Abstract
VKORC1 (vitamin K epoxide reductase complex subunit 1, 16p11.2) is the main genetic determinant of human response to oral anticoagulants of antivitamin K type (AVK). This gene was recently suggested to be a putative target of positive selection in East Asian populations. In this study, we genotyped the HGDP-CEPH Panel for six VKORC1 SNPs and downloaded chromosome 16 genotypes from the HGDP-CEPH database in order to characterize the geographic distribution of footprints of positive selection within and around this locus. A unique VKORC1 haplotype carrying the promoter mutation associated with AVK sensitivity showed especially high frequencies in all the 17 HGDP-CEPH East Asian population samples. VKORC1 and 24 neighboring genes were found to lie in a 505 kb region of strong linkage disequilibrium in these populations. Patterns of allele frequency differentiation and haplotype structure suggest that this genomic region has been submitted to a near complete selective sweep in all East Asian populations and only in this geographic area. The most extreme scores of the different selection tests are found within a smaller 45 kb region that contains VKORC1 and three other genes (BCKDK, MYST1 (KAT8), and PRSS8) with different functions. Because of the strong linkage disequilibrium, it is not possible to determine if VKORC1 or one of the three other genes is the target of this strong positive selection that could explain present-day differences among human populations in AVK dose requirement. Our results show that the extended region surrounding a presumable single target of positive selection should be analyzed for genetic variation in a wide range of genetically diverse populations in order to account for other neighboring and confounding selective events and the hitchhiking effect.
Introduction
Oral anticoagulants of antivitamin K type (AVK) − such as warfarin and acenocoumarol − are widely prescribed drugs for the prevention and treatment of arterial and venous thromboembolic disorders [1], [2]. They exert their anticoagulant effect by inhibiting the vitamin K 2,3-epoxide reductase complex 1 (VKORC1). Besides well-known physiopathological and environmental factors, including age, sex, body mass index, disease states, co-medications and diet, genetic factors have been identified as major determinants of AVK dose variability [3]. Candidate-gene and genome-wide association studies have identified four main genes − CYP2C9, CYP4F2, CYP2C18 and VKORC1− which explain together between 28.2% and 43.5% of the AVK dose variance [3], [4], [5], [6], [7]. CYP2C9, CYP4F2 and CYP2C18 encode proteins involved in the hepatic metabolism of AVK [8], [9], [10]. VKORC1 encodes the VKORC1 enzyme, which is the direct pharmacologic target of AVK [11], [12]. Differences in the worldwide distribution of the most important polymorphisms influencing AVK dosing are likely to underlie the wide interethnic variability in AVK dose requirements: current population-based trends in warfarin dosing, as reported by the International Warfarin Pharmacogenetics Consortium, indicate a mean weekly dose of 21 mg in Asians, 31.5 mg in Europeans and 40 mg in individuals of African ancestry [13].
Recently, Ross et al. [14] documented the distribution of four functional variants located in the three main genes known to influence AVK dose requirement − rs9923231 (VKORC1), rs1799853 and rs1057910 (CYP2C9), and rs2108622 (CYP4F2) − in a large set of samples from the Human Genome Diversity Project - Centre d’Etude du Polymorphisme Humain (HGDP-CEPH) Panel, representing 52 world populations [15]. They observed a pattern of genetic differentiation among human populations for the VKORC1 single nucleotide polymorphism (SNP) rs9923231. They applied three formal tests of positive selection to the VKORC1 gene − the locus-specific branch length (LSBL) test [16], the log of the ratio of heterozygosities (lnRH) test [17], and Tajima’s D [18] − using genome-wide data available for the West African, European and East Asian HapMap samples [19]. The tests yielded significant results in the East Asian sample. Interestingly, the rs9923231 SNP (g.-1639G>A), which was found to be a putative target of positive selection [14], is the main genetic determinant of AVK dose requirement and can alone explain between 25% to 30% of the dose variance among patients [4], [5], [6], [7]. This SNP, located in the promoter region, alters a VKORC1 transcription factor binding site, leading to lower protein expression [20]. By decreasing VKORC1 activity, the derived -1639A allele thus confers an increased AVK sensitivity phenotype and patients carrying one and two -1639A alleles require on average respectively 25% and 50% lower daily warfarin doses than -1639G homozygous carriers to obtain the same anticoagulant effect [21], [22]. Understanding the processes of local adaption that may result in high levels of population differentiation and important interethnic differences in the required AVK dose is thus of particular relevance.
During these last few years, newer methods than those proposed by Ross et al. have been developed to detect the molecular footprints of positive selection. These methods are particularly well suited to detect classical signatures of selective sweeps, i.e. when a new advantageous mutation spreads rapidly to fixation in particular populations (the so-called ‘hard sweep’ model) [23]. Such a selective sweep occurs too quickly to leave enough time for recombination events to break down the linkage disequilibrium (LD), leading to a similar increase in frequency of alleles at nearby variants. Therefore, the pattern of genetic variation in the genomic region surrounding the selected allele may differ among populations [24], and the selected allele is expected to be carried by a long and frequent haplotype only in those populations that experienced the local adaptive event [25]. Signals of positive selection can thus be detected by looking for an increased genetic differentiation among populations (using methods such as FST [26] and the Cross-Population Composite Likelihood Ratio (XP-CLR) test [24]), and an extended haplotype homozygosity (EHH) at the putatively selected locus (using methods such as the Cross-Population Extended Haplotype Homozygosity (XP-EHH) test [27] and the integrated Haplotype Score (iHS) [28]). These methods have proved to be powerful and largely complementary to detect and localize a selective sweep, and are more robust to ascertainment bias in SNP discovery than methods based on the allele frequency spectrum such as the Tajima’s D used by Ross et al. [14], [29].
In this study, we investigated whether and how positive selection has acted on the VKORC1 gene locus using these complementary analytic methods. Our first objective was to determine (1) if the selective sweep is restricted to East Asia or if it is detected in other geographic regions, in particular Central South Asia and America, which are geographically close to East Asia, and (2) if it occurred in all East Asian populations or only in a few of them. Thus, we genotyped six VKORC1 SNPs in the HGDP-CEPH Panel [30] which covers a much wider range of world populations – including 17 populations from East Asia – than the HapMap Panel in which positive selection at the VKORC1 locus was initially evidenced. Furthermore, by expanding the analysis to a 2 Mb region encompassing the VKORC1 gene, we sought to determine if the selective sweep identified around VKORC1 was due to positive selection directly acting on this gene, or if it was caused by positive selection at a nearby linked gene resulting in genetic hitchhiking [23]. Finally, we discuss combining different methods for uncovering distinct selection signatures, in order to both increase power to detect a selective signal and precisely define its genomic location. We address the difficulty, even with such detailed analyses, in identifying the specific target of selection.
Results
VKORC1 Haplotype Study
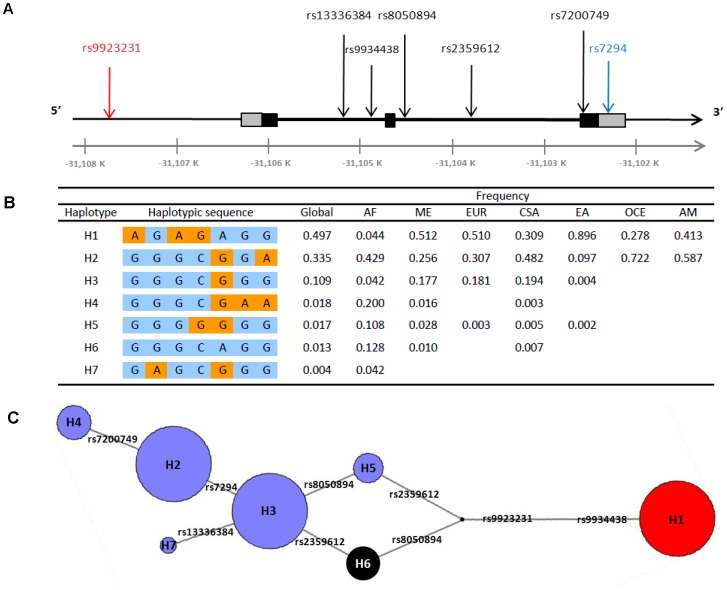
A haplotype study of the 4.1 kb VKORC1 gene was carried out with seven VKORC1 SNPs genotyped in the 52 HGDP-CEPH population samples (Figure 1A). Haplotypes were reconstructed from these SNPs. Seven of these haplotypes had a frequency above 1% in at least one geographic region and were labeled H1 to H7 according to their frequency at the global level (Figure 1B). Four haplotypes are found in at least five geographic regions and only two are shared among all regions. The highest and lowest haplotype diversity values are observed in Sub-Saharan Africa (0.75±0.02) and East Asia (0.19±0.02), respectively. Most individuals carrying the ancestral haplotype (H6), i.e. the haplotype carrying the ancestral allele at each SNP, are from Sub-Saharan Africa (Figure 1B and Figure S1). Interestingly, the -1639A allele (rs9923231) conferring the increased sensitivity to AVK is carried by a unique haplotype (H1). This haplotype associated with AVK sensitivity is the most frequent at the worldwide level (49.7%) and shows an extremely high differentiation among geographic regions (Figure 1B). While rare in Sub-Saharan Africa (4.4%), it is found at intermediate frequencies in the Middle East, Europe, Central South Asia, Oceania and America (from 27.8% to 51.2%), and is largely predominant in East Asia (89.6%). The prevalence of H1 tends to be high in all of the 17 East Asian population samples investigated, ranging from 75% in She to 100% in Oroqen (Figure S1). However, the sample size is small for most of them, with 10 or less individuals.
Figure 1. Results of the VKORC1 haplotype study.
(A) Position of the seven SNPs along the VKORC1 gene. VKORC1 is a 4.1 kb gene (GenBank accession number AY587020) located at 16p11.2. The three exons of the gene are represented as boxes, with 5′UTR and 3′UTR regions colored in grey and coding regions in black. Flanking and intronic regions are represented as thin and thick lines, respectively. The seven studied SNPs are shown in their sequential order along the VKORC1 gene. The functional polymorphism rs9923231 located in the promoter, is highlighted in red and the SNP already present in the Illumina 650K chip in blue. Physical position along chromosome 16 is indicated in kb below. (B) Distribution of VKORC1 haplotypes at the global and regional level. For each haplotype, SNPs are listed in the same sequential order than in Figure 1A. Ancestral and derived alleles are shown in blue and orange, respectively. Haplotype labels H1 to H7 were given according to the global haplotype frequency. AF, sub-Saharan Africa; ME, Middle East; EUR, Europe; CSA, Central South Asia; EA, East Asia; OCE, Oceania; AM, America. (C) Median-joining network of the inferred VKORC1 haplotypes at the global level. Circles areas are proportional to the global haplotype frequency and branch lengths to the number of mutations separating haplotypes. Labels of haplotypes are indicated in corresponding circles, and labels of mutations on the network branches. The haplotype carrying the -1639A allele conferring the AVK sensitivity phenotype (H1) is shown in red and the ancestral haplotype (H6) in black.
The median-joining haplotype network describes the mutational relationships between the different VKORC1 haplotypes inferred (Figure 1C). Haplotype H1 differs from the others by two nucleotide substitutions at the functional rs9923231 SNP and at the rs9934438 SNP, which are found in complete LD in all geographic regions (D’ = 1 and r 2 = 1, Figure S2).
Detection of Signatures of Positive Selection
To support the hypothesis that positive selection has played a role in shaping patterns of genetic variation at VKORC1, four complementary methods were applied to detect signatures of selective sweeps in the genome. FST and XP-CLR are both based on allele frequency differentiation, whereas XP-EHH and iHS are based on haplotype structure. Scores for the four test statistics were computed at both the regional and population levels for the seven VKORC1 SNPs and for some other available SNPs [15] representing the expected neutral genomic background. For each score, a p-value was derived from the empirical distribution obtained from the genomic background (cf. Material and Methods). We considered as significant any p-value below 0.05. The results of the four tests are presented in Table 1 and Table 2.
Table 1. Results of the inter-regional FST, intra-regional FST, XP-EHH and iHS tests in the seven geographic regions.
| Region | SNP | DAFa | Inter-regional FST b | Inter-regional FST p-valuec | Intra-regional FST d | Intra- regional FST p-valuec | XP-EHH score | XP-EHH p-valuee | iHS score | iHS p-valuee |
| Africa | rs7294 | 0.63 | 0.18 | 0.215 | 0.13 | 0.074 | −0.94 | 0.833 | −1.24 | 0.183 |
| rs7200749 | 0.20 | 0.48 | 0.217 | 0.02 | 0.643 | −1.50 | 0.923 | −0.31 | 0.748 | |
| rs2359612 | 0.82 | 0.23 | 0.173 | 0.09 | 0.123 | −1.15 | 0.875 | −0.96 | 0.305 | |
| rs8050894 | 0.16 | 0.25 | 0.143 | 0.09 | 0.125 | −1.14 | 0.872 | 0.11 | 0.909 | |
| rs9934438 | 0.04 | 0.36 | 0.029 * | 0.10 | 0.058 | −1.05 | 0.855 | −0.03 | 0.974 | |
| rs13336384 | 0.04 | 0.16 | 0.329 | 0.02 | 0.448 | −1.06 | 0.858 | 0.14 | 0.883 | |
| rs9923231 | 0.04 | 0.36 | 0.029 * | 0.10 | 0.058 | −1.01 | 0.845 | −0.01 | 0.989 | |
| Middle East | rs7294 | 0.27 | 0.02 | 0.411 | 0.00 | 0.906 | 0.94 | 0.171 | 1.55 | 0.103 |
| rs7200749 | 0.02 | 0.00 | 0.670 | 0.02 | 0.310 | 1.58 | 0.069 | 0.65 | 0.492 | |
| rs2359612 | 0.48 | 0.00 | 1.000 | 0.006 | 0.595 | 1.17 | 0.127 | 2.69 | 0.009 ** | |
| rs8050894 | 0.54 | 0.00 | 0.849 | 0.002 | 0.667 | 1.15 | 0.132 | −1.40 | 0.141 | |
| rs9934438 | 0.51 | 0.00 | 0.946 | 0.01 | 0.481 | 1.05 | 0.149 | −1.76 | 0.066 | |
| rs13336384 | 0.00 | 0.005 | 0.044 * | 0.00 | 1.000 | 1.07 | 0.145 | NA | NA | |
| rs9923231 | 0.51 | 0.00 | 0.946 | 0.01 | 0.481 | 1.01 | 0.156 | −1.76 | 0.066 | |
| Europe | rs7294 | 0.30 | 0.005 | 0.670 | 0.004 | 0.570 | 0.94 | 0.167 | 0.67 | 0.474 |
| rs7200749 | 0.00 | 0.02 | 0.477 | 0.00 | 1.000 | 1.50 | 0.077 | NA | NA | |
| rs2359612 | 0.49 | 0.00 | 1.000 | 0.02 | 0.304 | 1.15 | 0.125 | 2.00 | 0.039 * | |
| rs8050894 | 0.51 | 0.00 | 1.000 | 0.02 | 0.286 | 1.14 | 0.128 | −0.98 | 0.298 | |
| rs9934438 | 0.51 | 0.00 | 0.993 | 0.02 | 0.304 | 1.05 | 0.145 | −1.02 | 0.281 | |
| rs13336384 | 0.00 | 0.005 | 0.071 | 0.00 | 1.000 | 1.06 | 0.142 | NA | NA | |
| rs9923231 | 0.51 | 0.00 | 0.993 | 0.02 | 0.304 | 1.01 | 0.155 | −1.02 | 0.281 | |
| Central South Asia | rs7294 | 0.49 | 0.06 | 0.042 * | 0.07 | 0.026* | 0.62 | 0.260 | −0.54 | 0.550 |
| rs7200749 | 0.003 | 0.02 | 0.261 | 0.02 | 0.041* | 1.25 | 0.116 | NA | NA | |
| rs2359612 | 0.69 | 0.12 | 0.002 ** | 0.07 | 0.025* | 0.89 | 0.187 | 1.00 | 0.280 | |
| rs8050894 | 0.32 | 0.12 | 0.003 ** | 0.08 | 0.015* | 0.87 | 0.191 | −0.13 | 0.893 | |
| rs9934438 | 0.31 | 0.10 | 0.006 ** | 0.07 | 0.020* | 0.78 | 0.215 | −0.20 | 0.834 | |
| rs13336384 | 0.00 | 0.005 | 0.088 | 0.00 | 1.000 | 0.79 | 0.210 | NA | NA | |
| rs9923231 | 0.31 | 0.10 | 0.006 ** | 0.07 | 0.020* | 0.73 | 0.227 | −0.20 | 0.834 | |
| East Asia | rs7294 | 0.10 | 0.21 | 0.063 | 0.02 | 0.311 | 2.68 | 0.011 * | 1.99 | 0.040 * |
| rs7200749 | 0.00 | 0.02 | 0.576 | 0.00 | 1.000 | 3.10 | 0.005 ** | NA | NA | |
| rs2359612 | 0.10 | 0.39 | 0.005 ** | 0.02 | 0.317 | 2.89 | 0.008 ** | 1.92 | 0.047 * | |
| rs8050894 | 0.90 | 0.38 | 0.005 ** | 0.02 | 0.331 | 2.88 | 0.008 ** | −1.20 | 0.200 | |
| rs9934438 | 0.90 | 0.41 | 0.003 ** | 0.02 | 0.300 | 2.81 | 0.009 ** | −1.27 | 0.174 | |
| rs13336384 | 0.00 | 0.005 | 0.252 | 0.00 | 1.000 | 2.81 | 0.009 ** | NA | NA | |
| rs9923231 | 0.90 | 0.41 | 0.003 ** | 0.02 | 0.300 | 2.773 | 0.010 * | −1.274 | 0.174 | |
| Oceania | rs7294 | 0.72 | 0.23 | 0.090 | 0.00 | 0.771 | 0.03 | 0.456 | 0.05 | 0.961 |
| rs7200749 | 0.00 | 0.005 | 0.401 | 0.00 | 1.000 | 0.51 | 0.285 | NA | NA | |
| rs2359612 | 0.72 | 0.09 | 0.404 | 0.00 | 0.771 | 0.32 | 0.346 | 0.05 | 0.961 | |
| rs8050894 | 0.25 | 0.12 | 0.327 | 0.02 | 0.530 | 0.29 | 0.355 | 0.50 | 0.584 | |
| rs9934438 | 0.28 | 0.08 | 0.438 | 0.00 | 0.749 | 0.20 | 0.388 | 0.50 | 0.584 | |
| rs13336384 | 0.00 | 0.009 | 0.014 * | 0.00 | 1.000 | 0.21 | 0.384 | NA | NA | |
| rs9923231 | 0.28 | 0.08 | 0.438 | 0.00 | 0.749 | 0.16 | 0.404 | 0.50 | 0.584 | |
| America | rs7294 | 0.58 | 0.11 | 0.320 | 0.17 | 0.195 | 0.76 | 0.207 | NA | NA |
| rs7200749 | 0.00 | 0.01 | 0.553 | 0.00 | 1.000 | 1.15 | 0.125 | NA | NA | |
| rs2359612 | 0.59 | 0.02 | 0.674 | 0.17 | 0.190 | 0.96 | 0.162 | NA | NA | |
| rs8050894 | 0.41 | 0.02 | 0.667 | 0.17 | 0.190 | 0.95 | 0.163 | NA | NA | |
| rs9934438 | 0.41 | 0.01 | 0.743 | 0.17 | 0.190 | 0.88 | 0.178 | NA | NA | |
| rs13336384 | 0.00 | 0.006 | 0.013 * | 0.00 | 1.000 | 0.89 | 0.176 | NA | NA | |
| rs9923231 | 0.41 | 0.01 | 0.743 | 0.17 | 0.190 | 0.85 | 0.185 | NA | NA |
Derived allele frequency estimated at the global level.
FST estimated at the inter-regional level, i.e. between a given geographic region and the remaining ones.
P-values are derived from the genome-wide empirical distribution of FST values.
FST estimated at the intra-regional level, i.e. among populations within a region.
P-values are derived from the empirical distribution of the iHS and XP-EHH scores along the chromosome 16.
p<0.05; ** p<0.01; *** p<0.005.
NA: Not Applicable (for iHS: when a gap>200 kb between successive SNPs is found in the region in the region delimited by the SNPs where the EHH value drops below 0.05 around the core SNP).
Table 2. Results of the XP-CLR test in a 16 kb region centered on VKORC1 in the seven geographic regions.
| Region | Physicalposition | XP-CLRscore | XP-CLRp-valuea |
| Africa | 31005354 | 0.00 | 1.000 |
| 31009354 | 0.00 | 1.000 | |
| 31013354 | 0.96 | 0.289 | |
| 31017354 | 0.58 | 0.348 | |
| 31021354 | 0.09 | 0.470 | |
| Middle East | 31005354 | 4.00 | 0.138 |
| 31009354 | 0.85 | 0.306 | |
| 31013354 | 3.28 | 0.158 | |
| 31017354 | 0.27 | 0.403 | |
| 31021354 | 6.25 | 0.092 | |
| Europe | 31005354 | 0.54 | 0.351 |
| 31009354 | 0.00 | 1.000 | |
| 31013354 | 2.63 | 0.186 | |
| 31017354 | 0.15 | 0.427 | |
| 31021354 | 2.40 | 0.198 | |
| Central South Asia | 31005354 | 0.03 | 0.464 |
| 31009354 | 0.00 | 1.000 | |
| 31013354 | 0.00 | 1.000 | |
| 31017354 | 0.01 | 0.476 | |
| 31021354 | 0.00 | 0.490 | |
| East Asia | 31005354 | 24.08 | 0.032 * |
| 31009354 | 16.53 | 0.050 * | |
| 31013354 | 30.49 | 0.023 * | |
| 31017354 | 26.82 | 0.028 * | |
| 31021354 | 43.44 | 0.012 * | |
| Oceania | 31005263 | 0.00 | 1.000 |
| 31009263 | 0.00 | 1.000 | |
| 31013263 | 0.00 | 1.000 | |
| 31017263 | 0.00 | 1.000 | |
| 31021263 | 0.00 | 1.000 | |
| America | 31005354 | 0.00 | 1.000 |
| 31009354 | 0.00 | 1.000 | |
| 31013354 | 0.00 | 1.000 | |
| 31017354 | 0.01 | 0.587 | |
| 31021354 | 0.00 | 0.597 |
P-values are derived from the empirical distribution of the XP-CLR scores along the chromosome 16.
p<0.05; ** p<0.01; *** p<0.005.
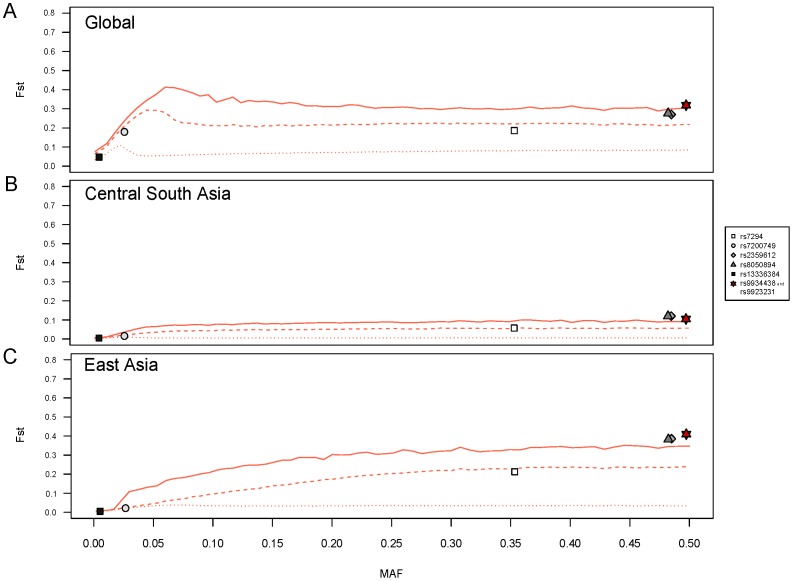
At the global level, when we evaluated the level of genetic differentiation among the seven HGDP-CEPH Panel geographic regions, an atypical pattern of genetic differentiation was detected for four VKORC1 SNPs: rs2359612, rs8050894, rs9934438 and rs9923231 (p<0.05). The functional rs9923231 polymorphism and the rs9934438 SNP, in complete LD with each other, displayed FST values falling above the 99th percentile of the empirical genome-wide distribution (FST = 0.32, p = 0.008) (Figure 2A). When global FST values were computed among the 52 world populations, very similar results were obtained (Table S1). At the inter-regional level, i.e. between a given geographic region and the remaining ones, the same four VKORC1 SNPs showed highly significant FST values (p<0.01) when comparing Central South Asia and East Asia to the rest of the world (Table 1, Figures 2B and 2C). Regarding East Asia, the highest FST values (FST = 0.41, p = 0.003) were also observed for the two SNPs, rs9923231 and rs9934438. For the other geographic regions, no VKORC1 SNP displayed an inter-regional FST value as much significant as the ones observed for Central South Asia and East Asia (Table 1 and Figure S3). At the intra-regional level, i.e. among populations within a region, no extreme pattern of genetic differentiation (p<0.01) was observed for any VKORC1 SNP in any geographic region (Table 1 and Figure S4).
Figure 2. Atypical patterns of genetic differentiation observed for VKORC1 SNPs.
Genome-wide empirical distributions of FST values were constructed from 644,143 SNPs having a MAF ≥0.001 at the global level. Individual values of FST calculated for each of the seven VKORC1 SNPs are plotted against their global MAF. The functional rs9923231 SNP is shown in red. The 50th, 95th and 99th percentiles are indicated as dotted, dashed and full red lines, respectively.
The XP-CLR test applied to each geographic region also provided evidence of an atypical pattern of genetic differentiation at the VKORC1 gene locus, with XP-CLR scores in East Asia ranging from 16.53 (p = 0.050) to 43.44 (p = 0.012) in the 16 kb genomic region centered on VKORC1 (Table 2). For each of the other six geographic regions, the XP-CLR scores were very low, supporting the existence of a selective sweep restricted to East Asia. In this geographic region, when the XP-CLR test was performed for each population, all of the 17 population samples, except Oroqen, showed this extreme pattern of genetic differentiation, with at least three significant XP-CLR scores out of the five scores computed in the 16 kb genomic region surrounding VKORC1 (Table S2). As most of the SNPs in the VKORC1 genomic region have reached fixation in the Oroqen sample, XP-CLR scores could be calculated for only very few SNPs on either side of VKORC1, making difficult the interpretation of XP-CLR results in this sample.
Regional results obtained with the extended haplotype-based XP-EHH test indicated that the unusual pattern of genetic differentiation observed at the VKORC1 gene locus resulted from a selective sweep in East Asia. Significant XP-EHH scores, ranging from 2.68 (p = 0.011) to 3.10 (p = 0.005), were observed for the seven VKORC1 SNPs in East Asia, while no significant values were observed for any other geographic region (Table 1). For East Asian populations, evidence for a selective sweep was detected in all 17 population samples with significant XP-EHH scores for each of the seven VKORC1 SNPs, ranging from 1.84 (p = 0. 049) in the Dai sample for rs7294, to 3.78 (p = 0.004) in the Tujia sample for rs8050894 (Table S3).
With the iHS test, only two VKORC1 SNPs (rs7294 and rs2359612) exhibited significant iHS scores in East Asia (p = 0.040 and 0.047, respectively; Table 1). Two other significant scores were observed for the rs2359612 SNP in the Middle East (2.69, p = 0.009) and Europe (2.00, p = 0.039). At the population level in East Asia, only three samples (Hezhen, Lahu, and Yakut) displayed significant iHS scores for two, three and four SNPs, respectively (Table S3).
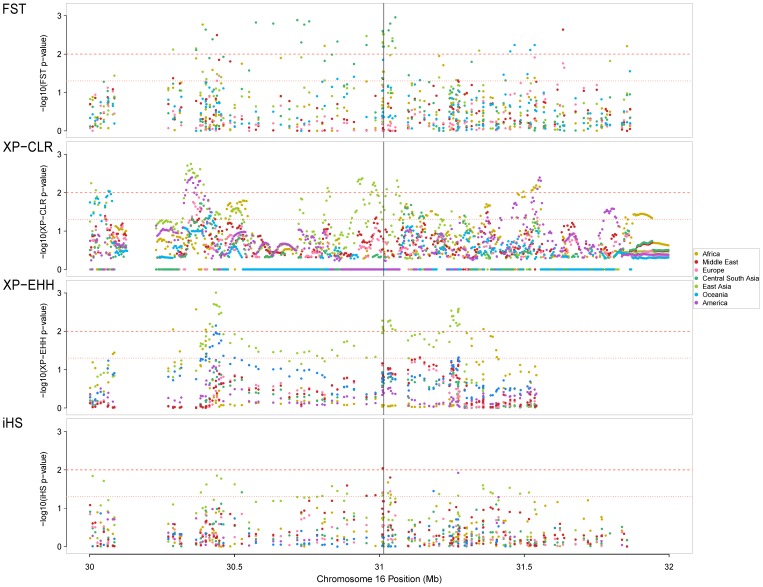
The four selection tests consistently evidenced the signature of a selective sweep involving the VKORC1 genomic region in East Asia. However, this result did not allow us to determine with certainty that VKORC1 is the direct target of positive selection. A linked gene could be the target instead, resulting in genetic hitchhiking of VKORC1 [23]. In an attempt to seek the true target of positive selection, we probed the downloaded chromosome 16 genotypes [15] with the four tests for selection and examined the results over an extended 2 Mb genomic region centered on VKORC1. We focused on clusters of selection test scores with highly significant p-values (p<0.01) for East Asia only. Three clusters were observed (Figure 3): (i) ∼ 570 kb downstream of VKORC1, the first cluster was found with partially overlapping clusters of extreme XP-CLR and XP-EHH scores over a region of 64 and 39 kb, respectively, involving the genes ITGAL, ZNF768, and ZNF747; (ii) at or close to VKORC1 genomic position, the second cluster was determined by overlapping clusters of extreme FST values when comparing East Asia to the rest of the world (with the lowest p-values observed for the same two VKORC1 SNPs evidenced before, rs9923231 and rs9934438) and extreme XP-CLR and XP-EHH scores. These clusters ranged in size from 45 to 244 kb; (iii) ∼ 230 kb upstream of VKORC1, the third cluster of 32 kb was found with XP-EHH and concerned the genes ITGAM and ITGAX. If SNPs within clusters are in high LD (D’≥0.97, except for one SNP in the third cluster), only limited LD exists between the SNPs located in the different clusters (Figure 4 and Figure S5) and several recombination hotspots are present between these clusters (Figure 4). This suggests that each of the three clusters represents a different adaptive event.
Figure 3. Distribution of –log10 (p-values) for four selection tests across a 2 Mb region centered on VKORC1.
A black vertical line indicates the physical position of VKORC1 on chromosome 16. Horizontal red dotted and dashed lines show 0.05 and 0.01 chromosome-wide significance levels, respectively. The selection tests (inter-regional FST, XP-CLR, XP-EHH and iHS, respectively) were separately applied in each of the seven geographic regions.
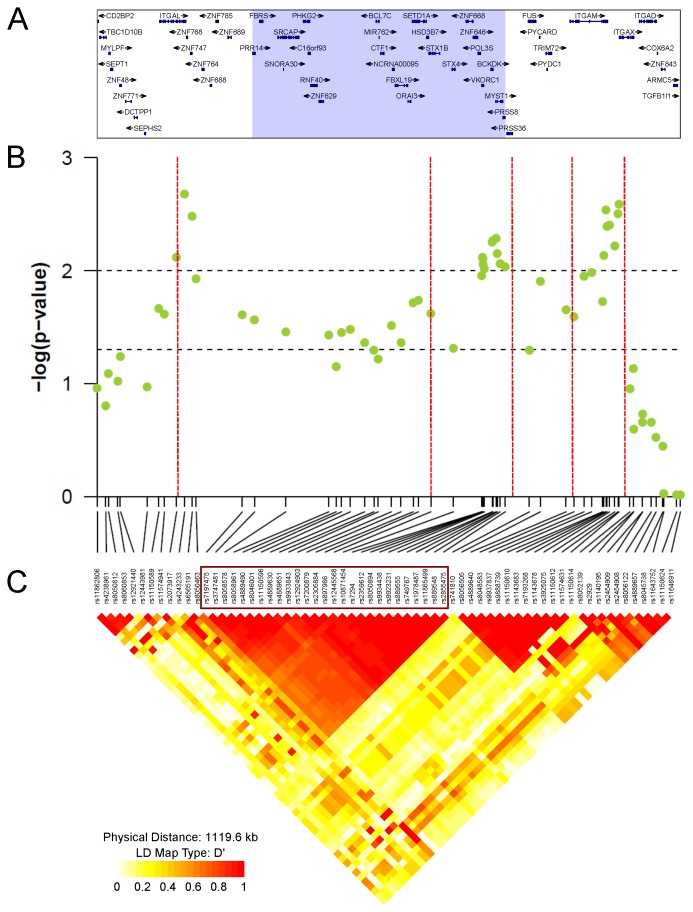
Figure 4. Detailed analysis of a 1.1 Mb genomic region surrounding the VKORC1 gene locus in East Asia.
The boundaries of the region displayed (chr16:30,271,572-31,391,123; UCSC human genome build hg18) were chosen so as to include the three clusters of significant scores detected in East Asia by the selection tests in the 2 Mb region centered on VKORC1 (Figure 3). (A) Name and location of genes. Exons are displayed as blue boxes and the transcribed strand is indicated with an arrow. Genes located in the block of strong LD encompassing VKORC1 and including the SNPs in the red box shown in Figure 4C, are highlighted in the grey area. (B) XP-EHH results in East Asia. The significance of the XP-EHH scores (−log10 empirical p-value) are shown for individual SNPs with a MAF ≥0.01 in East Asia. Horizontal dashed lines indicate 0.05 and 0.01 chromosome-wide significance levels. Recombination hotspots detected in HapMap Phase II data are indicated by red vertical dotted lines. The data and methods used to derive these hotspots are available from the HapMap website (http://www.hapmap.org/) [83], [84]. (C) LD plot. Pairwise LD values, depicted as D’, are shown for SNPs with a MAF ≥0.01 in East Asia. D’ values are displayed in different colors from yellow to red for D’ = 0 to D’ = 1, respectively. The red box highlights SNPs included in the LD block encompassing VKORC1. The plot was produced using the snp.plotter R package [74].
Examination of the second cluster showed that VKORC1 is contained in a block of strong LD spanning ∼ 505 kb in East Asia (Figure 4 and Figure S5). Similar LD blocks were observed for Central South Asia and Europe, and to a lesser extent, for the Middle East (Figure S5). This LD block encompasses 25 genes (Figure 4). We used the most extreme FST, XP-CLR and XP-EHH scores in order to spatially localize a target of selection within the LD block. Significant XP-CLR scores (p<0.05) were found in a 350 kb region encompassing 19 genes including VKORC1 (Table S4). XP-EHH scores were almost all significant at the 0.05 threshold but four adjacent genes VKORC1, BCKDK, MYST1 (KAT8) and PRSS8 displayed most extreme XP-EHH scores (p<0.01). Clusters of highly significant FST values when comparing East Asia to the rest of the world (p<0.01) and significant global FST values (p<0.05) were also found for these four genes (Table S5). It is thus probable that the selective pressure has targeted one of these genes.
When did the -1639A VKORC1 Allele begin to Increase in East Asia?
The time at which the frequency of the -1639A allele started to increase in East Asia was estimated by using a maximum-likelihood method [31] with the 17 East Asian HGDP-CEPH sample data. Our analysis yielded an age estimate of 181 generations (95% CI: 128–256 generations). Assuming a generation time of 25 years, the expansion therefore occurred about 4,525 years ago (95% CI: 3,200–6,400 years).
Discussion
Numerous genes involved in absorption, distribution, metabolism and excretion (ADME) of drugs, exhibit evidence of recent positive selection and/or high population differentiation levels [32]. However, there are fewer examples of the action of natural selection on genes involved in the pharmacodynamics of drugs, such as VKORC1. Although numerous surveys have examined the genetic polymorphism of VKORC1 in samples from diverse ethnic origins [13], [20], [33], [34], [35], [36], [37], these studies provided an incomplete picture of haplotype diversity because different sets of SNPs were used and worldwide coverage was incomplete. In this study, we took advantage of the worldwide coverage of the HGDP-CEPH Panel to provide the first detailed analysis of VKORC1 population diversity using the same set of SNPs. Haplotype analysis revealed that the -1639A derived allele that confers AVK sensitivity is carried by a unique haplotype in all 52 population samples investigated. This haplotype associated with AVK sensitivity is predominant in East Asia, rare in Sub-Saharan Africa and occurs at intermediate frequencies in other geographic regions. Because it is found in Sub-Saharan Africa and other world populations, this haplotype is probably rather old. Its geographic distribution leads to striking differences between East Asian and non East Asian samples for genetic susceptibility to AVK sensitivity.
One explanation for worldwide diversity of this haplotype could be positive selection. This hypothesis was supported by five genome-wide scans that found atypical patterns of the allele frequency spectrum [38], extended LD [39], [40], and unusual genetic differentiation [40], [41], [42] in a 450 kb genomic region encompassing VKORC1. When specified, the target population was Asian [38], [40]. Ross et al. [14] found evidence of positive selection at VKORC1 in the East Asian HapMap sample, based on the level of genetic diversity (lnRH test [17]), genetic differentiation (LSBL test [16]) and allele frequency spectrum (Tajima’s D [18]).
In this study, we provided compelling evidence of positive selection at the VKORC1 gene locus in East Asia and only in this geographic region. A footprint of natural selection was found in each of the widely distributed 17 HGDP-CEPH East Asian population samples. By using four different tests of positive selection and by assessing significance at a given locus on the basis of an empirical distribution derived from the genomic background, we believe we can be confident that positive selection, rather than demographic forces, accounts for the data presented here. Indeed, it is well known that large allele frequency differences between populations are not infallible proofs of positive selection: these can also result from genetic drift, migration and other neutral demographic processes [43], [44]. This might be the explanation for the significant inter-regional FST values observed in Central South Asia (Table 1 and Figure 2B).
Because the XP-EHH test is designed to detect fixation events that are relatively young (∼ 30,000 years) [27], the selective event we have detected is likely to be rather recent. This is indeed supported by an age estimate of 4,525 years (95% CI: 3,200–6,400 years) for the time at which the VKORC1 -1639A allele started to increase in frequency in East Asia. The poor performance of the iHS test that detected only very few signals of positive selection in this study could have been predicted since its power to detect selective sweeps involving alleles near fixation is known to be low [28], [45]. By contrast, XP-EHH and XP-CLR perform better when the allele targeted by selection is near fixation and indeed showed strong evidence of a selective sweep in this study [24], [27].
In an attempt to determine if the VKORC1 gene has been the direct target of positive selection or if it reflects genetic hitchhiking [23], we extended our analysis to a 2 Mb region surrounding the VKORC1 gene (Figure 3). Apart from the highly significant footprint of positive selection localized in the VKORC1 region, two other significant signals, at ∼ 570 kb downstream and ∼ 230 kb upstream of VKORC1, were detected with XP-CLR and/or XP-EHH in East Asia. These two regions contains genes that belong to the same integrin family – specifically to the CD11 gene cluster: ITGAL downstream, and adjoining genes ITGAM and ITGAX upstream – involved in immune functions and being thus good candidates for positive selection [46], [47], [48]. However, since SNPs located in these integrin genes show limited LD with those of VKORC1, a single adaptive event is unlikely. Apart from East Asia, the ITGAL region showed signals of positive selection in other geographic regions (America with XP-CLR, and Sub-Saharan Africa and Oceania with XP-EHH), arguing for a different evolutionary history from that of VKORC1, which was only found in East Asia. This observation emphasizes the need for studying the geographic distribution of a selective event in a wide range of genetically diverse populations, as per Scheinfeldt et al. [49] who, after performing a detailed analysis of a 3 Mb region surrounding a gene showing strong footprints of positive selection, discovered patterns of genetic variation consistent with the presence of a cluster of three independent selective events occurring in different populations. By extending their analysis to the entire genome, they identified several other genomic regions exhibiting evidence for the presence of multiple and independent selective targets, suggesting that clusters of adaptive evolution, such as the one detected herein, are widespread in the human genome.
After delimitating the selective signal for VKORC1 by analyzing selective events identified in the 2 Mb region just described, we aimed at precisely mapping the gene targeted by positive selection. VKORC1 is located in a ∼ 505 kb LD block in East Asia containing 25 genes (Figure 4), and the selective pressure could have targeted any gene in this LD block. We used FST, XP-CLR and XP-EHH scores to spatially localize possible targets of positive selection within the LD region. A block of four adjacent genes –VKORC1, BCKDK, MYST1, and PRSS8– was found to be the most likely selective target (Table S4).
BCKDK codes for the mitochondrial branched chain ketoacid dehydrogenase kinase. MYST1 and PRSS8 are two immunity-related genes, listed as candidates for positive selection in several databases [40], [42], [50]. If, indeed, one of these three genes is the target of the selective sweep detected here, it should contain a functional variant of high frequency in East Asia and we did not find such a variant in HapMap data.
Assuming that selection has directly targeted the VKORC1 gene, the advantage would then probably be related to vitamin K metabolism,vitamin K being the only known substrate of VKORC1. This vitamin plays a crucial role in the synthesis of vitamin K-dependent (VKD) proteins, especially blood coagulation factors, which requires VKORC1 activity [51], [52]. Large geographic differences in dietary vitamin K intake, especially in vitamin K2, exist between human populations, with the highest plasma levels found in Asian populations, as compared to Europeans and Africans [53], [54]. These differences could be explained by the wide consumption of fermented soybean food (natto) - a major source of vitamin K2 - in East Asia [55], [56]. It is then possible that, at some points in the history of East Asian populations, these high levels of vitamin K intakecould have been deleterious and created a selective pressure against VKORC1 gene expression and coagulant activity. There is, however, no report so far of a deleterious effect associated with a high consumption of vitamin K and it is more the low dietary vitamin K intake that is problematic, hampering the adequate synthesis of VKD proteins in extrahepatic tissues notably bone and arterial vessels [57]. An alternative hypothesis could be that a naturally occurring environmental molecule of AVK type - such as a coumarin derivative - specifically found in East Asia, exerted a selective pressure on the VKORC1 gene in populations of this region during their recent history. Such molecules are present in the nature, as illustrated by the example of the sweet clover disease that affected cattle in Canada and North America in the 1920’s. Sweet clover hay, used to feed cattle, contains a natural coumarin that is oxidized in mouldy hay to form dicoumarol, a hemorrhagic agent. Its discovery led to the synthesis of coumarin derivatives used in clinical application as oral anticoagulants since the 1940’s [58], [59]. Evidence of an effect of warfarin in shaping VKORC1 genetic diversity could be found in rats and mices. Indeed, since the introduction in the 1950’s of this molecule as rodenticide, mutations in the VKORC1 gene conferring warfarin resistance have spread in rodent populations but the mechanisms by which they lead to warfarin resistance are still not elucidated [60], [61], [62], [63].
In conclusion, we found that the VKORC1 genomic region exhibits diversity patterns consistent with the action of positive selection in East Asia. Nearly complete selective sweeps, such as the one described herein, are believed to be rare in recent human adaptive history [64], [65], [66], [67]. This selective event is probably responsible for the spread of the derived -1639A allele conferring the increased AVK-sensitive phenotype in East Asian populations and contributes to present-day differences among human populations in the genetic sensitivity to AVK. A detailed analysis of the extended VKORC1 genomic region revealed selective signals at several independent genetic loci, indicating a complex evolutionary history for this chromosome 16 region. Our evolutionary analysis emphasizes the importance of considering the surrounding genomic region of a candidate gene for selection in order to avoid erroneous conclusions about the true target of selection. We show here that the gene targeted by selection could be either VKORC1 or another gene located in the 45 kb region covered by selective sweep detected in East Asia. Our ability to identify the target of selection may be limited by the number of genetic polymorphisms investigated. Examining the selective signal with more genetic variation using whole-genome sequences from the 1000 Genomes Project [68] may well improve the mapping of the gene targeted by selection. Furthermore, allele frequency spectrum bias tends to be minimized with whole genome sequences, which may allow the use of tests for natural selection based on this spectrum.
Materials and Methods
The HGDP-CEPH Panel
We used the HGDP-CEPH Panel that presently includes 1,064 individuals from 52 populations worldwide [69]. For the analysis presented here, the standardized subset panel H952 containing no first nor second degree relative pairs, was used [70]. This subpanel includes 952 individuals grouped into seven broad geographic regions as defined by Li et al. [15]: Sub-Saharan Africa (N = 105), the Middle East and Mozabites from north Africa (N = 163), Europe (N = 158), Central South Asia (N = 202), East Asia (N = 232), Oceania (N = 28) and America (N = 64). A full description of the 52 samples included in the HGDP-CEPH Panel is provided in Table S6.
SNP Genotyping
A total of 940 individuals from the original H952 subpanel were previously genotyped by Li et al. [15] with the Illumina HumanHap 650 K platform and their genotypes at 644,258 autosomal SNPs were downloaded from the public HGDP-CEPH database (http://www.cephb.fr/en/hgdp/). Only one SNP (rs7294) from this dataset is located in the VKORC1 gene. We additionally genotyped six SNPs in VKORC1 in the 940 individuals, using the TaqMan® SNP Genotyping Assay-by-Design method in 5 µl reaction volumes according to the manufacturer's protocol (Applied Biosystems, Foster City, CA): rs9923231 (g.-1639G>A) located in the promoter region, rs13336384 and rs9934438 in the first intron, rs2359612 and rs8050894 in the second intron, and rs7200749 in the third exon (Figure 1A). Missing genotype rates varied from 0.5% to 2.2% for SNPs rs7200749 and rs9934438, respectively. Since the two SNPs rs9923231 and rs9934438 were found in complete LD in the seven geographic regions (Figure S2), we were able to impute the missing genotypes of a given SNP using available information from the other, leading to a total of 0.96% missing genotypes for these two SNPs. No significant deviations from the Hardy-Weinberg proportions were observed for any VKORC1 SNP in any of the 52 population samples at the 0.01 significance level (data not shown). Allele frequency distributions of the seven VKORC1 SNPs in the 52 population samples are shown in Figure S6.
Statistical Analysis
VKORC1 haplotype study
To investigate the worldwide diversity of the VKORC1 gene, we conducted a haplotype study using the seven genotyped SNPs. A total of 931 individuals with less than three missing genotypes were included in the haplotype reconstruction. For each geographic region, haplotype frequencies were estimated with the Bayesian statistical method implemented in Phase v2.1 [71] using defaults parameters. To avoid the convergence of the algorithm to a local maximum, we ran it 10 times with different random seeds and kept the output from the run with the best average value. The worldwide haplotype frequencies were then calculated as the weighted average of the frequencies estimated in each of the seven geographic regions. Similar results were obtained when a single pooled sample of all individuals was considered in the haplotype frequency estimation (data not shown). Since information on ancestral allele state is required to distinguish between ancestral and derived haplotypes, we used the snp131OrthoPt2Pa2Rm2.txt file downloaded from the UCSC genome browser (http://genome.ucsc.edu/) which provides the orthologous alleles in chimpanzee, orangutan and rhesus macaque. For each SNP, the allele shared by the three species was identified as the ancestral allele. Haplotype networks were drawn with the Network v4.5.1.6 software (http://www.fluxus-engineering.com/), using the median-joining algorithm which builds the minimum spanning network from the given haplotypes by favoring short connections [72]. LD analyses were performed with Haploview v4.1 [73] and the snp.plotter R package [74], using Lewontin’s disequilibrium coefficient D’ [75] and the correlation coefficient r 2 [76].
Detection of Signatures of Positive Selection
To explore whether VKORC1 has evolved under positive selection in humans, we looked for two distinct genetic patterns of a selective sweep that are expected to remain detectable in the genome over different time scales after the action of natural selection: (i) an important genetic differentiation among populations nearby the locus of interest, and (ii) the presence of unusually frequent and long haplotypes in the surrounding genomic region. For each method, we used an outlier approach to calculate the p-values of the computed scores. Under this approach, an empirical distribution is constructed using other SNPs in the genome that are assumed to be neutral and to represent the genomic background under neutrality. An empirical p-value is computed that corresponds to the proportion of values from the empirical distribution that are higher than the value observed at the locus of interest. If the value obtained for the SNP of interest is greater than the 95th percentile (p<0.05) of the empirical distribution, positive selection is invoked. For that purpose, we used the empirical distributions obtained from the scores calculated either on a genome-wide (all autosomal chromosomes) or chromosome-wide (chromosome 16, where VKORC1 is located) basis.
First, we used two statistics, FST and XP-CLR, which measure the genetic differentiation among human populations [24], [26]. These methods are able to detect selective sweeps that have occurred up to 75,000 years ago [77]. The fixation index FST [78] quantifies the proportion of genetic variance explained by allele frequency differences among populations. FST ranges from 0 (for genetically identical populations) to 1 (for completely differentiated populations). We calculated FST values using the BioPerl module PopGen [79] for each autosomal SNP with a minor allele frequency (MAF) ≥10−3 (644,143 SNPs) at three different levels: (i) global level (either among the seven HGDP-CEPH Panel geographic regions or among the 52 Panel populations), (ii) inter-regional level (each geographic region versus the remaining ones), and (iii) intra-regional level (among populations within a region). Since FST strongly correlates with heterozygosity [41], [80], [81], empirical p-values were calculated within bins of 10,000 SNPs grouped according to MAF. The resulting distributions represent the average genetic differentiation of human populations corrected for heterozygosity.
We next applied the XP-CLR test [24] which identifies selective sweeps in a population by detecting significant genetic differentiation in an extended genomic region of interest as compared to a reference population. This method presents both the advantages of being robust to ascertainment bias and of not requiring any information on haplotypes, thus avoiding errors of haplotype estimation from genotype data. XP-CLR scores were computed at regularly spaced grid points (every 4 kb) across chromosome 16 using the genotypes from SNPs within overlapping windows of 0.1 cM around each grid point. To account for different SNP densities among genomic regions, we restricted to 200 the maximal number of SNPs used to compute a XP-CLR score within the 0.1 cM genomic region, by removing excess SNPs at random. We applied this method by considering all SNPs with a MAF ≥10−3 on chromosome 16 at both the regional and population levels (17,729 SNPs). P-values were calculated from the empirical distribution of the collected scores obtained with these SNPs. XP-CLR requires the definition of a reference population: the Sub-Saharan African samples were used as a reference for non Sub-Saharan African regions, and the European samples as a reference for Sub-Saharan Africa. For the analyses performed at the population level, we defined the Yoruba as the reference for non Sub-Saharan African samples, and the French for Sub-Saharan African samples.
The second class of methods that we used is based on EHH, i.e. the sharing of identical alleles across relatively long distances by most haplotypes in population samples [25]. In brief, the EHH is computed for a given SNP (the core SNP) of a sequence being interrogated for a selective sweep. In the absence of a selective sweep, recombination events break down haplotypes relatively rapidly with time and with increasing distance from the core SNP. In the case of a selective sweep, LD tends to maintain the haplotype carrying the selected allele, and the relative frequency of this (favored) haplotype will increase with time leading to so-called EHH. Integration of genetic distance in both directions from the core SNP can be used to discriminate between selected and non-selected alleles, and be applied to ancestral and derived alleles. Analytic methods based on EHH are able to detect recent selective sweeps (i.e. those occurring less than 30,000 years ago [77]). Such analyses require haplotype data. We used fastPHASE v1.3.0 EM algorithm [82] to infer haplotypes with chromosome 16 SNPs for individuals from each geographic region. For each region, the K-selection procedure was first run several times in order to define the optimal number of clusters of similar haplotypes by minimizing chance error rates. Ultimately, phase was determined with K = 6 for Oceania, K = 14 for Europe and Central-South Asia and K = 12 for the remaining regions. Using these values, the EM algorithm was then run with 20 random starts and 25 iterations.
Once haplotypes were reconstructed, we computed the XP-EHH statistic [27] that compares the integrated EHH computed in a test population versus that of a reference population. Therefore, this method detects a sweep in which the selected allele has risen to near fixation in one population but remains polymorphic in the other. XP-EHH scores were computed using the same parameters as those described in Sabeti et al. (2007). Reference populations were defined as for XP-CLR.
We finally applied the iHS [28] that compares the rate of EHH decay observed for both the derived and ancestral allele at the core SNP. An extremely positive or negative value at the core SNP provides evidence of positive selection with unusually long haplotypes carrying the ancestral or the derived allele, respectively. The raw iHS scores were computed using the iHS option implemented in the WHAMM software developed by Voight et al. (2006). The scores were standardized to have null mean and unit variance in 5% bins of the derived allele frequency at the core SNP. Information on ancestral allele state was obtained from the snp131OrthoPt2Pa2Rm2.txt file downloaded from the UCSC website. We were unable to determine with certainty the ancestral allele status of 111 SNPs on chromosome 16 and we removed them from the analysis.
XP-EHH and iHS scores were calculated for all available SNPs on chromosome 16 (19,733 and 19,622, respectively) at both the regional and population levels. The resulting distributions were used to calculate empirical p-values.
The genetic map used for applying XP-CLR, XP-EHH and iHS was retrieved from release 22, build 36 of HapMap (www.hapmap.org).
Age of the Expansion of the -1639A VKORC1 Allele in East Asia
We inferred the age at which the -1639A allele started to increase in frequency in East Asia by estimating the age of the most recent common ancestor carrying this allele in East Asia using the likelihood-based method implemented in the Estiage program [31]. This method assumes that all individuals derive from a common ancestor who introduced the mutation n generations ago. Estimation of n is based on the length of the haplotype shared by the individuals, which is estimated through the identification of recombination events on the ancestral haplotype by taking into account allele frequencies and recombination rates. We estimated n using only one haplotype per East Asian population sample (i.e., 17 haplotypes). For each population, this one haplotype was constructed by taking at each locus over a 6 Mb region the allele the most frequently seen in individuals from the population carrying the -1639A allele. A mutation rate of 10−6 per individual and per generation, and a 25-year generation time were assumed.
Supporting Information
Distribution of VKORC1 haplotypes in the 52 HGDP-CEPH samples. The haplotype carrying the -1639A allele (H1) is represented in red and the ancestral haplotype (H6) in black.
(TIF)
Pairwise LD between the seven VKORC1 SNPs at the regional and global level. Red squares indicate statistically significant (logarithm of odds >2) LD between the pair of SNPs, as measured by the D’ statistic [75] with the Haploview software [73]; darker colors of red indicate higher values of D’, up to a maximum of 1. White squares indicate pairwise D’ values of <1 with no statistically significant evidence of LD. Blue squares indicate pairwise D’ values of 1 but without statistical significance.
(TIF)
Genome-wide empirical distributions of inter-regional FST values against MAF in the seven geographic regions. Empirical distributions of FST were constructed by calculating an FST value for 644,413 SNPs having a MAF ≥0.001 at the global level. Individual values of FST calculated for each of the seven VKORC1 SNPs are plotted against their global MAF. The functional rs9923231 SNP is shown in red. The 50th, 95th and 99th percentiles are indicated as dotted, dashed and full red lines, respectively.
(TIFF)
Genome-wide empirical distributions of intra-regional FST values against MAF in the seven geographic regions. Empirical distributions of FST were constructed by calculating an FST value for all SNPs having a MAF ≥0.001 at the intra-regional level. Individual values of FST calculated for each of the seven VKORC1 SNPs are plotted against the regional MAF. The functional rs9923231 SNP is shown in red. The 50th, 95th and 99th percentiles are indicated as dotted, dashed and full red lines, respectively.
(TIFF)
LD patterns over a 2 Mb region centered on VKORC1 in the seven geographic regions. Pairwise LD, depicted as D’, is shown for SNPs with a MAF ≥0.05 at the global level. D’ values are displayed in different colors from yellow to red for D’ = 0 to D’ = 1, respectively. The plot was produced using the snp.plotter R package [74]. The vertical dashed lines delineate VKORC1 gene position.
(TIF)
Allele frequency distribution of the seven VKORC1 SNPs in the 52 HGDP-CEPH samples: rs9923231 (A), rs13336384, (B) rs9934438 (C), rs8050894 (D), rs2359612 (E), rs7200749 (F) and rs7294 (G). The derived and ancestral alleles are represented in orange and blue, respectively.
(TIF)
Global FST values among populations and among regions for the seven VKORC1 SNPs.
(XLS)
Results of the XP-CLR test in a 16 kb region centered on VKORC1 in the 52 HGDP-CEPH samples.
(XLS)
Results of the XP-EHH and iHS tests in the 52 HGDP-CEPH samples.
(XLS)
Results of the XP-CLR test in the ∼ 500 kb genomic region of the LD block encompassing VKORC1 in East Asia.
(XLS)
Results of the XP-EHH, iHS tests, inter-regional FST and global FST for all SNPs located in the linkage disequilibrium block encompassing VKORC1 in East Asia.
(XLS)
Description of the 52 HGDP-CEPH samples grouped into seven main geographic regions.
(XLS)
Acknowledgments
PL thanks Txema Heredia, Angel Carreno and Jordi Rambla for computational support. BP thanks Steven Gazal for his valuable assistance in writing scripts, Quentin Vincent for his advices on the datation protocol, and Marie-Claude Babron and Remi Kazma for their helpful comments on the manuscript.
Funding Statement
This work was supported by the Spanish National Institute for Bioinformatics (www.inab.org). PL is supported by a PhD fellowship from “Acción Estratégica de Salud, en el Marco del Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica 2008–2011” from Instituto de Salud Carlos III. BP is supported by a PhD fellowship from the doctoral program in Public Health (ED420: www.ed-sante-publique.u-psud.fr) from Paris Sud University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hirsh J, Dalen JE, Anderson DR, Poller L, Bussey H, et al. (1998) Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 114: 445S–469S. [DOI] [PubMed] [Google Scholar]
- 2. Hyers TM, Agnelli G, Hull RD, Weg JG, Morris TA, et al. (1998) Antithrombotic therapy for venous thromboembolic disease. Chest 114: 561S–578S. [DOI] [PubMed] [Google Scholar]
- 3. D'Andrea G, D'Ambrosio R, Margaglione M (2008) Oral anticoagulants: Pharmacogenetics Relationship between genetic and non-genetic factors. Blood Rev 22: 127–140. [DOI] [PubMed] [Google Scholar]
- 4. Cooper GM, Johnson JA, Langaee TY, Feng H, Stanaway IB, et al. (2008) A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood 112: 1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, et al. (2009) A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet 5: e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teichert M, Eijgelsheim M, Rivadeneira F, Uitterlinden AG, van Schaik RH, et al. (2009) A genome-wide association study of acenocoumarol maintenance dosage. Hum Mol Genet 18: 3758–3768. [DOI] [PubMed] [Google Scholar]
- 7. Cha PC, Mushiroda T, Takahashi A, Kubo M, Minami S, et al. (2010) Genome-wide association study identifies genetic determinants of warfarin responsiveness for Japanese. Hum Mol Genet 19: 4735–4744. [DOI] [PubMed] [Google Scholar]
- 8. Goldstein JA, de Morais SM (1994) Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics 4: 285–299. [DOI] [PubMed] [Google Scholar]
- 9. Stec DE, Roman RJ, Flasch A, Rieder MJ (2007) Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics 30: 74–81. [DOI] [PubMed] [Google Scholar]
- 10. Bardowell SA, Stec DE, Parker RS (2010) Common variants of cytochrome P450 4F2 exhibit altered vitamin E-{omega}-hydroxylase specific activity. J Nutr 140: 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, et al. (2004) Identification of the gene for vitamin K epoxide reductase. Nature 427: 541–544. [DOI] [PubMed] [Google Scholar]
- 12. Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hortnagel K, et al. (2004) Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427: 537–541. [DOI] [PubMed] [Google Scholar]
- 13. Limdi NA, Wadelius M, Cavallari L, Eriksson N, Crawford DC, et al. (2010) Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood 115: 3827–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ross KA, Bigham AW, Edwards M, Gozdzik A, Suarez-Kurtz G, et al. (2010) Worldwide allele frequency distribution of four polymorphisms associated with warfarin dose requirements. J Hum Genet 55: 582–589. [DOI] [PubMed] [Google Scholar]
- 15. Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, et al. (2008) Worldwide human relationships inferred from genome-wide patterns of variation. Science 319: 1100–1104. [DOI] [PubMed] [Google Scholar]
- 16. Shriver MD, Kennedy GC, Parra EJ, Lawson HA, Sonpar V, et al. (2004) The genomic distribution of population substructure in four populations using 8,525 autosomal SNPs. Hum Genomics 1: 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Storz JF, Payseur BA, Nachman MW (2004) Genome scans of DNA variability in humans reveal evidence for selective sweeps outside of Africa. Mol Biol Evol 21: 1800–1811. [DOI] [PubMed] [Google Scholar]
- 18. Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, et al. (2007) A second generation human haplotype map of over 3.1 million SNPs. Nature 449: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, et al. (2005) Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 352: 2285–2293. [DOI] [PubMed] [Google Scholar]
- 21. Wu AH, Wang P, Smith A, Haller C, Drake K, et al. (2008) Dosing algorithm for warfarin using CYP2C9 and VKORC1 genotyping from a multi-ethnic population: comparison with other equations. Pharmacogenomics 9: 169–178. [DOI] [PubMed] [Google Scholar]
- 22. Yang L, Ge W, Yu F, Zhu H (2010) Impact of VKORC1 gene polymorphism on interindividual and interethnic warfarin dosage requirement–a systematic review and meta analysis. Thromb Res 125: e159–166. [DOI] [PubMed] [Google Scholar]
- 23. Smith JM, Haigh J (1974) The hitch-hiking effect of a favourable gene. Genet Res 23: 23–35. [PubMed] [Google Scholar]
- 24. Chen H, Patterson N, Reich D (2010) Population differentiation as a test for selective sweeps. Genome Res 20: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sabeti PC, Reich DE, Higgins JM, Levine HZ, Richter DJ, et al. (2002) Detecting recent positive selection in the human genome from haplotype structure. Nature 419: 832–837. [DOI] [PubMed] [Google Scholar]
- 26. Weir BS, Hill WG (2002) Estimating F-statistics. Annu Rev Genet 36: 721–750. [DOI] [PubMed] [Google Scholar]
- 27. Sabeti PC, Varilly P, Fry B, Lohmueller J, Hostetter E, et al. (2007) Genome-wide detection and characterization of positive selection in human populations. Nature 449: 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voight BF, Kudaravalli S, Wen X, Pritchard JK (2006) A map of recent positive selection in the human genome. PLoS Biol 4: e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clark AG, Hubisz MJ, Bustamante CD, Williamson SH, Nielsen R (2005) Ascertainment bias in studies of human genome-wide polymorphism. Genome Res 15: 1496–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cann HM, de Toma C, Cazes L, Legrand MF, Morel V, et al. (2002) A human genome diversity cell line panel. Science 296: 261–262. [DOI] [PubMed] [Google Scholar]
- 31. Genin E, Tullio-Pelet A, Begeot F, Lyonnet S, Abel L (2004) Estimating the age of rare disease mutations: the example of Triple-A syndrome. J Med Genet 41: 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li J, Zhang L, Zhou H, Stoneking M, Tang K (2011) Global patterns of genetic diversity and signals of natural selection for human ADME genes. Hum Mol Genet 20: 528–540. [DOI] [PubMed] [Google Scholar]
- 33. Marsh S, King CR, Porche-Sorbet RM, Scott-Horton TJ, Eby CS (2006) Population variation in VKORC1 haplotype structure. J Thromb Haemost 4: 473–474. [DOI] [PubMed] [Google Scholar]
- 34. Limdi NA, Beasley TM, Crowley MR, Goldstein JA, Rieder MJ, et al. (2008) VKORC1 polymorphisms, haplotypes and haplotype groups on warfarin dose among African-Americans and European-Americans. Pharmacogenomics 9: 1445–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, et al. (2008) Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med 358: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Geisen C, Watzka M, Sittinger K, Steffens M, Daugela L, et al. (2005) VKORC1 haplotypes and their impact on the inter-individual and inter-ethnical variability of oral anticoagulation. Thromb Haemost 94: 773–779. [DOI] [PubMed] [Google Scholar]
- 37. Bodin L, Verstuyft C, Tregouet DA, Robert A, Dubert L, et al. (2005) Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood 106: 135–140. [DOI] [PubMed] [Google Scholar]
- 38. Carlson CS, Thomas DJ, Eberle MA, Swanson JE, Livingston RJ, et al. (2005) Genomic regions exhibiting positive selection identified from dense genotype data. Genome Res 15: 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang ET, Kodama G, Baldi P, Moyzis RK (2006) Global landscape of recent inferred Darwinian selection for Homo sapiens. Proc Natl Acad Sci U S A 103: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Teo YY, Sim X, Ong RT, Tan AK, Chen J, et al. (2009) Singapore Genome Variation Project: a haplotype map of three Southeast Asian populations. Genome Res 19: 2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barreiro LB, Laval G, Quach H, Patin E, Quintana-Murci L (2008) Natural selection has driven population differentiation in modern humans. Nat Genet 40: 340–345. [DOI] [PubMed] [Google Scholar]
- 42. Akey JM (2009) Constructing genomic maps of positive selection in humans: where do we go from here? Genome Res 19: 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xue Y, Zhang X, Huang N, Daly A, Gillson CJ, et al. (2009) Population differentiation as an indicator of recent positive selection in humans: an empirical evaluation. Genetics 183: 1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hofer T, Ray N, Wegmann D, Excoffier L (2009) Large allele frequency differences between human continental groups are more likely to have occurred by drift during range expansions than by selection. Ann Hum Genet 73: 95–108. [DOI] [PubMed] [Google Scholar]
- 45. Pickrell JK, Coop G, Novembre J, Kudaravalli S, Li JZ, et al. (2009) Signals of recent positive selection in a worldwide sample of human populations. Genome Res 19: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nath SK, Han S, Kim-Howard X, Kelly JA, Viswanathan P, et al. (2008) A nonsynonymous functional variant in integrin-alpha(M) (encoded by ITGAM) is associated with systemic lupus erythematosus. Nat Genet 40: 152–154. [DOI] [PubMed] [Google Scholar]
- 47. Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, et al. (2008) Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med 358: 900–909. [DOI] [PubMed] [Google Scholar]
- 48. Jarvinen TM, Hellquist A, Koskenmies S, Einarsdottir E, Panelius J, et al. (2010) Polymorphisms of the ITGAM gene confer higher risk of discoid cutaneous than of systemic lupus erythematosus. PLoS One 5: e14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scheinfeldt LB, Biswas S, Madeoy J, Connelly CF, Akey JM (2011) Clusters of adaptive evolution in the human genome. Front Genet 2: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barreiro LB, Quintana-Murci L (2010) From evolutionary genetics to human immunology: how selection shapes host defence genes. Nat Rev Genet 11: 17–30. [DOI] [PubMed] [Google Scholar]
- 51. Suttie JW (1985) Vitamin K-dependent carboxylase. Annu Rev Biochem 54: 459–477. [DOI] [PubMed] [Google Scholar]
- 52. Oldenburg J, Marinova M, Muller-Reible C, Watzka M (2008) The vitamin K cycle. Vitam Horm 78: 35–62. [DOI] [PubMed] [Google Scholar]
- 53. Yan L, Zhou B, Greenberg D, Wang L, Nigdikar S, et al. (2004) Vitamin K status of older individuals in northern China is superior to that of older individuals in the UK. Br J Nutr 92: 939–945. [DOI] [PubMed] [Google Scholar]
- 54. Beavan SR, Prentice A, Stirling DM, Dibba B, Yan L, et al. (2005) Ethnic differences in osteocalcin gamma-carboxylation, plasma phylloquinone (vitamin K1) and apolipoprotein E genotype. Eur J Clin Nutr 59: 72–81. [DOI] [PubMed] [Google Scholar]
- 55. Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, et al. (2001) Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition 17: 315–321. [DOI] [PubMed] [Google Scholar]
- 56.Fujita Y, Iki M, Tamaki J, Kouda K, Yura A, et al. (2011) Association between vitamin K intake from fermented soybeans, natto, and bone mineral density in elderly Japanese men: the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) study. Osteoporos Int. [DOI] [PubMed]
- 57. Vermeer C, Shearer MJ, Zittermann A, Bolton-Smith C, Szulc P, et al. (2004) Beyond deficiency: potential benefits of increased intakes of vitamin K for bone and vascular health. Eur J Nutr 43: 325–335. [DOI] [PubMed] [Google Scholar]
- 58. Wardrop D, Keeling D (2008) The story of the discovery of heparin and warfarin. Br J Haematol 141: 757–763. [DOI] [PubMed] [Google Scholar]
- 59. Mueller RL, Scheidt S (1994) History of drugs for thrombotic disease. Discovery, development, and directions for the future. Circulation 89: 432–449. [DOI] [PubMed] [Google Scholar]
- 60. Kohn MH, Pelz HJ, Wayne RK (2000) Natural selection mapping of the warfarin-resistance gene. Proc Natl Acad Sci U S A 97: 7911–7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kohn MH, Pelz HJ, Wayne RK (2003) Locus-specific genetic differentiation at Rw among warfarin-resistant rat (Rattus norvegicus) populations. Genetics 164: 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Diaz JC, Song Y, Moore A, Borchert JN, Kohn MH (2010) Analysis of vkorc1 polymorphisms in Norway rats using the roof rat as outgroup. BMC Genet 11: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Song Y, Endepols S, Klemann N, Richter D, Matuschka FR, et al. (2011) Adaptive introgression of anticoagulant rodent poison resistance by hybridization between old world mice. Curr Biol 21: 1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hernandez RD, Kelley JL, Elyashiv E, Melton SC, Auton A, et al. (2011) Classic selective sweeps were rare in recent human evolution. Science 331: 920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pritchard JK, Di Rienzo A (2010) Adaptation - not by sweeps alone. Nat Rev Genet 11: 665–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pritchard JK, Pickrell JK, Coop G (2010) The genetics of human adaptation: hard sweeps, soft sweeps, and polygenic adaptation. Curr Biol 20: R208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Coop G, Pickrell JK, Novembre J, Kudaravalli S, Li J, et al. (2009) The role of geography in human adaptation. PLoS Genet 5: e1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Consortium TGP (2010) A map of human genome variation from population-scale sequencing. Nature 467: 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cann HM (1998) Human genome diversity. C R Acad Sci III 321: 443–446. [DOI] [PubMed] [Google Scholar]
- 70. Rosenberg NA (2006) Standardized subsets of the HGDP-CEPH Human Genome Diversity Cell Line Panel, accounting for atypical and duplicated samples and pairs of close relatives. Ann Hum Genet 70: 841–847. [DOI] [PubMed] [Google Scholar]
- 71. Stephens M, Donnelly P (2003) A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet 73: 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bandelt HJ, Forster P, Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16: 37–48. [DOI] [PubMed] [Google Scholar]
- 73. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 74. Luna A, Nicodemus KK (2007) snp.plotter: an R-based SNP/haplotype association and linkage disequilibrium plotting package. Bioinformatics 23: 774–776. [DOI] [PubMed] [Google Scholar]
- 75. Lewontin RC (1964) The Interaction of Selection and Linkage. I. General Considerations; Heterotic Models. Genetics 49: 49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hill WG, Robertson A (1968) Linkage disequilibrium in finite populations Theoretical and Applied Genetics. 38: 226–231. [DOI] [PubMed] [Google Scholar]
- 77. Sabeti PC, Schaffner SF, Fry B, Lohmueller J, Varilly P, et al. (2006) Positive natural selection in the human lineage. Science 312: 1614–1620. [DOI] [PubMed] [Google Scholar]
- 78.Wright S (1951) The genetical structure of populations. Annals of Eugenics 15 323–354. [DOI] [PubMed]
- 79. Stajich JE, Block D, Boulez K, Brenner SE, Chervitz SA, et al. (2002) The Bioperl toolkit: Perl modules for the life sciences. Genome Res 12: 1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Beaumont MA, Nichols RA (1996) Evaluating loci for use in the genetic analysis of population structure. The Royal Society 263: 1619–1626. [Google Scholar]
- 81. Gardner M, Bertranpetit J, Comas D (2008) Worldwide genetic variation in dopamine and serotonin pathway genes: implications for association studies. Am J Med Genet B Neuropsychiatr Genet 147B: 1070–1075. [DOI] [PubMed] [Google Scholar]
- 82. Scheet P, Stephens M (2006) A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. Am J Hum Genet 78: 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. McVean GA, Myers SR, Hunt S, Deloukas P, Bentley DR, et al. (2004) The fine-scale structure of recombination rate variation in the human genome. Science 304: 581–584. [DOI] [PubMed] [Google Scholar]
- 84. Winckler W, Myers SR, Richter DJ, Onofrio RC, McDonald GJ, et al. (2005) Comparison of fine-scale recombination rates in humans and chimpanzees. Science 308: 107–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of VKORC1 haplotypes in the 52 HGDP-CEPH samples. The haplotype carrying the -1639A allele (H1) is represented in red and the ancestral haplotype (H6) in black.
(TIF)
Pairwise LD between the seven VKORC1 SNPs at the regional and global level. Red squares indicate statistically significant (logarithm of odds >2) LD between the pair of SNPs, as measured by the D’ statistic [75] with the Haploview software [73]; darker colors of red indicate higher values of D’, up to a maximum of 1. White squares indicate pairwise D’ values of <1 with no statistically significant evidence of LD. Blue squares indicate pairwise D’ values of 1 but without statistical significance.
(TIF)
Genome-wide empirical distributions of inter-regional FST values against MAF in the seven geographic regions. Empirical distributions of FST were constructed by calculating an FST value for 644,413 SNPs having a MAF ≥0.001 at the global level. Individual values of FST calculated for each of the seven VKORC1 SNPs are plotted against their global MAF. The functional rs9923231 SNP is shown in red. The 50th, 95th and 99th percentiles are indicated as dotted, dashed and full red lines, respectively.
(TIFF)
Genome-wide empirical distributions of intra-regional FST values against MAF in the seven geographic regions. Empirical distributions of FST were constructed by calculating an FST value for all SNPs having a MAF ≥0.001 at the intra-regional level. Individual values of FST calculated for each of the seven VKORC1 SNPs are plotted against the regional MAF. The functional rs9923231 SNP is shown in red. The 50th, 95th and 99th percentiles are indicated as dotted, dashed and full red lines, respectively.
(TIFF)
LD patterns over a 2 Mb region centered on VKORC1 in the seven geographic regions. Pairwise LD, depicted as D’, is shown for SNPs with a MAF ≥0.05 at the global level. D’ values are displayed in different colors from yellow to red for D’ = 0 to D’ = 1, respectively. The plot was produced using the snp.plotter R package [74]. The vertical dashed lines delineate VKORC1 gene position.
(TIF)
Allele frequency distribution of the seven VKORC1 SNPs in the 52 HGDP-CEPH samples: rs9923231 (A), rs13336384, (B) rs9934438 (C), rs8050894 (D), rs2359612 (E), rs7200749 (F) and rs7294 (G). The derived and ancestral alleles are represented in orange and blue, respectively.
(TIF)
Global FST values among populations and among regions for the seven VKORC1 SNPs.
(XLS)
Results of the XP-CLR test in a 16 kb region centered on VKORC1 in the 52 HGDP-CEPH samples.
(XLS)
Results of the XP-EHH and iHS tests in the 52 HGDP-CEPH samples.
(XLS)
Results of the XP-CLR test in the ∼ 500 kb genomic region of the LD block encompassing VKORC1 in East Asia.
(XLS)
Results of the XP-EHH, iHS tests, inter-regional FST and global FST for all SNPs located in the linkage disequilibrium block encompassing VKORC1 in East Asia.
(XLS)
Description of the 52 HGDP-CEPH samples grouped into seven main geographic regions.
(XLS)