Abstract
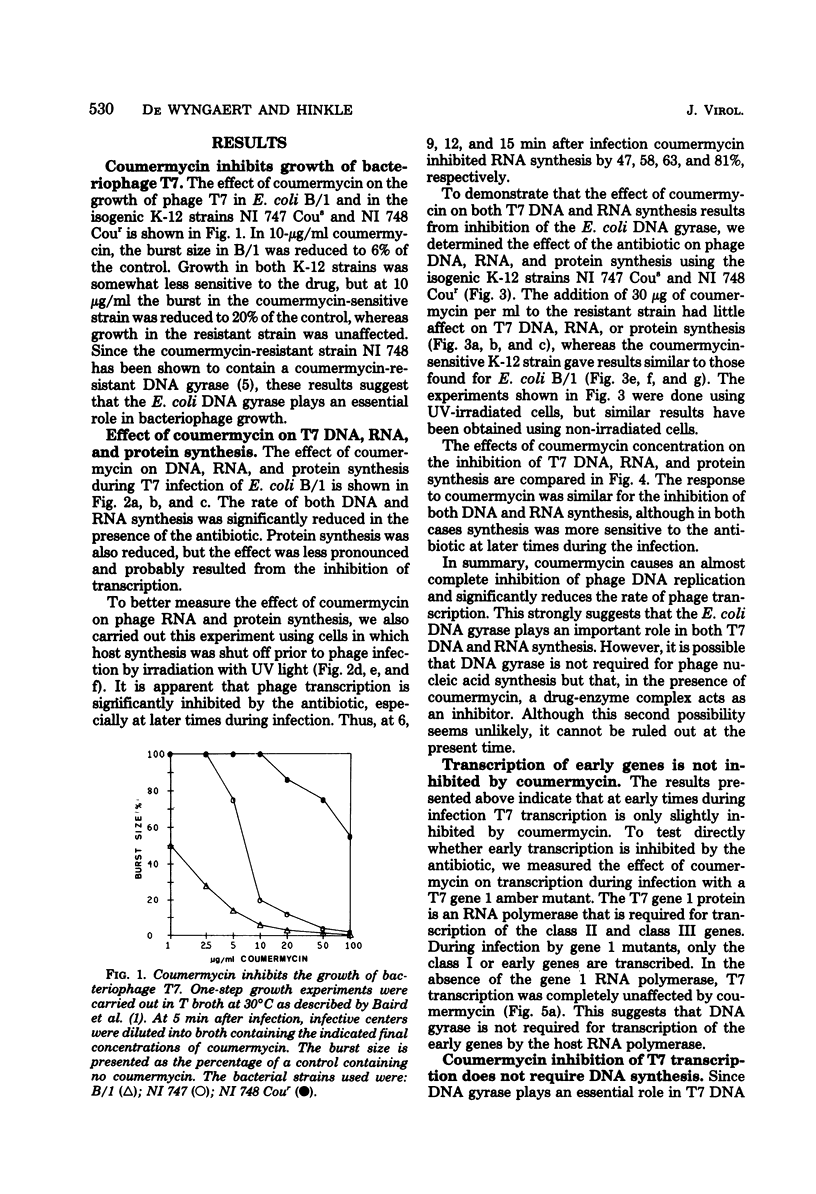
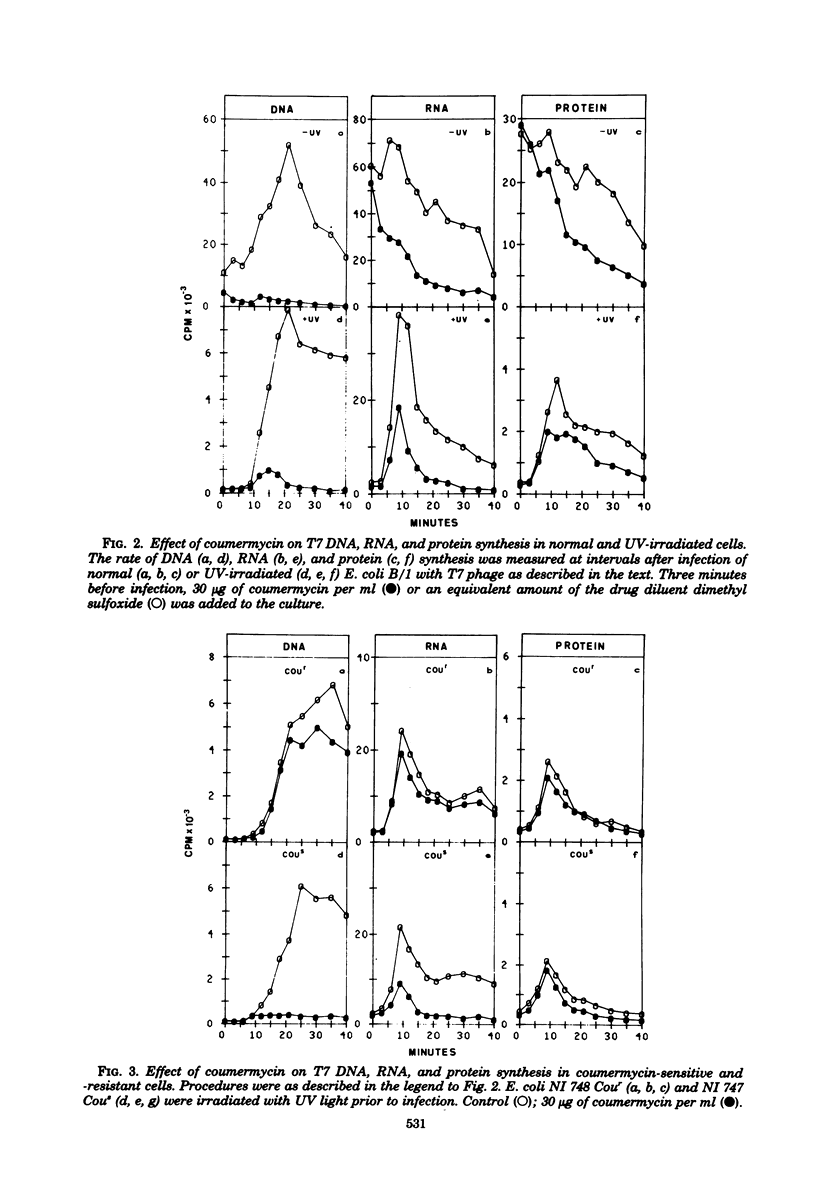
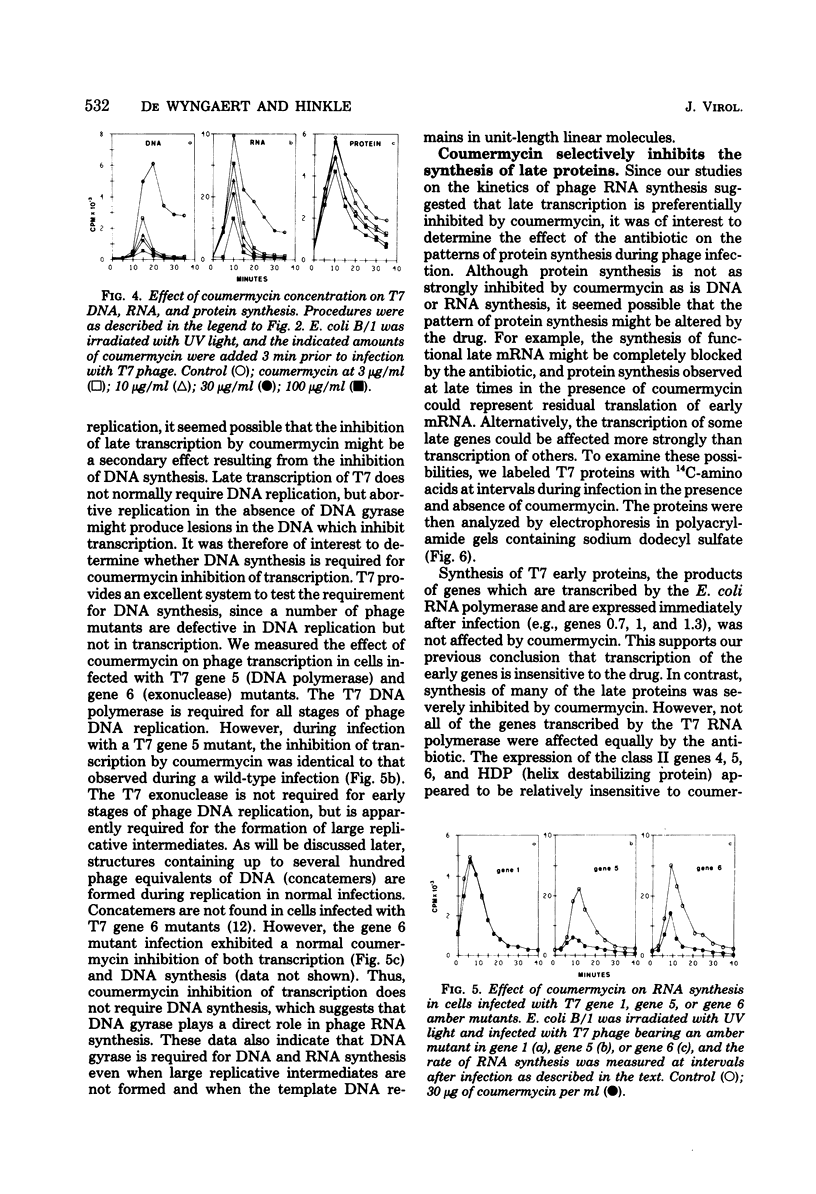
Growth of bacteriophage T7 is inhibited by the antibiotic coumermycin A1, an inhibitor of the Escherichia coli DNA gyrase. Since growth of the phage is insensitive to the antibiotic in strains containing a coumermycin-resistant DNA gyrase, this enzyme appears to be required for phage growth. We have investigated the effect of coumermycin on the kinetics of DNA, RNA, and protein synthesis during T7 infection. DNA synthesis is completely inhibited by the antibiotic. In addition, coumermycin significantly inhibits transcription of late but not early genes. Thus, E. coli DNA gyrase may play an important role in transcription as well as in replication of T7 DNA.
Full text
PDF



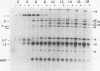
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird J. P., Bourguignon G. J., Sternglanz R. Effect of nalidixic acid on the growth of deoxyribonucleic acid bacteriophages. J Virol. 1972 Jan;9(1):17–21. doi: 10.1128/jvi.9.1.17-21.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan P., Wang J. C., Echols H. Effect of circularity and superhelicity on transcription from bacteriophagelambda DNA. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3077–3081. doi: 10.1073/pnas.70.11.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Snyder M. Superhelical Escherichia coli DNA: relaxation by coumermycin. J Mol Biol. 1978 Apr 5;120(2):145–154. doi: 10.1016/0022-2836(78)90061-x. [DOI] [PubMed] [Google Scholar]
- Fröhlich B., Powling A., Knippers R. Formation of concatemeric DNA in bacteriophage T7-infected bacteria. Virology. 1975 Jun;65(2):455–468. doi: 10.1016/0042-6822(75)90051-3. [DOI] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D. C., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro. Requirements for deoxyribonucleic acid synthesis and characterization of the product. J Biol Chem. 1974 May 10;249(9):2974–2980. [PubMed] [Google Scholar]
- Holloman W. K., Wiegand R., Hoessli C., Radding C. M. Uptake of homologous single-stranded fragments by superhelical DNA: a possible mechanism for initiation of genetic recombination. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2394–2398. doi: 10.1073/pnas.72.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. I. Involvement of DNA gyrase in bacteriophage T7 DNA replication. Nature. 1977 Nov 3;270(5632):78–80. doi: 10.1038/270078a0. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Marians K. J., Ikeda J. E., Schlagman S., Hurwitz J. Role of DNA gyrase in phiX replicative-form replication in vitro. Proc Natl Acad Sci U S A. 1977 May;74(5):1965–1968. doi: 10.1073/pnas.74.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro VI. Synthesis of biologically active T7 DNA. J Mol Biol. 1976 Feb 5;100(4):557–567. doi: 10.1016/s0022-2836(76)80045-9. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr, Lee M. The role of bacteriophage T7 exonuclease (gene 6) in genetic recombination and production of concatemers. J Mol Biol. 1976 Feb 25;101(2):223–234. doi: 10.1016/0022-2836(76)90374-0. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Gellert M., Nash H. A. Involement of supertwisted DNA in integrative recombination of bacteriophage lambda. J Mol Biol. 1978 May 25;121(3):375–392. doi: 10.1016/0022-2836(78)90370-4. [DOI] [PubMed] [Google Scholar]
- Nash H. A. Integrative recombination of bacteriophage lambda DNA in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1072–1076. doi: 10.1073/pnas.72.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetkau V., Langman L., Bradley R., Scraba D., Miller R. C., Jr Folded, concatenated genomes as replication intermediates of bacteriophage T7 DNA. J Virol. 1977 Apr;22(1):130–141. doi: 10.1128/jvi.22.1.130-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Initiation of transcription by Escherichia coli RNA polymerase from supercoiled and non-supercoiled bacteriophage PM2 DNA. J Mol Biol. 1975 Feb 5;91(4):477–487. doi: 10.1016/0022-2836(75)90274-0. [DOI] [PubMed] [Google Scholar]
- Ryan M. J. Coumermycin A1: A preferential inhibitor of replicative DNA synthesis in Escherichia coli. I. In vivo characterization. Biochemistry. 1976 Aug 24;15(17):3769–3777. doi: 10.1021/bi00662a020. [DOI] [PubMed] [Google Scholar]
- Serwer P. Fast sedimenting bacteriophage T7 DNA from T7-infected Escherichia coli. Virology. 1974 May;59(1):70–88. doi: 10.1016/0042-6822(74)90207-4. [DOI] [PubMed] [Google Scholar]
- Strätling W., Krause E., Knippers R. Fast sedimenting deoxyribonucleic acid in bacteriophage T7-infected cells. Virology. 1973 Jan;51(1):109–119. doi: 10.1016/0042-6822(73)90371-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971 Feb 14;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]