., 2009) was amplified and cloned into the EcoRI-XhoI site of pLacZi2μ (Lin et al., 2007). LacZ reporter genes driven by mutant COP1 promoters were generated using the COP1p:LacZ reporter plasmid as the template and the QuikChange site-directed mutagenesis kit (Stratagene). The AD-FHY3, AD-HY5, and AD-HYH constructs were described previously (Wang and Deng, 2002; Li et al., 2010). For EMSA assays, the GST-HY5 and GST-FHY3N constructs were described previously (Ang et al., 1998; Lin et al., 2007). For LCI assays, NLuc, CLuc, HY5- NLuc, and CLuc-FHY3N were described previously (Li et al., 2010). For dual-luciferase assays, to generate the COP1p:LUC reporter construct, the reported COP1 promoter region (Kang et al., 2009) was again amplified and cloned into the HindIII-XhoI site of the pGreenII 0800-LUC (Hellens et al., 2005). LUC reporter genes driven by mutant COP1 promoters were generated using the COP1p:LUC reporter plasmid as the template and the QuikChange site-directed mutagenesis kit (Stratagene). The 35S:FHY3 and 35S:HY5 constructs were described previously (Li et al., 2010).
All the primers are listed in Supplemental Table 1 online, and all the constructs were confirmed by sequencing analyses.
Immunoblot Analysis
For anti-FHY3 immunoblots, Arabidopsis seedlings were ground to a fine powder and total proteins were eluted in 2× SDS loading buffer. For all other immunoblots, Arabidopsis seedlings were homogenized in a protein extraction buffer containing 50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 10 mM NaF, 25 mM β-glycerophosphate, 2 mM Na3VO4, 10 mM NaF, 10% glycerol, 0.1% Tween 20, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, and 1×complete protease inhibitor cocktail (Roche).
Primary antibodies used in this study were anti-flag (Sigma-Aldrich), anti-HY5 (Osterlund et al., 2000), anti-FHY3 (Saijo et al., 2008), anti-COP1, and anti-RPN6 (Chen et al., 2006) antibodies.
Quantitative Real-Time PCR
Total RNA was extracted from Arabidopsis seedlings using the RNeasy plant mini kit (Qiagen). Reverse transcription was performed using the SuperScript II first-strand cDNA synthesis system (Invitrogen) according to the manufacturer’s instructions. Real-time qPCR analysis was performed using Power SYBR Green PCR Master Mix (Applied Biosystems) with a Bio-Rad CFX96 real-time PCR detection system. Each experiment was repeated with three independent samples, and RT-PCR reactions were performed in three technical replicates for each sample. The primers used for quantitative RT-PCR are listed in Supplemental Table 1 online.
Yeast One-Hybrid Assays
Plasmids of AD fusions were cotransformed with the LacZ reporter genes driven by wild-type and mutant COP1 promoters into the yeast strain EGY48. Transformants were grown on proper dropout plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside for blue color development. Yeast transformation was conducted as described in the Yeast Protocols Handbook (Clontech).
ChIP
Arabidopsis seedlings grown under −UV-B and +UV-B for 4 d were used for ChIP assays following the procedure described previously (Lee et al., 2007). Briefly, 5 g of seedlings were first cross-linked with 1% formaldehyde under vacuum, and then the samples were ground to powder in liquid nitrogen. The chromatin complexes were isolated and sonicated and then incubated with anti-H3 (Millipore), anti-flag (Sigma-Aldrich), anti-FHY3, and anti-HY5 antibodies. The precipitated DNA was recovered and analyzed by PCR methods using the primers in Supplemental Table 1 online. Real-time PCR was performed as described above.
EMSA
EMSAs were performed using the biotin-labeled probes and the Lightshift Chemiluminescent EMSA kit (Pierce) according to the manufacturer’s instructions. Briefly, 0.5 μg of GST or GST fusion proteins were incubated together with biotin-labeled probes in 20-μL reaction mixtures containing 10 mM Tris-HCl, 150 mM KCl, 1 mM DTT, 50 ng/μL poly (dI-dC), 2.5% glycerol, 0.05% Nonidet P-40, 100 μM ZnCl2, and 0.5 μg/μL BSA for 20 min at room temperature and separated on 6% native polyacrylamide gels in Tris-Gly buffer. The labeled probes were detected according to the instructions provided with the EMSA kit. Sequences of the complementary oligonucleotides used to generate the biotin-labeled probes are shown in Supplemental Table 1 online.
Transient Transcription Dual-Luciferase Assay
Transient dual-luciferase assays in Nicotiana benthamiana and Arabidopsis were performed as described previously (Hellens et al., 2005). After infiltration, plants were left under −UV-B or +UV-B for 3 d, and then leaf samples were collected. Firefly luciferase and Renillia luciferase were assayed using the dual luciferase assay reagents (Promega) and were performed as previously described (Liu et al., 2008). Briefly, leaf discs (1 to 2 cm in diameter) were excised, ground in liquid nitrogen, and homogenized in 100 μL of the passive lysis buffer. Eight microliters of this crude extract was mixed with 40 μL of luciferase assay buffer, and the firefly LUC activity was measured using a GLOMAX 20/20 luminometer (Promega). Forty microliters of Stop and Glow Buffer was then added to the reaction, and the Renillia luciferase (REN) activity was measured. Four biological replicates were measured for each sample.
Anthocyanin and Hypocotyl Measurement
Anthocyanins were extracted and quantified as previously described (Noh and Spalding, 1998). Briefly, Arabidopsis seedlings were harvested and placed into extraction solution (18% 1-propanol and 1% HCl) and boiled for 3 min. Then, the mixture was left in darkness for at least 3 h at room temperature. After a brief centrifugation to pellet the tissue debris, the supernatant was removed and diluted with the extraction solution. The anthocyanin content was presented as A535 − 2(A650) g−1 fresh weight.
For each line under each condition (−UV-B or +UV-B), hypocotyl length was analyzed using three biological replicates. In each replicate, at least 30 Arabidopsis seedlings were measured. The relative hypocotyl length was presented as the percentage of the hypocotyl length under +UV-B with respect to that under −UV-B (percentage of −UV-B).
LCI Assays
Transient LCI assays in N. benthamiana were performed as described previously (Chen et al., 2008). Briefly, Agrobacterium tumefaciens containing the indicated constructs were infiltrated into N. benthamiana leaves. After infiltration, plants were grown under −UV-B and +UV-B for 3 d, and luciferase signals were then viewed in an IVIS Spectrum imaging system (Caliper LifeSciences) and quantified with the Living Image 4.0 software.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: COP1 (At2g32950), ELIP2 (At4g14690), At4g15480, DREB2A (At5g05410), CHS (At5g13930), FHY3 (At3g22170), HY5 (At5g11260), HYH (At3g17609), and FHY1 (At2g37678).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. 3FLAG-FHY3-3HA Associates with the COP1 Promoter under −UV-B and +UV-B.
Supplemental Figure 2. HYH Does Not Bind to the COP1 Promoter.
Supplemental Figure 3. FHY1 Expression Is Not Induced by Photomorphogenic UV-B.
Supplemental Figure 4. Relative Hypocotyl Length of Wild-Type and Various Mutant Seedlings.
Supplemental Figure 5. Additional fhy3 Alleles Are Defective in UV-B–Induced Hypocotyl Growth.
Supplemental Figure 6. FAR1 Is Barely Involved in UV-B–Induced Photomorphogenesis.
Supplemental Figure 7. UV-B–Induced Photomorphogenesis Is Impaired in hy5-ks50.
Supplemental Figure 8. COP1 Is Overexpressed in COP1OE and GUS-COP1/cop1-5.
Supplemental Figure 9. COP1, HY5, and FHY3 Show Rapid Expression Responses to Photomorphogenic UV-B.
Supplemental Table 1. Summary of Primers Used in This Study.
Acknowledgments
We thank Jian-Min Zhou for 35S:NLuc and 35S:CLuc plasmids, Chentao Lin for pGreenII 0800-LUC plasmid, and Tian Xu for kindly sharing their imaging system. We also thank Cynthia Nezames for helpful comments on the article and all the Deng lab members for their constructive suggestions on the project. This work was supported by a National Institutes of Health grant (GM47850) to X.W.D., in part by the Ministry of Science and Technology of China (2012CB910900) and the State Key Laboratory of Protein and Plant Gene Research at Peking University, and in part by a China Postdoctoral Science Foundation grant (2012M510266) to X.O. and The Postdoctoral Fellowship at Center for Life Sciences to X.H. and X.O. X.H. and X.O. were Monsanto Fellows during their visit to Yale University.
AUTHOR CONTRIBUTIONS
X.H., X.O., and X.W.D. designed the research. X.H., X.O., P.Y., O.S.L., G.L., J.L., and H.C. performed the research. X.H. and X.O. analyzed the data. X.H. and X.W.D. wrote the article.
Glossary
- ACE
ACGT-containing element
- FBS
FHY3 binding site
- AD
activation domain
- EMSA
electrophoretic mobility shift assay
- GST
glutathione S-transferase
- ChIP
chromatin immunoprecipitation
- qPCR
quantitative PCR
- DEX
dexamethasone
- Ws
Wassilewskija
- LCI
luciferase complementation imaging
- LRE
light-responsive cis-element
- Col
Columbia
- No-0
Nossen-0
References
- Allen T., Koustenis A., Theodorou G., Somers D.E., Kay S.A., Whitelam G.C., Devlin P.F. (2006). Arabidopsis FHY3 specifically gates phytochrome signaling to the circadian clock. Plant Cell 18: 2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L.H., Chattopadhyay S., Wei N., Oyama T., Okada K., Batschauer A., Deng X.W. (1998). Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell 1: 213–222 [DOI] [PubMed] [Google Scholar]
- Brosché M., Schuler M.A., Kalbina I., Connor L., Strid A. (2002). Gene regulation by low level UV-B radiation: Identification by DNA array analysis. Photochem. Photobiol. Sci. 1: 656–664 [DOI] [PubMed] [Google Scholar]
- Brown B.A., Cloix C., Jiang G.H., Kaiserli E., Herzyk P., Kliebenstein D.J., Jenkins G.I. (2005). A UV-B-specific signaling component orchestrates plant UV protection. Proc. Natl. Acad. Sci. USA 102: 18225–18230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B.A., Jenkins G.I. (2008). UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol. 146: 576–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati P., Campi M., Chu F., Suzuki N., Maltby D., Guan S., Burlingame A.L., Walbot V. (2008). Histone acetylation and chromatin remodeling are required for UV-B-dependent transcriptional activation of regulated genes in maize. Plant Cell 20: 827–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati P., Walbot V. (2003). Gene expression profiling in response to ultraviolet radiation in maize genotypes with varying flavonoid content. Plant Physiol. 132: 1739–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalá R., Medina J., Salinas J. (2011). Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 16475–16480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Huang X., Gusmaroli G., Terzaghi W., Lau O.S., Yanagawa Y., Zhang Y., Li J., Lee J.H., Zhu D., Deng X.W. (2010). Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 22: 108–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Shen Y., Tang X., Yu L., Wang J., Guo L., Zhang Y., Zhang H., Feng S., Strickland E., Zheng N., Deng X.W. (2006). Arabidopsis CULLIN4 forms an E3 ubiquitin ligase with RBX1 and the CDD complex in mediating light control of development. Plant Cell 18: 1991–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zou Y., Shang Y., Lin H., Wang Y., Cai R., Tang X., Zhou J.M. (2008). Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 146: 368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J.M., Arvai A.S., Baxter K.J., Heilmann M., Pratt A.J., O’Hara A., Kelly S.M., Hothorn M., Smith B.O., Hitomi K., Jenkins G.I., Getzoff E.D. (2012). Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335: 1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J.M., Jenkins G.I. (1996). Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 8: 1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloix C., Jenkins G.I. (2008). Interaction of the Arabidopsis UV-B-specific signaling component UVR8 with chromatin. Mol. Plant 1: 118–128 [DOI] [PubMed] [Google Scholar]
- Cluis C.P., Mouchel C.F., Hardtke C.S. (2004). The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J. 38: 332–347 [DOI] [PubMed] [Google Scholar]
- Favory J.J., et al. (2009). Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 28: 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehér B., Kozma-Bognár L., Kevei E., Hajdu A., Binkert M., Davis S.J., Schäfer E., Ulm R., Nagy F. (2011). Functional interaction of the circadian clock and UV RESISTANCE LOCUS 8-controlled UV-B signaling pathways in Arabidopsis thaliana. Plant J. 67: 37–48 [DOI] [PubMed] [Google Scholar]
- Franklin K.A., Praekelt U., Stoddart W.M., Billingham O.E., Halliday K.J., Whitelam G.C. (2003). Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 131: 1340–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnmeyer H., Staiger D. (2003). Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 133: 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G., Lin C., Tobin E.M., Loehrer H., Brinkman D. (2009). Photobiological properties of the inhibition of etiolated Arabidopsis seedling growth by ultraviolet-B irradiation. Plant Cell Environ. 32: 1573–1583 [DOI] [PubMed] [Google Scholar]
- Genoud T., Schweizer F., Tscheuschler A., Debrieux D., Casal J.J., Schäfer E., Hiltbrunner A., Fankhauser C. (2008). FHY1 mediates nuclear import of the light-activated phytochrome A photoreceptor. PLoS Genet. 4: e1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber H., Heijde M., Heller W., Albert A., Seidlitz H.K., Ulm R. (2010). Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Proc. Natl. Acad. Sci. USA 107: 20132–20137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke C.S., Gohda K., Osterlund M.T., Oyama T., Okada K., Deng X.W. (2000). HY5 stability and activity in Arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J. 19: 4997–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hectors K., Prinsen E., De Coen W., Jansen M.A., Guisez Y. (2007). Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol. 175: 255–270 [DOI] [PubMed] [Google Scholar]
- Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltbrunner A., Viczián A., Bury E., Tscheuschler A., Kircher S., Tóth R., Honsberger A., Nagy F., Fankhauser C., Schäfer E. (2005). Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr. Biol. 15: 2125–2130 [DOI] [PubMed] [Google Scholar]
- Jenkins G.I. (2009). Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 60: 407–431 [DOI] [PubMed] [Google Scholar]
- Jiang L., Wang Y., Li Q.F., Björn L.O., He J.X., Li S.S. (2012). Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 22: 1046–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Lau O.S., Deng X.W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Jiao Y., et al. (2003). A genome-wide analysis of blue-light regulation of Arabidopsis transcription factor gene expression during seedling development. Plant Physiol. 133: 1480–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserli E., Jenkins G.I. (2007). UV-B promotes rapid nuclear translocation of the Arabidopsis UV-B specific signaling component UVR8 and activates its function in the nucleus. Plant Cell 19: 2662–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C.Y., Lian H.L., Wang F.F., Huang J.R., Yang H.Q. (2009). Cryptochromes, phytochromes, and COP1 regulate light-controlled stomatal development in Arabidopsis. Plant Cell 21: 2624–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.C., Tennessen D.J., Last R.L. (1998). UV-B-induced photomorphogenesis in Arabidopsis thaliana. Plant J. 15: 667–674 [DOI] [PubMed] [Google Scholar]
- Kliebenstein D.J., Lim J.E., Landry L.G., Last R.L. (2002). Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol. 130: 234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O.S., Deng X.W. (2010). Plant hormone signaling lightens up: integrators of light and hormones. Curr. Opin. Plant Biol. 13: 571–577 [DOI] [PubMed] [Google Scholar]
- Lee J., He K., Stolc V., Lee H., Figueroa P., Gao Y., Tongprasit W., Zhao H., Lee I., Deng X.W. (2007). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., et al. (2011). Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis. Nat. Cell Biol. 13: 616–622 [DOI] [PubMed] [Google Scholar]
- Li J., Li G., Gao S., Martinez C., He G., Zhou Z., Huang X., Lee J.H., Zhang H., Shen Y., Wang H., Deng X.W. (2010). Arabidopsis transcription factor ELONGATED HYPOCOTYL5 plays a role in the feedback regulation of phytochrome A signaling. Plant Cell 22: 3634–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Ding L., Casola C., Ripoll D.R., Feschotte C., Wang H. (2007). Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318: 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Teng Y., Park H.J., Ding L., Black C., Fang P., Wang H. (2008). Discrete and essential roles of the multiple domains of Arabidopsis FHY3 in mediating phytochrome A signal transduction. Plant Physiol. 148: 981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Wang H. (2004). Arabidopsis FHY3/FAR1 gene family and distinct roles of its members in light control of Arabidopsis development. Plant Physiol. 136: 4010–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Yu X., Li K., Klejnot J., Yang H., Lisiero D., Lin C. (2008). Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322: 1535–1539 [DOI] [PubMed] [Google Scholar]
- Liu Z., Hall J.D., Mount D.W. (2001). Arabidopsis UVH3 gene is a homolog of the Saccharomyces cerevisiae RAD2 and human XPG DNA repair genes. Plant J. 26: 329–338 [DOI] [PubMed] [Google Scholar]
- Lloyd A.M., Schena M., Walbot V., Davis R.W. (1994). Epidermal cell fate determination in Arabidopsis: Patterns defined by a steroid-inducible regulator. Science 266: 436–439 [DOI] [PubMed] [Google Scholar]
- Makarova K.S., Aravind L., Koonin E.V. (2002). SWIM, a novel Zn-chelating domain present in bacteria, archaea and eukaryotes. Trends Biochem. Sci. 27: 384–386 [DOI] [PubMed] [Google Scholar]
- Mao J., Zhang Y.C., Sang Y., Li Q.H., Yang H.Q. (2005). From The Cover: A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl. Acad. Sci. USA 102: 12270–12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNellis T.W., von Arnim A.G., Deng X.W. (1994). Overexpression of Arabidopsis COP1 results in partial suppression of light-mediated development: Evidence for a light-inactivable repressor of photomorphogenesis. Plant Cell 6: 1391–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B., Spalding E.P. (1998). Anion channels and the stimulation of anthocyanin accumulation by blue light in Arabidopsis seedlings. Plant Physiol. 116: 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oravecz A., Baumann A., Máté Z., Brzezinska A., Molinier J., Oakeley E.J., Adám E., Schäfer E., Nagy F., Ulm R. (2006). CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell 18: 1975–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Ouyang X., et al. (2011). Genome-wide binding site analysis of FAR-RED ELONGATED HYPOCOTYL3 reveals its novel function in Arabidopsis development. Plant Cell 23: 2514–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T., Shimura Y., Okada K. (1997). The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente P., Wei N., Deng X.W. (1996). Combinatorial interplay of promoter elements constitutes the minimal determinants for light and developmental control of gene expression in Arabidopsis. EMBO J. 15: 3732–3743 [PMC free article] [PubMed] [Google Scholar]
- Reed J.W., Nagatani A., Elich T.D., Fagan M., Chory J. (1994). Phytochrome A and Phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 104: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini L., Favory J.J., Cloix C., Faggionato D., O’Hara A., Kaiserli E., Baumeister R., Schäfer E., Nagy F., Jenkins G.I., Ulm R. (2011). Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106 [DOI] [PubMed] [Google Scholar]
- Safrany J., Haasz V., Mate Z., Ciolfi A., Feher B., Oravecz A., Stec A., Dallmann G., Morelli G., Ulm R., Nagy F. (2008). Identification of a novel cis-regulatory element for UV-B-induced transcription in Arabidopsis. Plant J. 54: 402–414 [DOI] [PubMed] [Google Scholar]
- Saijo Y., Sullivan J.A., Wang H., Yang J., Shen Y., Rubio V., Ma L., Hoecker U., Deng X.W. (2003). The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 17: 2642–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y., Zhu D., Li J., Rubio V., Zhou Z., Shen Y., Hoecker U., Wang H., Deng X.W. (2008). Arabidopsis COP1/SPA1 complex and FHY1/FHY3 associate with distinct phosphorylated forms of phytochrome A in balancing light signaling. Mol. Cell 31: 607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkle J.R., Edwards M.C., Koenig A., Shaltz A., Barnes P.W. (2010). Photomorphogenic regulation of increases in UV-absorbing pigments in cucumber (Cucumis sativus) and Arabidopsis thaliana seedlings induced by different UV-B and UV-C wavebands. Physiol. Plant. 138: 113–121 [DOI] [PubMed] [Google Scholar]
- Stirnberg P., Zhao S., Williamson L., Ward S., Leyser O. (2012). FHY3 promotes shoot branching and stress tolerance in Arabidopsis in an AXR1-dependent manner. Plant J. 71: 907–920 [DOI] [PubMed] [Google Scholar]
- Stracke R., Favory J.J., Gruber H., Bartelniewoehner L., Bartels S., Binkert M., Funk M., Weisshaar B., Ulm R. (2010). The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ. 33: 88–103 [DOI] [PubMed] [Google Scholar]
- Tang W., Wang W., Chen D., Ji Q., Jing Y., Wang H., Lin R. (2012). Transposase-derived proteins FHY3/FAR1 interact with PHYTOCHROME-INTERACTING FACTOR1 to regulate chlorophyll biosynthesis by modulating HEMB1 during deetiolation in Arabidopsis. Plant Cell 24: 1984–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman J.M., Hudson M.E., Khanna R., Zhu T., Chang S.H., Wang X., Quail P.H. (2004). Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J. 38: 725–739 [DOI] [PubMed] [Google Scholar]
- Tepperman J.M., Zhu T., Chang H.S., Wang X., Quail P.H. (2001). Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc. Natl. Acad. Sci. USA 98: 9437–9442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R., Baumann A., Oravecz A., Máté Z., Adám E., Oakeley E.J., Schäfer E., Nagy F. (2004). Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R., Nagy F. (2005). Signalling and gene regulation in response to ultraviolet light. Curr. Opin. Plant Biol. 8: 477–482 [DOI] [PubMed] [Google Scholar]
- von Arnim A.G., Deng X.W. (1994). Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specificregulation of its nucleocytoplasmic partitioning. Cell 79: 1035–1045 [DOI] [PubMed] [Google Scholar]
- von Arnim A.G., Osterlund M.T., Kwok S.F., Deng X.W. (1997). Genetic and developmental control of nuclear accumulation of COP1, a repressor of photomorphogenesis in Arabidopsis. Plant Physiol. 114: 779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Deng X.W. (2002). Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1. EMBO J. 21: 1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ma L.G., Li J.M., Zhao H.Y., Deng X.W. (2001). Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294: 154–158 [DOI] [PubMed] [Google Scholar]
- Whitelam G.C., Johnson E., Peng J., Carol P., Anderson M.L., Cowl J.S., Harberd N.P. (1993). Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5: 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf L., Rizzini L., Stracke R., Ulm R., Rensing S.A. (2011). The molecular and physiological responses of Physcomitrella patens to ultraviolet-B radiation. Plant Physiol. 153: 1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Hu Q., Yan Z., Chen W., Yan C., Huang X., Zhang J., Yang P., Deng H., Wang J., Deng X., Shi Y. (2012). Structural basis of ultraviolet-B perception by UVR8. Nature 484: 214–219 [DOI] [PubMed] [Google Scholar]
- Wu M., Grahn E., Eriksson L.A., Strid A. (2011). Computational evidence for the role of Arabidopsis thaliana UVR8 as UV-B photoreceptor and identification of its chromophore amino acids. J. Chem. Inf. Model. 51: 1287–1295 [DOI] [PubMed] [Google Scholar]
- Yang H.Q., Tang R.H., Cashmore A.R. (2001). The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13: 2573–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Deng X.W. (2005). COP1 - From plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 15: 618–625 [DOI] [PubMed] [Google Scholar]
- Zhang H., He H., Wang X., Wang X., Yang X., Li L., Deng X.W. (2011). Genome-wide mapping of the HY5-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J. 65: 346–358 [DOI] [PubMed] [Google Scholar]
- Zhu D., Maier A., Lee J.H., Laubinger S., Saijo Y., Wang H., Qu L.J., Hoecker U., Deng X.W. (2008). Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell 20: 2307–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]