Abstract
In Solanaceae, the self-incompatibility S-RNase and S-locus F-box interactions define self-pollen recognition and rejection in an S-specific manner. This interaction triggers a cascade of events involving other gene products unlinked to the S-locus that are crucial to the self-incompatibility response. To date, two essential pistil-modifier genes, 120K and High Top-Band (HT-B), have been identified in Nicotiana species. However, biochemistry and genetics indicate that additional modifier genes are required. We recently reported a Kunitz-type proteinase inhibitor, named NaStEP (for Nicotiana alata Stigma-Expressed Protein), that is highly expressed in the stigmas of self-incompatible Nicotiana species. Here, we report the proteinase inhibitor activity of NaStEP. NaStEP is taken up by both compatible and incompatible pollen tubes, but its suppression in Nicotiana spp. transgenic plants disrupts S-specific pollen rejection; therefore, NaStEP is a novel pistil-modifier gene. Furthermore, HT-B levels within the pollen tubes are reduced when NaStEP-suppressed pistils are pollinated with either compatible or incompatible pollen. In wild-type self-incompatible N. alata, in contrast, HT-B degradation occurs preferentially in compatible pollinations. Taken together, these data show that the presence of NaStEP is required for the stability of HT-B inside pollen tubes during the rejection response, but the underlying mechanism is currently unknown.
To avoid low-fitness progeny, many plants have developed a cell-cell interaction mechanism to promote outcrossing, through the recognition and discrimination of both self and nonself pollen. This recognition system is controlled by the highly polymorphic self-incompatibility S-locus, which determines pollination specificity in both the pollen and pistil. Pollen is rejected when male and female S-haplotypes coincide (de Nettancourt, 1977, 2001; Franklin et al., 1995).
In Solanaceae, Plantaginaceae, and Rosaceae, the S-locus product in the pistil is an extracellular glycoprotein named S-RNase (Anderson et al., 1986; McClure et al., 1989). During pollination, S-RNase is taken up by both compatible and incompatible pollen tubes (Luu et al., 2000) and targeted to a vacuole (Goldraij et al., 2006). In the later stages of an incompatible cross, the S-RNase-containing vacuole is disrupted and the S-RNases are released to the pollen tube cytoplasm, where RNA degradation can occur (McClure et al., 2011).
The S-pollen gene encodes an SLF or SFB (SLF/SFB; for S-locus F-box) protein, which is a member of the F-box protein family (Entani et al., 2003; Sijacic et al., 2004). In vitro binding assays show that PiSLF in Petunia inflata physically interacts with S-RNases, although this interaction is stronger with nonself S-RNases than with self S-RNases (Hua and Kao, 2006). Additional protein-protein interaction assays suggest that SLF/SFB may be a component of an SCF (for Skp1-Cullin1-F-box) or SCF-like complex (Qiao et al., 2004; Hua and Kao, 2006). Notably, data from Zhao et al. (2010) in Petunia hybrida show that reduction of PhSSK1 (for P. hybrida SLF-interacting Skp-like1) and its Antirrhinum hispanicum ortholog, AhSSK1, is also required for cross-pollen compatibility.
Although S-RNase and SLF/SFB define pollen rejection S-specificity, modifier genes unlinked to the S-locus are required for self-incompatibility (SI; Martin, 1968; Ai et al., 1991; Murfett et al., 1996; Tsukamoto et al., 1999).
To date, only two pistil-modifier genes have been identified: High Top-Band (HT-B) and 120K. In Nicotiana spp., HT-B is an 8.6-kD acidic protein with a domain consisting of 20 Asn and Asp residues toward its C terminus (McClure et al., 1999; Kondo and McClure, 2008). Loss-of-function assays prove HT-B to be essential for pollen rejection in Nicotiana spp., Solanum spp., and Petunia spp. (McClure et al., 1999; Kondo et al., 2002; O’Brien et al., 2002; Sassa and Hirano, 2006; Puerta et al., 2009), although it is not expressed in SI Solanum habrochaites, prompting the speculation that in this species a related gene, HT-A, may function as a substitute (Covey et al., 2010). Immunolocalization shows that HT-B is readily taken up by pollen tubes during pollination. Its steady-state levels decrease slightly in pollen tubes from incompatible pollinations. However, in compatible crosses, HT-B levels decrease 75% to 97%, probably as a result of protein degradation (Goldraij et al., 2006).
120K is a style-specific 120-kD arabinogalactan protein (Schultz et al., 1997) that is taken up by pollen tubes (Lind et al., 1996) and appears to be associated with S-RNase-containing vacuoles (Goldraij et al., 2006). 120K forms complexes with S-RNases and other proteins (Cruz-Garcia et al., 2005) in vitro, and suppression of 120K expression prevents S-specific pollen rejection (Hancock et al., 2005). Protein-protein interaction assays demonstrate that 120K interacts with the pollen-specific protein NaPCCP (a pollen C2 domain-containing protein), a protein that binds phosphatidylinositol 3-phosphate and is associated with the pollen tube endomembrane system (Lee et al., 2008, 2009).
Two models have been proposed to explain pollen rejection in Solanaceae. (1) The S-RNase degradation model (Hua and Kao, 2006; Hua et al., 2007, 2008; Kubo et al., 2010) focuses on S-RNase-SLF interactions that bring about preferential nonself S-RNase degradation. In this model, strong nonself S-RNase-SLF interactions lead to the degradation of nonself S-RNases by the ubiquitin-26S proteasome system, allowing pollen tubes to escape from its cytotoxic effect. Weak self S-RNase-SLF interactions, in contrast, permit the persistence of sufficient free S-RNase that pollen tube RNA is degraded, resulting in self-pollen rejection. Notably, by functional and protein-protein interaction assays in Petunia spp., Kubo et al. (2010) found at least three types of divergent SLF proteins encoded at the S-locus, each recognizing a subgroup of nonself S-RNases. The authors proposed the collaborative nonself recognition model, where multiple SLF proteins interact with nonself S-RNases to protect nonself pollen from degradation (Kubo et al., 2010). (2) The compartmentalization model incorporates the observations that pollen tubes internalize both self and nonself S-RNases and targets them to vacuoles and that HT-B is degraded in compatible crosses but is stable in incompatible crosses (Goldraij et al., 2006). In incompatible crosses, the S-RNase-containing vacuoles are ultimately disrupted and S-RNases are released to the cytoplasm, where they degrade RNA, leading to rejection of self-pollen. In compatible crosses, the integrity of the S-RNase-containing vacuoles is preserved, allowing pollen tube growth to continue. Thus, in this model, self or nonself S-RNase-SLF interactions determine the specificity of pollen rejection indirectly.
Biochemical and genetic data show that pistil-modifier genes apart from HT-B and 120K are required for SI. We recently described NaStEP (for N. alata Stigma-Expressed Protein), an abundant, pistil-specific stigma protein found in SI Nicotiana spp. (Busot et al., 2008). Its abundance in SI species made NaStEP a strong modifier gene candidate. Here, we demonstrate that NaStEP is taken up by pollen tubes, has subtilisin inhibitory activity, and that suppressing its expression in transgenic hybrids disrupts pollen rejection. Moreover, when NaStEP-suppressed hybrids are pollinated, HT-B protein is degraded in both compatible and incompatible pollen tubes, while in wild-type SI N. alata, HT-B is preferentially stabilized in incompatible pollen tubes.
RESULTS
NaStEP Suppression
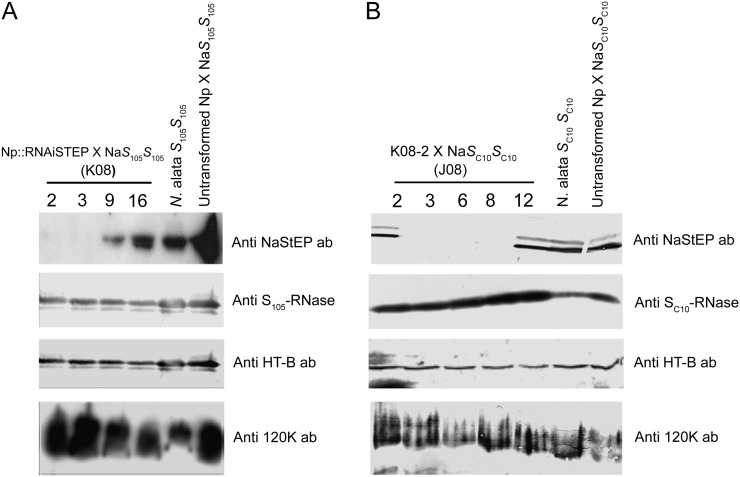
To test whether NaStEP is required for S-specific pollen rejection, we introduced an RNA interference (RNAi)::NaStEP construct into self-compatible (SC) Nicotiana plumbaginifolia (i.e. S0S0) and crossed T0 transformants with SI N. alata S105S105 to generate S105S0 T1 progeny family K08. Figure 1A shows an analysis of pistil extracts from two hybrids with no detectable NaStEP, K08-2 and K08-3; a partially suppressed hybrid, K08-9; and an unsuppressed hybrid, K08-16. Population J08 was obtained by crossing hybrid K08-2 to SI N. alata SC10SC10. This second population enabled testing the effects of NaStEP suppression on a second S-haplotype in a further generation. The entire J08 population consisted of 25 plants (13 SC10S0 and 12 SC10S105). Ten J08 plants showed suppression and 15 expressed normal levels of NaStEP (Fig. 1B; Supplemental Fig. S1A). Five representatives are shown in Figure 1B. Plants J08-3, J08-6, and J08-8 showed no detectable NaStEP, while plants J08-2 and -12, with nearly normal expression levels, were used as controls (Fig. 1B). The RNAi effect was specific to NaStEP in both the K08 and J08 populations, as little or no change in S-RNase, HT-B, or 120K levels was observed (Fig. 1).
Figure 1.
NaStEP suppression in Nicotiana spp. A, Ten-milligram total protein extracts from pistil in transformed SC N. plumbaginifolia × SI N. alata (S105S105) hybrids. NaStEP is not detectable in plants K08-2 and K08-3 and is partially detectable in K08-9. S-RNase, HT-B, and 120K protein levels were not greatly affected. B, Ten-milligram total protein extracts from pistil in K08-2 × SI N. alata (SC10SC10) progeny. NaStEP is not detectable in plants J08-3, J08-6, and J08-8. S-RNase, HT-B, and 120K protein levels were not greatly affected. The untransformed control is SC N. plumbaginifolia × SI N. alata (S105S105 or SC10SC10).
NaStEP Is Required for S-Specific Pollen Rejection
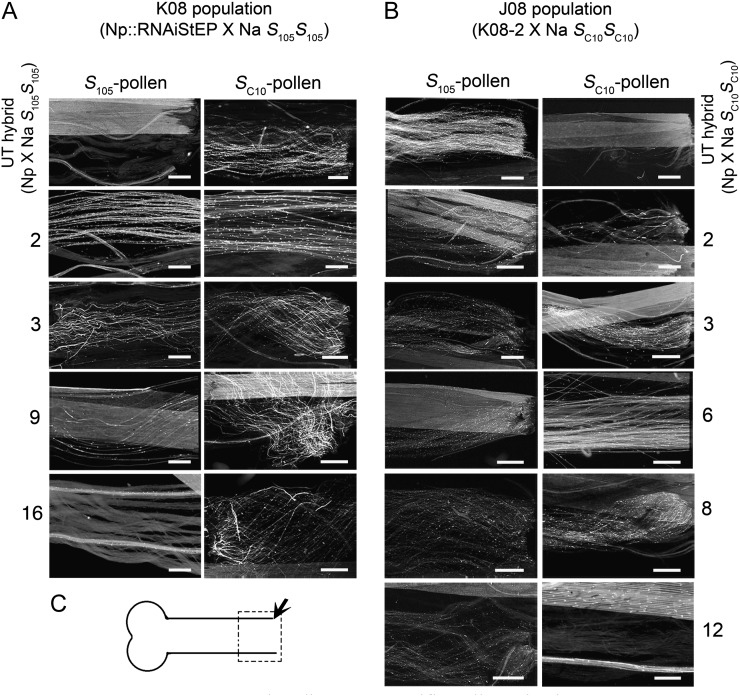
Pollination tests showed that NaStEP expression is essential for S-specific pollen rejection in Nicotiana spp. The effect on pollination phenotype was assessed by observing pollen tubes at the base of the style (Fig. 2C) 72 h post pollination after challenging with SC10 or S105 pollen (Fig. 2, A and B; Supplemental Tables S1 and S2). As expected, untransformed control S105S0 and SC10S0 hybrids (i.e. N. plumbaginifolia × SI N. alata S105S105 or N. plumbaginifolia × SI N. alata SC10SC10 hybrids expressing S105-RNase or SC10-RNase, respectively) displayed S-specific pollen rejection by only rejecting pollen with a matching S-haplotype (Fig. 2, A and B, top panels). In contrast, fully suppressed hybrids (K08-2, K08-3, J08-3, J08-6, and J08-8) showed many (more than 50) pollen tubes at the base of the style, regardless of the pollen S-haplotype, and thus did not display S-specific pollen rejection (Fig. 2, A and B; Supplemental Tables S1 and S2). Moreover, the S105S0 K08-9 hybrid that displayed partially suppressed NaStEP allowed a few pollen tubes to reach the base of the style (Fig. 2A).
Figure 2.
NaStEP suppression disrupts S-specific pollen rejection. A, NaStEP RNAi N. plumbaginifolia × SI N. alata (S105S105) hybrids were pollinated with S105 or SC10 pollen and prepared for imaging after 72 h. Images were taken at or near the base of the style (arrow in C). The plants K08-2 (S105S0) and K08-3 (S105S0) show both S105 and SC10 pollen tubes reaching the base of the style. B, Plants from the J08 population were pollinated with SC10 or S105 pollen. The plants J08-3 (SC10S0), J08-6 (SC10S0), and J08-8 (SC10S0) show both S105 and SC10 pollen tubes reaching the base of the style. Untransformed (UT) hybrid (N. plumbaginifolia × SI N. alata S105S105 or SC10SC10) pollinated with S105 or SC10 pollen was used as a control. Bars = 50 µm. Results represent a replicate of three pollination assays. C, Pistil diagram showing the observation area for pollen tube growth.
NaStEP in Interspecific Pollen Rejection
To test the role of NaStEP in interspecific pollen rejection, fully suppressed S105S0 hybrids were pollinated with SI Rastroensis (Nicotiana rastroensis), SC N. longiflora, SC N. plumbaginifolia, SC N. tabacum, SC N. benthamiana, or SC N. glauca pollen. Table I shows that untransformed S105S0 hybrids and SI N. alata S105S105 accepted pollen from SI Rastroensis and SC N. longiflora but rejected pollen from all other SC species. NaStEP-suppressed hybrids behaved the same with one notable exception, N. plumbaginifolia. Fully suppressed hybrids accepted N. plumbaginifolia pollen, and the partially suppressed hybrid showed partial compatibility (Table I). Thus, NaStEP is required for interspecific rejection of N. plumbaginifolia pollen but not for the rejection of pollen from the other SC species that were tested.
Table I. NaStEP does not account for interspecific pollen rejection.
Transformed K08-2 (S105S0), K08-3 (S105S0), and K08-16 (S105S0) hybrids were pollinated with SI Rastroensis (R), SC N. longiflora (Nl), SC N. plumbaginifolia (Np), SC N. tabacum (Nt), SC N. benthamiana (Nb), and SC N. glauca (Ng) pollen. After 72 h of pollination, styles were prepared for imaging. Images were taken at or near the base of the style. Compatible (+), incompatible (−), and semicompatible (+/−) pollinations are indicated. Each symbol (+ or −) represents a replicate of each pollination assay.
| Pistil | SI R | SC Nl | SC Np | SC Nt | SC Nb | SC Ng |
|---|---|---|---|---|---|---|
| SI Na S105S105 | +,+,+ | +,+,+ | −,−,− | −,−,− | −,−,− | −,−,− |
| Untransformed hybrid | ||||||
| Np × NaS105S105 | +,+,+ | +,+,+ | −,−,− | −,−,− | −,−,− | −,−,− |
| K08-2 S105S0 | +,+,+ | +,+,+ | +,+,+ | −,−,− | −,−,− | −,−,− |
| K08-3 S105S0 | +,+,+ | +,+,+ | +,+,+ | −,−,− | −,−,− | −,−,− |
| K08-16 S105S0 | +,+,+ | +,+,+ | +/−,−,− | −,−,− | −,−,− | −,−,− |
NaStEP Is Taken Up by Pollen Tubes
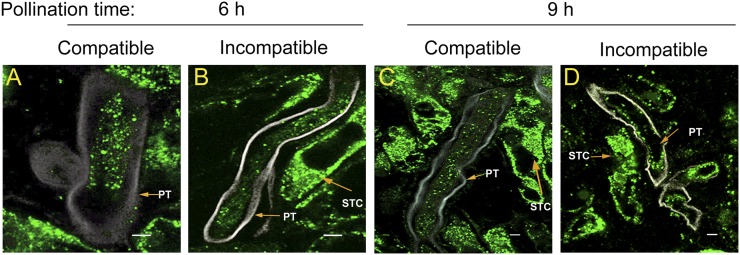
We recently demonstrated that NaStEP is sorted to a vacuole in mature papillary stigmatic cells. Upon pollination, the papillar cell wall becomes perforated and NaStEP is relocalized onto the stigma apoplast (Busot et al., 2008). Since NaStEP is essential for pollen rejection (Fig. 2), we hypothesized that it might be taken up and function inside the pollen tube. We performed double-label experiments using antibodies for callose and NaStEP in compatible (N. alata S105S105 × N. alata SC10SC10) and incompatible (N. alata S105S105 × N. alata S105S105) crosses 6 and 9 h after pollination (Fig. 3, green signal). At these times, pollen grains had germinated and pollen tubes had entered the upper style. Figure 3 shows that NaStEP accumulates abundantly in the stigmatic cells. Figure 3 also reveals that NaStEP enters both compatible and incompatible pollen tubes, and once there, its appearance looks like small dots. Relocalization of NaStEP from the stigma surface to the pollen tube would position it to function in internal pollen tube processes.
Figure 3.
NaStEP is taken up by pollen tubes. A and B, Six-hours-postpollination pistils. A, SI N. alata S105S105 pistils pollinated with SC10 pollen (compatible). B, SI N. alata S105S105 pistils pollinated with S105 pollen (incompatible). C and D, Nine-hours-postpollination pistils. C, SI N. alata S105S105 pistils pollinated with SC10 pollen (compatible). D, SI N. alata S105S105 pistils pollinated with S105 pollen (incompatible). Sections from pollinated pistils were simultaneously probed with anti-NaStEP (green) or anti-callose (white) antibodies. PT, Pollen tube; STC, stigmatic cell. Bars = 5 mm.
NaStEP Positively Regulates HT-B Stability in Pollen Tubes
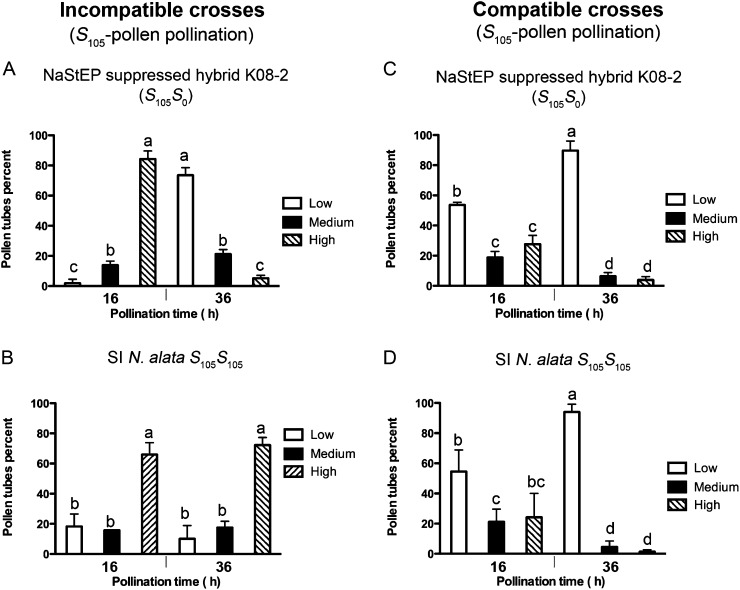
NaStEP is a Kunitz-type proteinase inhibitor family member (Busot et al., 2008) and could modulate the stability of other proteins in the pollen tubes. HT-B is of special interest since it shows differential stability in compatible versus incompatible pollen tubes (Goldraij et al., 2006). To test this possibility, we carried out immunolocalization experiments to evaluate HT-B levels in compatible and incompatible pollen tubes. HT-B levels were classified by staining intensity as low or not stained, medium, or high (sample images are shown in Supplemental Fig. S2). In suppressed S105S0 hybrid challenged with S105 pollen, most pollen tubes contained high levels of HT-B 16 h post pollination, but by 36 h, a striking shift occurred and HT-B was almost undetectable in most pollen tubes (RNAi S105S0 × S105 pollen; Fig. 4A). As reported previously (Goldraij et al., 2006), no such shift occurred in control crosses where SI N. alata S105S105 was challenged with S105 pollen, as HT-B is stabilized in incompatible crosses (SI N. alata S105S105 × S105 pollen; Fig. 4B). Compatible crosses also behaved as expected and displayed HT-B degradation. When suppressed S105S0 hybrid or SI N. alata S105S105 plants were challenged with SC10 pollen, most pollen tubes showed little or no detectable HT-B by 36 h (Fig. 4, C and D, respectively). Thus, NaStEP suppression interferes with S-specific stabilization of HT-B in otherwise incompatible pollen tubes; HT-B degradation occurred regardless of S-haplotype. These results are consistent with a role for NaStEP in HT-B stability.
Figure 4.
NaStEP suppression negatively affects HT-B levels in both compatible and incompatible pollen tubes. A, Hybrid K08-2 (S105S0; no detectable NaStEP) was pollinated with S105 or SC10 pollen (C). B, Wild-type SI N. alata S105S105 was pollinated with S105 or SC10 pollen (D). Analyses were performed at 16 and 36 h post pollination. Results reflect analyses of 200 pollen tubes. Error bars indicate se of three biological replicates. Values with different letters are significantly different (P < 0.05). Analysis was made by Tukey’s multiple comparison test after two-way ANOVA (n = 3). To perform both analyses, the results expressed as percentages were arcosin transformed in order to have data normally distributed.
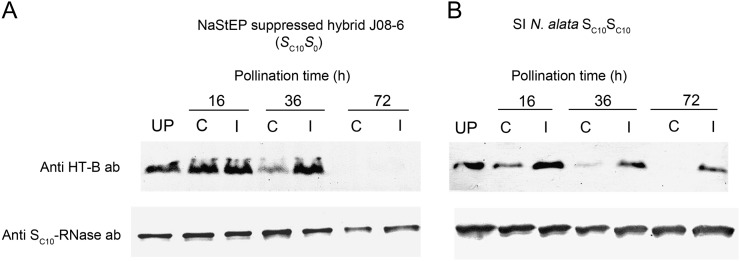
Experiments on pistil extracts also showed HT-B degradation regardless of pollen S-genotype in suppressed hybrids. Figure 5 shows immunoblot analyses of extracts prepared 16, 36, and 72 h post pollination. SI N. alata SC10SC10 controls showed preferential degradation of HT-B after compatible pollination, as reported previously (Fig. 5B; Goldraij et al., 2006). Compatible pollination of NaStEP-suppressed hybrid (SC10S0) showed very similar results (Fig. 5A; S105 pollen). Incompatible SC10 pollen gave similar results as controls at 36 h, but HT-B was undetectable after 72 h. Thus, degradation of HT-B occurred regardless of pollen S-genotype in the NaStEP-suppressed hybrid, although the effect at the whole-organ level was delayed compared with observations of HT-B inside pollen tubes (Fig. 4 versus Fig. 5).
Figure 5.
HT-B levels decrease after pollination of an RNAi NaStEP-suppressed hybrid. A, Hybrid J08-6 (SC10S0; no detectable NaStEP) pistils from not detectable NaStEP RNAi hybrid were heavily pollinated with S105 or SC10 pollen and probed to evaluate HT-B and SC10-RNase levels at 16, 36, and 72 h after pollination. B, Wild-type SI N. alata SC10SC10 pistils were heavily pollinated with S105 or SC10 pollen and probed to evaluate HT-B and SC10-RNase levels at 16, 36, and 72 h after pollination. Representative results from three totally independent assays are shown. UP, Unpollinated; C, compatible pollination with S105 pollen; I, incompatible pollination with SC10 pollen. Ten-milligram total protein extracts were SDS-PAGE fractioned and blotted onto a membrane in each assay.
Model of the NaStEP Three-Dimensional Structure
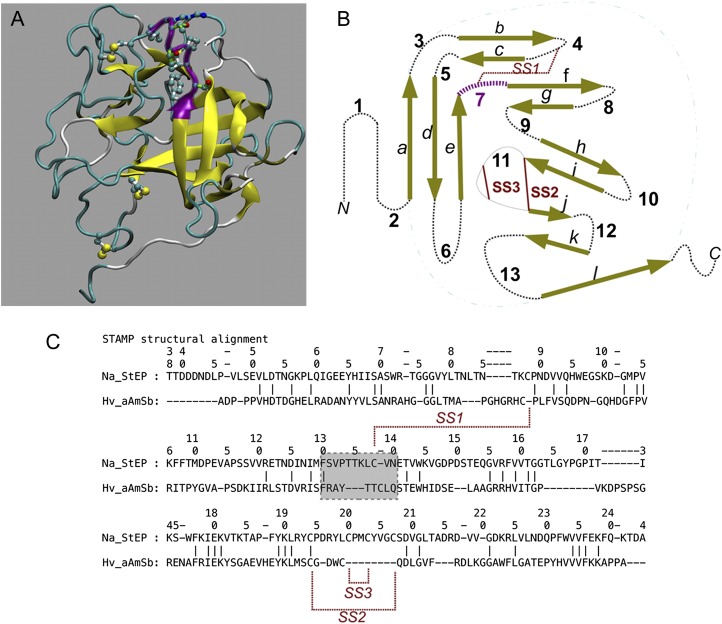
A model for the NaStEP three-dimensional structure was produced and used for structure-based comparisons with other Kunitz-type inhibitors to infer its possible function. Per-residue GROMOS energies revealed a well-refined final three-dimensional model (Fig. 6). The Rd.HMM score (Martínez-Castilla and Rodríguez-Sotres, 2010) for the NaStEP amino acid sequence was nearly 0.3 times the sequence length before relaxation (E value of 1.8 × 10−8) and 0.92 after relaxation (E value of 6.4 × 10−51). The Rd.HMM structurally aware alignment (Martínez-Castilla and Rodríguez-Sotres, 2010) of the model was in frame with the NaStEP amino acid sequence, and no other sequence in the National Center for Biotechnology Information nonredundant database had a higher score. Using a cutoff E value of 10, only orthologs from N. alata (GenBank accession no. ABX76298.1) and N. glutinosa (GenBank accession no. AAF15901.1) were identified as possible sequence fits with the proposed three-dimensional model. However, the scores for these sequences were only one-half of the score obtained with the NaStEP sequence. Molecular dynamics (MD) simulations (data not shown) also indicate that the proposed structure is very high quality.
Figure 6.
Model of the three-dimensional structure for NaStEP. A, Overall structure shown as cartoons with yellow β-strands. The putative subtilisin inhibition reactive site is shown in purple cartoons (shadowed) with side chains in balls-and-sticks format (the coloring scheme of the representation as defined in the visualized software: carbon, dark cyan; oxygen, red; nitrogen, blue; sulfur, yellow). Disulfide bridges are shown per the coloring scheme. SS1 appears at the top and SS3 at the bottom. This image was prepared using the visual MD software (VMD). B, Topology of the model in A, showing the classic connectivity of the Kunitz superfamily. Termini are labeled as N and C, β-strands are labeled with lowercase letters, turns/loops/coils (dotted black lines) are labeled with numbers, and disulfide bonds are indicated as red lines. In three dimensions, strand a pairs with strand l (cyan dotted-dashed lines). The putative subtilisin inhibitor reactive site is at loop 7, shown in purple. C, The STAMP plugin in VMD (Russell and Barton, 1992) of the model in A to the barley subtilisin/α-amylase inhibitor in Protein Data Bank entry 3BX1, chain C. In the STAMP comparison, amino acids are aligned if their backbones nearly overlap. The numbers at the top correspond to the NaStEP amino acid sequence. Disulfide bonds are indicated with red lines. The gray box encloses the subtilisin inhibitor barley reactive site motif and its corresponding NaStEP sequence, shown in purple in A and B.
Rd.HMM hits with poor scores suggested structural similarity with the barley (Hordeum vulgare) subtilisin/amylase inhibitor. Superimposition of the three-dimensional structure of the barley subtilisin/amylase inhibitor and the NaStEP three-dimensional model using STAMP (Russell and Barton, 1992) confirmed the close structural relationship. The barley subtilisin/amylase inhibitor structure was used to model the disulfide bridging pattern and to locate a putative subtilisin inhibitor reactive site in NaStEP (F130–K136). This site is shown in purple in Figure 6, A and B, and is boxed in Figure 6C. Comparison of the reactive sites reveals important sequence differences that could affect specificity. Both sequences have a basic residue (Fig. 6C), although its position differs. Importantly, STAMP does not align the TTKLC sequence in NaStEP with the TTCL sequence in the barley inhibitor, and this indicates a difference in conformation for these important residues. In particular, Thr-134 is not in the same conformation as the essential Thr-88 in the barley protein. In addition, the barley subtilisin/amylase inhibitor binds to subtilisin, forming a tetramer with 2:2 stoichiometry. The complex has two different contact interfaces between the inhibitor and the enzyme: one blocks the enzyme active site, and the other accounts for dimerization.
NaStEP Is a Subtilisin Inhibitor
NaStEP was purified by ammonium sulfate precipitation and anion-exchange chromatography and tested for its ability to inhibit subtilisin. Fractions were analyzed by SDS-PAGE, and those enriched in NaStEP (Supplemental Fig. S3, A and B) were tested for activity. The results showed that the major Coomassie blue-stainable bands corresponded to anti-NaStEP reactive bands. Active bands were also excised, fragmented, and sequenced by mass spectrometry. The results showed greater than 60% coverage and perfect agreement with the predicted NaStEP sequence (Supplemental Fig. S3C, red letters).
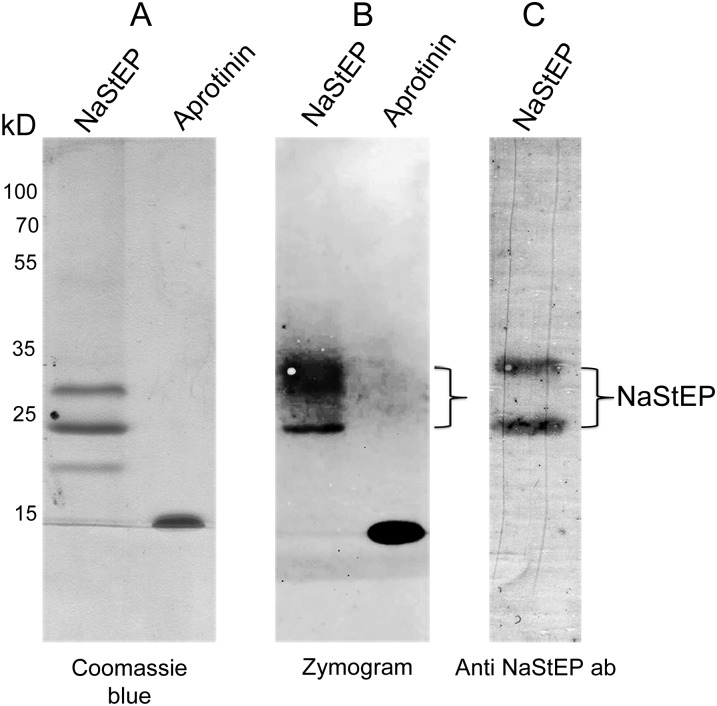
A reverse zymogram assay was used to test NaStEP for subtilisin inhibition activity. In this assay, proteins migrate through the gel and subtilisin inhibition is reflected by preventing the degradation of gelatin. Figure 7 shows two subtilisin inhibition bands with approximate molecular masses between 20 and 35 kD in the enriched NaStEP fractions. The aprotinin control ran near 15 kD and also showed strong inhibitory activity. An immunoblot of this gel stained with anti-NaStEP detected both inhibition bands, confirming the subtilisin inhibitory activity of this protein (Fig. 7, A and B versus C). It is not clear why NaStEP runs as multiple bands in these assays, but it may be related to protein oxidation or oligomerization.
Figure 7.
NaStEP is a subtilisin inhibitor. A, Coomassie blue staining of a NaStEP-purified fraction. B, Reverse zymography revealing NaStEP inhibition of subtilisin proteolytic activity. C, Immunoblotting against NaStEP after zymography.
DISCUSSION
Pollen rejection in Nicotiana spp. depends on S-specific interactions between S-RNase and SLF/SFB as well as other modifier genes. Until now, HT-B and 120K were the only identified modifier genes functionally tested in pollen rejection in Solanum spp., Nicotiana spp., and Petunia spp. (McClure et al., 1999; Kondo et al., 2002; O’Brien et al., 2002; Hancock et al., 2005; Sassa and Hirano, 2006; Puerta et al., 2009). Here, loss-of-function assays implicate NaStEP as a third modifier gene essential for the SI pollen rejection response in Nicotiana spp., since the suppressed hybrid (SC N. plumbaginifolia × SI N. alata) loses the ability to reject pollen in an S-specific manner. Likewise, we present data supporting NaStEP involvement in interspecific pollen rejection. Previous studies had shown undetectable transcript and protein levels of NaStEP in the SC species N. plumbaginifolia and N. tabacum but abundant expression in the mature stigmas of SI Nicotiana spp., where it is targeted to a vacuole in the papillar cells and released into the exudate upon pollination (Busot et al., 2008). The accumulation of NaStEP in stigmas suggests a function for this protein during the early stages of pollen germination or pollen tube growth. The roles of stigma-specific proteins in pollination are diverse and may or may not include a function in SI. As an example, the Brassica S-receptor kinase (SRK) protein functions at early pollination times and is essential to the pollen-rejection response (Stein et al., 1991; Takasaki et al., 2000), while LeSTIG1, a stigma protein from tomato (Solanum lycopersicum), interacts with the extracellular domain of the tomato pollen-specific receptor kinases LePRK1 and LePRK2 and stimulates pollen tube growth in vitro (Tang et al., 2004). However, because in the transgenic NaStEP-suppressed hybrids, pollen grains germinate on the stigmatic surface and grow down to the base of the style in high densities, a general role for NaStEP in pollen recognition and pollen tube growth may be excluded. Therefore, NaStEP suppression disrupts pollen rejection in an S-specific manner, and at least one main function of this gene is required for pollen rejection.
NaStEP function appears to be restricted to S-RNase-based self as well as interspecific pollen rejection, because its suppression in Nicotiana spp. did not result in any other evident phenotype changes. Interspecific pollen rejection occurs through multiple and complex mechanisms, which in Nicotiana spp. happen in both an S-RNase-dependent and an S-RNase-independent manner (Pandey, 1981; Murfett et al., 1996). Interspecific pollen rejection mediated by S-RNases has been shown in some instances to depend strictly on modifier genes common to SI, such as HT-B and 120K (McClure et al., 1999; Hancock et al., 2005). Suppressed HT-B or 120K transgenic hybrids of Nicotiana spp. (N. plumbaginifolia × SI N. alata) accept pollen from the SC N. plumbaginifolia, which are otherwise rejected by the untransformed control hybrids (SC N. plumbaginifolia × SI N. alata; Hancock et al., 2005). Our results using the NaStEP RNAi-silenced hybrid and pollen from different Nicotiana species (Table I) highlight the inability of NaStEP-suppressed hybrids to reject N. plumbaginifolia or N. alata pollen, in spite of normal S-RNase levels. These results show that NaStEP is required for S-specific pollen rejection and for rejecting pollen from N. plumbaginifolia and further highlight the similarity between the intraspecific SI mechanism and this type of interspecific pollen rejection. However, pollen from other SC species (N. tabacum, N. glauca, and N. benthamiana) clearly does not require NaStEP. This is consistent with other studies showing that multiple mechanisms contribute to interspecific pollen rejection (Murfett et al., 1996). Taken together, our results are congruent with a role for NaStEP as a pistil-modifier gene in SI and point to its involvement in an S-RNase interspecific pollen-rejection pathway dependent on modifier genes, as shown for HT-B and 120K.
Upon pollination, NaStEP is taken up by pollen tubes regardless of their S-haplotype. Although the subcellular destination of NaStEP within the pollen tube is currently unknown, in the S-RNase-based pollen-rejection systems, pistil proteins essential to SI and exerting their function within the pollen tube, such as S-RNases, HT-B, and 120K, have been shown to associate with the pollen tube endomembrane system (Goldraij et al., 2006). Although the molecular mechanism leading to pollen rejection is completely different between gametophytic and sporophytic systems, the events that occur in association with the endomembrane system seem to be relevant to the pollen-rejection response in both systems. Samuel et al. (2009) showed in Brassica spp. that Exo70 A1, a subunit of an exocyst complex involved in polarized secretion in yeast (Saccharomyces cerevisiae) and animals (Novick et al., 2006; Zárský et al., 2009), functions in the polarized transport of vesicles in the stigma and is necessary for the hydration, germination, and growth of compatible pollen tubes. During the SI response, the S-specificity determinants SRK-SCR negatively regulate Exo70 A1, through the activation of ARC1 (for ARM repeat-containing1 protein), an E3 ubiquitin ligase. ARC1 targets Exo70 A1 for degradation, provoking a deregulation of the exocyst function, thereby promoting pollen rejection.
In N. alata, following both compatible and incompatible pollinations, S-RNases, HT-B, and 120K are taken up by the pollen tube and become associated with the endomembrane system. S-RNases are sequestered into a vacuole, where they remain contained until its destabilization releases them into the cytoplasm (Goldraij et al., 2006; McClure et al., 2011). Similarly, biochemical fractionation experiments give evidence of the HT-B association with the microsomal fraction (Kondo and McClure, 2008). Furthermore, immunolocalization studies show the colocalization of 120K with the S-RNases in the pollen tube vacuole (Goldraij et al., 2006). The incorporation of NaStEP into the pollen tube occurs early in pollination (6 h), and its function might be related, directly or indirectly, with the pollen tube endomembrane system. This is supported by the preservation of HT-B from degradation, once inside the pollen tube, when NaStEP is present, disclosing a link between NaStEP and HT-B stability. In wild-type SI N. alata, the HT-B remains stabilized in incompatible pollen tubes, even at times when pollen rejection has been effective, whereas it disappears from compatible pollen tubes (Goldraij et al., 2006; Kondo and McClure, 2008). By contrast, in the absence of NaStEP, HT-B signal loss was similar in both compatible and incompatible pollen tubes, which, according to the S-RNase compartmentalization model (Goldraij et al., 2006), would maintain the S-RNase vacuole stable, allowing pollen tube growth.
However, the mechanism by which NaStEP stabilizes HT-B remains to be elucidated. NaStEP is homologous to Kunitz-type protease inhibitors (Busot et al., 2008) and exhibits a subtilisin inhibition activity. A reasonable model could contemplate the inhibitory role of NaStEP on a subtilisin-like component of a proteolytic cascade targeting HT-B during compatible pollen tube growth. However, the putative subtilisin-like component has yet to be identified, and a control mechanism for the NaStEP action remains unknown, since this protein is also present during SI pollination events, where rejection does take place. Besides their possible involvement in SI, plant proteases and protease inhibitors have been increasingly associated with several developmental processes such as plant-pathogen-insect interactions (Li et al., 2008; Hartl et al., 2010) and more recently in program cell death (Chichkova et al., 2010), which makes it a very intense field of study.
Our results have led us to consider the timing of the events during the pollen-rejection response. We propose that NaStEP accumulates in the stigmatic exudate during pollination (Busot et al., 2008) and is taken in to the pollen tubes soon after germination in order to protect HT-B (directly or indirectly) in pollen tubes from degradation through its protease inhibitor activity. The HT-B protein is incorporated at later times, once the pollen tube has reached the transmitting tissue in the style. This temporal offset in the incorporation of stylar proteins would allow HT-B to avoid premature degradation and prevent the breakdown of the SI response, since this protein mediates in the rupture of the S-RNase-containing vacuole in the incompatible pollen tubes. This hypothesis is consistent with our observations in the NaStEP-suppressed hybrids that, in the majority of the pollen tubes from incompatible crosses, HT-B is not detected after 36 h. Likewise, our data show that HT-B might exerts its function in pollen rejection around 16 h post pollination, which is when it reaches high levels of accumulation in both compatible and incompatible pollen tubes, and after which its levels decline until they become undetectable. Although the exact time in which the S-RNase-SLF/SFB interaction occurs within the pollen tube and the destabilization of the S-RNase-containing vacuole are not known, we propose that these occur before HT-B degradation is set.
Finally, the development of a reliable and biologically appropriate three-dimensional model was particularly useful to interpret three pieces of experimental data: (1) the increased efficiency of the transit signal in the full protein, compared with the β-region alone, is consistent with a role for the NaStEP C terminus in the stability of the N terminus found in the three-dimensional model; (2) the role of the β-segment as a secretory signal sequence and (3) the activity of NaStEP as a subtilisin inhibitor are both in agreement with the intrinsically disordered nature of these regions and their high solvent accessibility, as deduced from the model. In addition, the model suggests a divergence between the barley subtilisin inhibitor and NaStEP, which suggests a process of subfunctionalization of an ancestral protein during the evolution of the SI mechanisms in Nicotiana spp. Accordingly, a similar process of divergence might have taken place on its target protease, and this information could be useful in the search for this putative component.
MATERIALS AND METHODS
Plant Materials
SI Nicotiana alata (S105S105, SC10SC10), SC Nicotiana glauca, and SC Nicotiana tabacum ‘Praecox’ have been described previously (Murfett et al., 1994, 1996; Beecher and McClure, 2001). SC Nicotiana plumbaginifolia (inventory no. TW107, accession 43B) and SC Nicotiana longiflora (inventory no. TW79, accession 30A) were obtained from the U.S. Tobacco Germplasm Collection (Crop Research Laboratory). SI Rastroensis (Nicotiana rastroensis) and SC Nicotiana benthamiana have been described previously (Juárez-Díaz et al., 2006; Lee et al., 2009). All plant materials were grown under greenhouse conditions.
RNAi Construct, Plant Transformation, and Transgenic Hybrid Generation
The complementary DNAs for the sense and antisense NaStEP were amplified as follows: sense oligonucleotide forward primer, 5′-CGCCCTCGAGATGAAATCCTTTATTTTCAGCTTCCTCTTG-3′; reverse, 5′-CGCCGGTACCTCAGGACAATACCTTAACTTGG-3′; antisense oligonucleotide forward primer, 5′-CGCCGGATCCATGAAATCCTTTATTTTCAGCTTCCTC-3′; reverse, 5′-CGCCATCGATTCAGGACAATACCTTAACTTGGTAGA-3′. The PCR products were cloned into a pHANNIBAL vector (Commonwealth Scientific and Industrial Research Organization Plant Industry; Wesley et al., 2001). N. plumbaginifolia was transformed as described (Murfett et al., 1992). Thirty independent lines were analyzed as primary transformants. We recovered one transformed N. plumbaginifolia line, which was crossed with SI N. alata S105S105. Four transgenic hybrids (N. plumbaginifolia × SI N. alata S105S105) were obtained and designated as K08-2 (S105S0), K08-3 (S105S0), K08-9 (S105S0), and K08-16 (S105S0). To generate an advanced hybrid transgenic population (J08), we crossed the SC fertile hybrid K08-2 (presumably tetraploid) N. plumbaginifolia × SI N. alata S105S105 with SI N. alata SC10SC10.
Pollination Phenotype
Mature flowers were pollinated with SI Rastroensis, SI N. longiflora, SC N. plumbaginifolia, SC N. tabacum, SC N. benthamiana, and SC N. glauca and N. alata (S105S105 or SC10SC10) pollen. Pollen tubes were stained with decolorized aniline blue as described (Kho and Baer, 1968) and counted at the base of the style 72 h after pollination, using an Olympus Provis AX70 microscope (Supplemental Tables S1 and S2). Pollinations were repeated at least three times as indicated. When the number of pollen tubes reaching the base of the style was over 200, the cross was considered as compatible (+); when less than 50 pollen tubes were observed at the base of the style, it was considered as incompatible (−); when over 50 but less than 200 pollen tubes were counted at the base of the style, the cross was considered as partially compatible (+/−).
Protein Isolation and Purification
Pistils were homogenized with extraction buffer (50 mm Tris-HCl, pH 8.0, 50 mm NaCl, and 1% 2-mercaptoethanol). Extracts were clarified by centrifugation, and supernatants were stored at –80°C until use. Protein concentration was estimated using the method of Bradford (1976) with bovine serum albumin as the standard.
Comparative Modeling of the NaStEP Three-Dimensional Structure
From BLAST analysis, NaStEP belonged to the Kunitz superfamily of protein inhibitors. However, the sequence homology of NaStEP to a protein with known three-dimensional structure was low. Therefore, we used the SAM-T08 server (Karplus, 2009) to obtain a starting three-dimensional model for NaStEP. Only amino acids 38 to 206 of the sequence in GenBank accession ABX76297.1 were included in the model. Structural defects may be present in SAM-T08 models (Chavelas-Adame et al., 2011), and this model was refined using a slow-convergence molecular mechanics (Hyperchem 7.5, AMBER 99 forcefield) minimization scheme described elsewhere (Rosales-León et al., 2012). The resulting model was superimposed to the barley (Hordeum vulgare) subtilisin/α-amylase inhibitor (Micheelsen et al., 2008; Protein Data Bank entry 3BX1, chain C) using STAMP (Russell and Barton, 1992) as implemented in MULTISEC (Roberts et al., 2006). The structural alignment allowed us to predict two disulfide bonds (Cys-88 to Cys-138 and Cys-194 to Cys-207). The minimized model was further refined using MD simulations in GROMACS 4.5 (Hess et al., 2008) under the GROMOS 53a6 forcefield (Oostenbrink et al., 2004). MD simulations were run in a mass-pressure-temperature conservative ensemble, under octahedral periodicity, with explicit solvent (single-point change water model) and with NaCl to make the system electroneutral and roughly 0.15 m salt. Electrostatics was handled using the particle-mesh Ewald summations, temperature was 313 K (Berendsen; velocity-rescaled thermal bath), with 1 bar constant pressure (Berendsen Barostat). The integration interval was 2 ps, and the simulation was extended for 25 ns. After 3- to 4-ns simulations, the structure root mean squared deviation and radius of gyration reached a plateau. Cluster analysis of the simulation indicated a third disulfide bond between residues Cys-200 and Cys-203, because their S atoms reached a disulfide bond-compatible geometry with a significant frequency. MD simulations of the fully reduced protein showed a modest impact on the overall folding stability, but with increased fluctuations in the putative subtilisin inhibitor reactive site. The most representative conformation of the model was obtained from a 20-ns MD simulation of the fully oxidized protein. After a final energy minimization, the model was relaxed with the ROSETTA fast-relax protocol (Raman et al., 2009) and scored for appropriateness with the Rd.HMM protocol (Martínez-Castilla and Rodríguez-Sotres, 2010), both before and after ROSETTA relaxation.
NaStEP Purification
For NaStEP purification, pistils were ground in liquid N2 and proteins were extracted with extraction buffer (with 2 mm phenylmethylsulfonyl fluoride and 5 mm EDTA). The extract was clarified (7,000g, 10 min, 4°C) to eliminate cellular debris, and ammonium sulfate (45% [w/v]) was added to the supernatant in order to precipitate the protein. After 1 h of incubation, the extract was centrifuged (20,000g, 30 min, 4°C), the supernatant was discarded, and the pellet was resuspended in column buffer (50 mm Tris-HCl, pH 8.5, 100 mm NaCl). Dialysis was performed overnight using the column buffer. The extract was loaded onto an HPLC system (1 mL min−1) using a 15-mL Source-Q column (GE Healthcare) preequilibrated with column buffer, and a linear NaCl elution gradient (200–600 mm) was used. Most of NaStEP eluted gradually before the NaCl gradient was initiated.
Protein Gel-Blot Analysis
Equal amounts of protein were separated using 12% or 15% SDS-PAGE and blotted onto nitrocellulose. Immunoblotting was performed essentially as described (Cruz-Garcia et al., 2005). Primary antibody dilutions were as follows: anti-120K (1:10,000), anti-HT-B (1:5,000), anti-NaStEP (1:15,000), anti-S105-RNase (1:10,000), and anti-SC10-RNase (1:10,000; Supplemental Fig. S1) as described (Cruz-Garcia et al., 2005; Goldraij et al., 2006; Busot et al., 2008).
Zymogram Analysis
Reverse zymograms were performed as suggested by Lantz and Ciborowski (1994). Two sets of gels were used, one without and one including gelatin (0.1% [w/v]) copolymerized with the polyacrylamide. After electrophoresis, the gels without gelatin were stained with Coomassie Brilliant Blue R-250 or blotted onto nitrocellulose to be immunostained with anti-NaStEP antibodies as described above. The gelatin gel was rinsed twice with 2.5% (v/v) Triton X-100 solution, twice with 2.5% (v/v) Triton X-100 + 50 mm Tris-HCl, pH 7.4, solution, and twice with 50 mm Tris-HCl, pH 7.4, each for 10 min, in order to remove SDS. The gelatin gel was incubated for 2 h at 37°C in a buffer solution (50 mm Tris-HCl, 200 mm NaCl) to which 2 μg mL−1 subtilisin was added. Gels were fixed (10% methanol, 10% acetic acid solution) and stained with Coomassie Brilliant Blue R-250. Destaining of the gels revealed the inhibition bands at sites where gelatin was not digested by the protease. Confirmation of NaStEP presence at these sites was obtained by immunostaining with anti-NaStEP antibodies as described above. Aprotinin was used as a positive control, because it is a commonly employed Ser protease inhibitor, generally used at a working concentration of 5 μg mL−1 (Deutscher, 1990). For the zymogram, we loaded 5 μg of aprotinin (Sigma).
Immunolocalization
Pollinated pistils were harvested and fixed in 4% (v/v) formaldehyde in phosphate-buffered saline after 6, 9, 16, and 36 h, dehydrated in an ethanol series, and embedded in Steedman’s wax (Electron Microscopy Sciences). Sections of 6 to 7 µm thickness were incubated with primary rabbit anti-NaStEP antibody (1:2,000; Busot et al., 2008) or primary anti-HT-B antibody (1:1,000; Goldraij et al., 2006) plus mouse anti-callose antibody (Biosupplies Australia; 1:1,000). They were then incubated with the secondary antibodies goat anti-rabbit Alexa 488 fluorochrome (1:200; Molecular Probes) for HT-B or NaStEP and goat anti-mouse Alexa 568 (1:200; Molecular Probes) for callose for 4 h at room temperature. HT-B amount in each pollen tube was classified as low when little or no fluorescence was observed, levels were considered as medium when fluorescence was intermediate, and levels were considered high when the staining was heavy (Supplemental Fig. S2). The fluorescence percentage was calculated taking the total fluorescent area between the pollen tube total area delimited by the anti-callose antibody signal and using Image Pro Plus6.3 software (Media Cybernetics). One hundred percent was taken as the highest immunostaining intensity. All sections were observed using a confocal fluorescence microscope (Olympus FV1000).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Immunoanalysis of NaStEP, SC10-RNase, and S105-RNase in pistils of J08 plants.
Supplemental Figure S2. HT-B staining intensity criteria.
Supplemental Figure S3. NaStEP purification.
Supplemental Table S1. Pollen tubes at the base of the style 72 h post pollination in a NaStEP-suppressed hybrid from the K08 population.
Supplemental Table S2. Pollen tubes at the base of the style 72 h post pollination in a NaStEP-suppressed hybrid from the J08 population.
Acknowledgments
We are grateful to Anabel Bieler-Antolin for her assistance with fluorescence microscopy, to Gabriel Orozco-Hoyuela for confocal microscopy help, to Maria Teresa Olivera-Flores and Laurel Fabila-Ibarra for plant transformation and greenhouse support, and to Guillermo Mendoza for mass spectrometry analysis. We particularly thank the anonymous reviewers for comments to a previous version of the manuscript.
Glossary
- SI
self-incompatibility
- RNAi
RNA interference
- MD
molecular dynamics
- SC
self-compatible
References
- Ai YJ, Kron E, Kao TH. (1991) S-Alleles are retained and expressed in a self-compatible cultivar of Petunia hybrida. Mol Gen Genet 230: 353–358 [DOI] [PubMed] [Google Scholar]
- Anderson MA, Cornish EC, Mav SL, Williams EG, Hoggart R, Atkinson A, Boning I, Greg B, Simpson RJ, Roche PJ, et al. (1986) Cloning of cDNA for a stylar glycoprotein associated with expression of self-incompatibility in Nicotiana alata. Nature 321: 38–44 [Google Scholar]
- Beecher B, McClure BA. (2001) Effects of RNases on rejection of pollen from Nicotiana tabacum and N. plumbaginifolia. Sex Plant Reprod 14: 69–76 [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Busot GY, McClure B, Ibarra-Sánchez CP, Jiménez-Durán K, Vázquez-Santana S, Cruz-García F. (2008) Pollination in Nicotiana alata stimulates synthesis and transfer to the stigmatic surface of NaStEP, a vacuolar Kunitz proteinase inhibitor homologue. J Exp Bot 59: 3187–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavelas-Adame EA, Hernández-Domínguez EE, Gaytán-Mondrangón S, Rosales-León L, Valencia-Turcotte L, Rodríguez-Sotres R. (2011) A hitchhiker’s guide to the modeling of the three-dimensional structure of proteins. Int Biotechnol Color J 1: 26–35 [Google Scholar]
- Chichkova NV, Shaw J, Galiullina RA, Drury GE, Tuzhikov AI, Kim SH, Kalkum M, Hong TB, Gorshkova EN, Torrance L, et al. (2010) Phytaspase, a relocalisable cell death promoting plant protease with caspase specificity. EMBO J 29: 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey PA, Kondo K, Welch L, Frank E, Sianta S, Kumar A, Nuñez R, Lopez-Casado G, van der Knaap E, Rose JK, et al. (2010) Multiple features that distinguish unilateral incongruity and self-incompatibility in the tomato clade. Plant J 64: 367–378 [DOI] [PubMed] [Google Scholar]
- Cruz-Garcia F, Hancock CN, Kim D, McClure B. (2005) Stylar glycoproteins bind to S-RNase in vitro. Plant J 42: 295–304 [DOI] [PubMed] [Google Scholar]
- de Nettancourt D. (1977) Incompatibility in Angiosperms: Monographs on Theoretical and Applied Genetics 3. Springer-Verlag, New York [Google Scholar]
- de Nettancourt D. (2001) Incompatibility and Incongruity in Wild and Cultivated Plants. Springer-Verlag, New York [Google Scholar]
- Deutscher MP. (1990) Maintaining protein stability. Methods Enzymol 182: 83–89 [DOI] [PubMed] [Google Scholar]
- Entani T, Iwano M, Shiba H, Che FS, Isogai A, Takayama S. (2003) Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 8: 203–213 [DOI] [PubMed] [Google Scholar]
- Franklin FCH, Lawrence MJ, Franklin-Tong VE. (1995) Cell and molecular biology of self-incompatibility in flowering plants. Int Rev Cytol 158: 1–64 [Google Scholar]
- Goldraij A, Kondo K, Lee CB, Hancock CN, Sivaguru M, Vazquez-Santana S, Kim S, Phillips TE, Cruz-Garcia F, McClure BA. (2006) Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana. Nature 439: 805–810 [DOI] [PubMed] [Google Scholar]
- Hancock CN, Kent L, McClure BA. (2005) The stylar 120 kDa glycoprotein is required for S-specific pollen rejection in Nicotiana. Plant J 43: 716–723 [DOI] [PubMed] [Google Scholar]
- Hartl M, Giri AP, Kaur H, Baldwin IT. (2010) Serine protease inhibitors specifically defend Solanum nigrum against generalist herbivores but do not influence plant growth and development. Plant Cell 22: 4158–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B, Kutzner C, van der Spoel D, Lindahl E. (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4: 435–447 [DOI] [PubMed] [Google Scholar]
- Hua ZH, Fields A, Kao TH. (2008) Biochemical models for S-RNase-based self-incompatibility. Mol Plant 1: 575–585 [DOI] [PubMed] [Google Scholar]
- Hua ZH, Kao TH. (2006) Identification and characterization of components of a putative Petunia S-locus F-box-containing E3 ligase complex involved in S-RNase-based self-incompatibility. Plant Cell 18: 2531–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua ZH, Meng XY, Kao TH. (2007) Comparison of Petunia inflata S-locus F-box protein (Pi SLF) with Pi SLF like proteins reveals its unique function in S-RNase based self-incompatibility. Plant Cell 19: 3593–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez-Díaz JA, McClure B, Vázquez-Santana S, Guevara-García A, León-Mejía P, Márquez-Guzmán J, Cruz-García F. (2006) A novel thioredoxin h is secreted in Nicotiana alata and reduces S-RNase in vitro. J Biol Chem 281: 3418–3424 [DOI] [PubMed] [Google Scholar]
- Karplus K. (2009) SAM-T08, HMM-based protein structure prediction. Nucleic Acids Res 37: W492–W497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho YO, Baer J. (1968) Observing pollen tubes by means of fluorescence. Euphytica 17: 298–302 [Google Scholar]
- Kondo K, McClure B. (2008) New microsome-associated HT-family proteins from Nicotiana respond to pollination and define an HT/NOD-24 protein family. Mol Plant 1: 634–644 [DOI] [PubMed] [Google Scholar]
- Kondo K, Yamamoto M, Matton DP, Sato T, Hirai M, Norioka S, Hattori T, Kowyama Y. (2002) Cultivated tomato has defects in both S-RNase and HT genes required for stylar function of self-incompatibility. Plant J 29: 627–636 [DOI] [PubMed] [Google Scholar]
- Kubo K, Entani T, Takara A, Wang N, Fields AM, Hua Z, Toyoda M, Kawashima S, Ando T, Isogai A, et al. (2010) Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science 330: 796–799 [DOI] [PubMed] [Google Scholar]
- Lantz MS, Ciborowski P. (1994) Zymographic techniques for detection and characterization of microbial proteases. Methods Enzymol 235: 563–594 [DOI] [PubMed] [Google Scholar]
- Lee CB, Kim S, McClure B. (2009) A pollen protein, NaPCCP, that binds pistil arabinogalactan proteins also binds phosphatidylinositol 3-phosphate and associates with the pollen tube endomembrane system. Plant Physiol 149: 791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CB, Swatek KN, McClure B. (2008) Pollen proteins bind to the C-terminal domain of Nicotiana alata pistil arabinogalactan proteins. J Biol Chem 283: 26965–26973 [DOI] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET. (2008) Kunitz trypsin inhibitor: an antagonist of cell death triggered by phytopathogens and fumonisin b1 in Arabidopsis. Mol Plant 1: 482–495 [DOI] [PubMed] [Google Scholar]
- Lind JL, Bönig I, Clarke AE, Anderson MA. (1996) A style-specific 120 kDA glycoprotein enters pollen tubes of Nicotiana alata in vivo. Sex Plant Reprod 9: 75–86 [Google Scholar]
- Luu D-T, Qin X, Morse D, Cappadocia M. (2000) S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature 407: 649–651 [DOI] [PubMed] [Google Scholar]
- Martin FW. (1968) The behavior of Lycopersicon incompatibility alleles in an alien genetic milieu. Genetics 60: 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Castilla LP, Rodríguez-Sotres R. (2010) A score of the ability of a three-dimensional protein model to retrieve its own sequence as a quantitative measure of its quality and appropriateness. PLoS ONE 5: e12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure BA, Cruz-García F, Romero C. (2011) Compatibility and incompatibility in S-RNase-based systems. Ann Bot (Lond) 108: 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure BA, Haring V, Ebert PR, Anderson MA, Simpson RJ, Sakiyama F, Clarke AE. (1989) Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature 342: 955–957 [DOI] [PubMed] [Google Scholar]
- McClure BA, Mou B, Canevascini S, Bernatzky R. (1999) A small asparagine-rich protein required for S-allele-specific pollen rejection in Nicotiana. Proc Natl Acad Sci USA 96: 13548–13553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheelsen PO, Vévodová J, De Maria L, Ostergaard PR, Friis EP, Wilson K, Skjøt M. (2008) Structural and mutational analyses of the interaction between the barley alpha-amylase/subtilisin inhibitor and the subtilisin savinase reveal a novel mode of inhibition. J Mol Biol 380: 681–690 [DOI] [PubMed] [Google Scholar]
- Murfett J, Atherton TL, Mou B, Gasser CS, McClure BA. (1994) S-RNase expressed in transgenic Nicotiana causes S-allele-specific pollen rejection. Nature 367: 563–566 [DOI] [PubMed] [Google Scholar]
- Murfett J, Cornish EC, Ebert PR, Bonig I, McClure BA, Clarke AE. (1992) Expression of a self-incompatibility glycoprotein (S2-ribonuclease) from Nicotiana alata in transgenic Nicotiana tabacum. Plant Cell 4: 1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murfett J, Strabala TJ, Zurek DM, Mou B, Beecher B, McClure BA. (1996) S RNase and interespecific pollen rejection in the genus Nicotiana: multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell 8: 943–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Medkova M, Dong G, Hutagalung A, Reinisch K, Grosshans B. (2006) Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem Soc Trans 34: 683–686 [DOI] [PubMed] [Google Scholar]
- O’Brien M, Kapfer C, Major G, Laurin M, Bertrand C, Kondo K, Kowyama Y, Matton DP. (2002) Molecular analysis of the stylar-expressed Solanum chacoense small asparagine-rich protein family related to the HT modifier of gametophytic self-incompatibility in Nicotiana. Plant J 32: 985–996 [DOI] [PubMed] [Google Scholar]
- Oostenbrink C, Villa A, Mark AE, van Gunsteren WF. (2004) A biomolecular force field based on the free enthalpy of hydration and solvation: the GROMOS force-field parameter sets 53A5 and 53A6. J Comput Chem 25: 1656–1676 [DOI] [PubMed] [Google Scholar]
- Pandey KK. (1981) Evolution of unilateral incompatibility in flowering plants: further evidence in favor of twin specificities controlling intra- and interspecific incompatibility. New Phytol 89: 705–728 [Google Scholar]
- Puerta AR, Ushijima K, Koba T, Sassa H. (2009) Identification and functional analysis of pistil self-incompatibility factor HT-B of Petunia. J Exp Bot 60: 1309–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Wang F, Zhao L, Zhou J, Lai Z, Zhang Y, Robbins TP, Xue Y. (2004) The F-box protein AhSLF-S2 controls the pollen function of S-RNase-based self-incompatibility. Plant Cell 16: 2307–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman S, Vernon R, Thompson J, Tyka M, Sadreyev R, Pei J, Kim D, Kellogg E, DiMaio F, Lange O, et al. (2009) Structure prediction for CASP8 with all-atom refinement using Rosetta. Proteins (Suppl 9) 77: 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Eargle J, Wright D, Luthey-Schulten Z. (2006) MultiSeq: unifying sequence and structure data for evolutionary analysis. BMC Bioinformatics 7: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales-León L, Hernández-Domínguez EE, Gaytán-Mondragón S, Rodríguez-Sotres R. (2012) Metal binding sites in plant soluble inorganic pyrophosphatases: an example of the use of ROSETTA design and hidden Markov models to guide the homology modeling of proteins. J Mex Chem Soc 56: 23–31 [Google Scholar]
- Russell RB, Barton GJ. (1992) Multiple protein sequence alignment from tertiary structure comparison: assignment of global and residue confidence levels. Proteins 14: 309–323 [DOI] [PubMed] [Google Scholar]
- Samuel MA, Chong YT, Haasen KE, Aldea-Brydges MG, Stone SL, Goring DR. (2009) Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. Plant Cell 21: 2655–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa H, Hirano H. (2006) Identification of a new class of pistil-specific proteins of Petunia inflata that is structurally similar to, but functionally distinct from, the self-incompatibility factor HT. Mol Genet Genomics 275: 97–104 [DOI] [PubMed] [Google Scholar]
- Schultz CJ, Hauser K, Lind JL, Atkinson AH, Pu Z-Y, Anderson MA, Clarke AE. (1997) Molecular characterisation of a cDNA sequence encoding the backbone of a style-specific 120 kDa glycoprotein which has features of both extensins and arabinogalactan proteins. Plant Mol Biol 35: 833–845 [DOI] [PubMed] [Google Scholar]
- Sijacic P, Wang X, Skirpan AL, Wang Y, Dowd PE, McCubbin AG, Huang S, Kao TH. (2004) Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature 429: 302–305 [DOI] [PubMed] [Google Scholar]
- Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB. (1991) Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci USA 88: 8816–8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki T, Hatakeyama K, Suzuki G, Watanabe M, Isogai A, Hinata K. (2000) The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403: 913–916 [DOI] [PubMed] [Google Scholar]
- Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S. (2004) LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J 39: 343–353 [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Ando T, Kokubun H, Watanabe H, Masada M, Zhu X, Marchesi E, Kao T. (1999) Breakdown of self-incompatibility in a natural population of Petunia axillaris (Solanaceae) in Uruguay containing both self-incompatible and self-compatible plants. Sex Plant Reprod 12: 6–13 [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, et al. (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27: 581–590 [DOI] [PubMed] [Google Scholar]
- Zárský V, Cvrcková F, Potocký M, Hála M. (2009) Exocytosis and cell polarity in plants: exocyst and recycling domains. New Phytol 183: 255–272 [DOI] [PubMed] [Google Scholar]
- Zhao L, Huang J, Zhao Z, Li Q, Sims TL, Xue Y. (2010) The Skp1-like protein SSK1 is required for cross-pollen compatibility in S-RNase-based self-incompatibility. Plant J 62: 52–63 [DOI] [PubMed] [Google Scholar]