Abstract
CCK is hypothesized to inhibit meal size by acting at CCK1 receptors (CCK1R) on vagal afferent neurons that innervate the gastrointestinal tract and project to the hindbrain. Earlier studies have shown that obese Otsuka Long-Evans Tokushima Fatty (OLETF) rats, which carry a spontaneous null mutation of the CCK1R, are hyperphagic and obese. Recent findings show that rats with CCK1R-null gene on a Fischer 344 background (Cck1r−/−) are lean and normophagic. In this study, the metabolic phenotype of this rat strain was further characterized. As expected, the CCK1R antagonist, devazepide, failed to stimulate food intake in the Cck1r−/− rats. Both Cck1r+/+ and Cck1r−/− rats became diet-induced obese (DIO) when maintained on a high-fat diet relative to chow-fed controls. Cck1r−/− rats consumed larger meals than controls during the dark cycle and smaller meals during the light cycle. These effects were accompanied by increased food intake, total spontaneous activity, and energy expenditure during the dark cycle and an apparent reduction in respiratory quotient during the light cycle. To assess whether enhanced responsiveness to anorexigenic factors may contribute to the lean phenotype, we examined the effects of melanotan II (MTII) on food intake and body weight. We found an enhanced effect of MTII in Cck1r−/− rats to suppress food intake and body weight following both central and peripheral administration. These results suggest that the lean phenotype is potentially driven by increases in total spontaneous activity and energy expenditure.
Keywords: satiety, meal size, cholecystokinin, CCK1 receptors
existing evidence supports the hypothesis that the postprandial satiety signal, cholecystokinin (CCK), inhibits food intake through stimulation of CCK1 receptors (CCK1R) (23, 30, 37, 45) and activation of hindbrain neurons (8, 12, 14, 17, 31, 41, 46, 50) with the net result being a reduction in meal size (54). Characterization studies with selective CCK1R agonists and antagonists have provided evidence in support of this receptor subtype mediating the effects of exogenous and endogenous CCK to inhibit food intake. CCK-8, which binds with nearly equal affinity to both CCK1R and CCK2R (19), fails to inhibit food intake and induce c-Fos (marker of neuronal activation) in the hindbrain following pretreatment with selective CCK1R antagonists (37, 44), whereas selective CCK2R antagonists are ineffective (37). Importantly, CCK1R antagonist administration stimulates food intake (37, 44), through a specific increase in meal size (36), thereby providing compelling support for a physiological role of endogenous CCK action at CCK1R in the regulation of food intake. More recently, CCK1R ablation (7, 9, 18, 25, 26, 30, 35) in both rats and mice, as well as replenishment of CCK1R into specific brain sites (56) in rats have confirmed the importance of CCK1R in the regulation of food intake, but questions remain as to whether CCK1Rs play a universal role in the regulation of body weight across species.
The role of CCK1R signaling in the regulation of food intake and body weight across species and strains is not fully understood. Recent studies have identified the CCK1R gene to a genetic region that is linked to obesity in humans (1) and mutations in the CCK1R (27, 28) or CCK gene (15) are likewise associated with obesity in humans. In contrast to these findings in humans, there are differing phenotypes in animal models that lack CCK1R, including the obese Otsuka Long-Evans Tokushima Fatty (OLETF) rats (30, 38), lean CCK1R-deficient mice (CCK1R−/−) (23), lean CCK-deficient mice (CCK−/−) (26), and the lean CCK1R-deficient Fischer 344 rat (Cck1r−/−) recently described in our laboratory (9). The obese OLETF rat that lacks CCK1R (18) is also associated with hyperphagia relative to their wild-type controls, the Long-Evans Tokushima Otsuka (LETO) rats (30, 38). Similar to the OLETF rat (38), the CCK1r−/− mice also show a failure in the ability of CCK-8 to reduce food intake (23). In contrast to OLETF rats, they have increased meal size evident only during the dark period (7), with no change in total daily food intake (7, 23). They also do not develop obesity when maintained on regular chow (7, 23). The more recently characterized CCK−/− mice are also lean and normophagic (29). While they show increased food intake during the light period, these effects appear to be offset by reduced food intake during the dark period (26). The Cck1r−/− (33, 34) rat model characterized by our laboratory and used in the present study is lean, fails to respond to CCK-8 (9), and has normal total daily food intake compared with controls (9). It is unclear to what extent this lean phenotype can be fully explained by offsetting effects on meal size. It is clear that multiple factors (33, 34, 40, 49, 51, 52), in addition to changes in food intake, account for the different behavioral and metabolic phenotypes attributed to loss of CCK function across species and strains.
While the obesity in the adult OLETF rat is prevented by both pair-feeding (4) and increased energy expenditure in the form of running-wheel activity (6), increases in energy expenditure and/or activity may also contribute to the lean phenotype evident in the CCK1R−/− mice, CCK−/− mice, and the Cck1r−/− rat model. Although there was no increase in overall energy expenditure in CCK−/− or CCK1R−/− mice (29) relative to wild-type counterparts, there were increases in energy expenditure at various times throughout the light and dark cycles (25) in CCK−/− mice. One of the unanswered questions is whether the lean phenotype of the Cck1r−/− rats may be attributed to increases in energy expenditure and/or spontaneous physical activity.
Enhanced sensitivity to the anorexigenic signal, the melanocortin 3/4 receptor (MC3/MC4R) agonist, melanotan II (MTII), is evident in the OLETF animal model (5), which is also associated with changes in MC4R expression levels in the CNS (24). Despite the increased MC3/MC4R signaling in these animals, they remain hyperphagic and obese, in part, because of overexpression of the orexigenic neuropeptide Y in the dorsomedial hypothalamus (DMH) (4). The extent to which increased MC3/MC4R signaling may contribute to the suppression of food intake and lean phenotype in our Cck1r−/− rat model has not been determined. One of the goals, therefore, of the present study was to determine whether there is also enhanced effectiveness to MTII in our model, which may provide a plausible mechanism to explain the lean phenotype.
We predicted that Cck1r−/− rats, relative to their Fischer 344 wild-type counterparts (Cck1r+/+), would show offsetting effects on meal size with respect to photoperiod, increased energy expenditure, and enhanced responsiveness to MC3/MC4R stimulation. The recent findings in the OLETF rats (3) and CCK1R−/− mice (3) led us to predict that both Cck1r−/− and Cck1r+/+ would show increased weight gain when chronically maintained on a high-fat diet (HFD). Using laser capture microdissection (LCM) and real-time PCR, we confirmed the absence of CCK1R mRNA expression in Cck1r−/− rats from two hypothalamic areas that normally express CCK1R [the arcuate nucleus (ARC) (20) and DMH (7)]. Furthermore, we confirmed the inability of the CCK1R antagonist, devazepide, to stimulate food intake in Cck1r−/− rats. In the present studies, we compared Cck1r−/− with Cck1r+/+ rats on the following behaviors: 1) meal patterns, energy expenditure, total spontaneous activity, and respiratory quotient (RQ); 2) predisposition to DIO in animals fed a HFD (45% kcal from fat) over 18 wk; and 3) response to increased anorexigenic signaling through MC3/4R stimulation.
MATERIALS AND METHODS
Experimental Animals
Experimental protocols were approved by the Institutional Animal Care and Use Committees from the University of Washington (UW) and the VA Puget Sound Health Care System (VAPSHCS). Adult male Fischer 344 rats (weight range 287–331 g) were used across all studies. All Cck1r−/− rats were obtained from a breeding colony at the UW, originally derived from Charles River (Charles River Laboratories International, Wilmington, MA), whereas Cck1r+/+ rats were obtained either from the UW colony or Charles River (studies 1B, 2, 3, and 5). The animals were housed individually in Plexiglas cages in a temperature-controlled room (21–23°C) under a 12:12-h light-dark cycle with the exception of studies 2 and 3, in which animals were housed individually in polycarbonate indirect-calorimetry chambers at 28 ± 1° C [Oxymax/Comprehensive Lab Animal Monitoring System (CLAMS); Columbus Instruments, Columbus, OH]. Animals were adapted to lights-off at 1300 and were fed a standard rat chow diet (3.02 kcal/g) (Harlan Teklad, Madison, WI) with the exception of study 4, in which a subset of these animals were fed a 45% kcal from fat HFD (5.24 kcal/g) (D12451; Research Diets, New Brunswick, NJ). All animals were age-matched, and animals within each respective genotype were weight-matched prior to study onset. Animals were fed ad libitum with the exception of studies 4C and 5. Tap water was freely available.
Drugs
Melanotan II (MTII; Bachem Americas, Torrance, CA) was dissolved in sterile saline. Devazepide (Tocris, Ellisville, MO) was initially dissolved in 100% DMSO before being diluted to 10% DMSO, 10% Tween 80, and 80% saline. ANG II (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline.
Injections
Animals were habituated to regular handling and sham injections for at least 1 wk prior to the commencement of studies. Intraperitoneal injections of devazepide were administered via a 1.0-ml syringe with a 25-gauge needle (1 ml/kg injection volume). Third ventricular (3V) injections (1 μl) of MTII and ANG II were administered via a 33-gauge injector (Plastics One, Roanoke, VA) connected by polyethylene 20 tubing to a 10-μl Hamilton syringe. Injections were completed over 30 s; the injector was slowly removed over 10 s and replaced with a 33-gauge dummy cap (Plastics One).
Surgery
For experiments that required 3V administration of MTII, animals were implanted with a cannula directed to the 3V, as previously described (56). Briefly, animals under isoflurane anesthesia were placed in a stereotaxic apparatus with the incisor bar positioned 3.3 mm below the interaural line. A sterile 26-gauge guide cannula (Plastics One) was stereotaxically positioned 1 mm dorsal to the 3V (6.8 mm anterior to the interaural line; 0.0 mm lateral to the midline, and 6.2 mm ventral to the skull surface) and secured to the surface of the skull with dental cement and stainless-steel screws. A sterile 33-gauge dummy obturator (Plastics One) was inserted into the cannula to maintain patency. Animals were injected with ketofen (5 mg/kg sc; Fort Dodge Animal Health, Fort Dodge, IA) at the completion of surgery (once per day for 3 days) and were allowed to recover at least 7 days prior to verification of cannula placement. Cannula placement was verified before the start of experiments by measuring the drinking response following 3V injection of ANG II at a dose of 20 ng/1 μl. Only animals that drank at least 5 ml of water over a 30-min period were used in the subsequent data analysis.
Body Composition
Determinations of lean body mass and fat mass were made on chow-fed and HFD-fed animals by quantitative magnetic resonance (QMR) using an EchoMRI 4-in-1 instrument (Echo Medical Systems, Houston, TX) performed by the Rodent Metabolic and Behavioral Phenotyping Core at the VAPSHCS. Unanesthetized rats were placed in plastic restrainer tubes; triplicate measurements were completed in less than 5 min.
Indirect Calorimetry
Rats were acclimated to indirect calorimetry cages prior to the study and data collection. Energy expenditure measures were obtained using a computer-controlled indirect calorimetry system (Oxymax/CLAMS, Columbus Instruments) located in the Rodent Metabolic and Behavioral Phenotyping Core at the VAPSHCS. The calorimetry system consisted of 16 polycarbonate cages with each being equipped with water bottles; food hoppers sitting upon Mettler analytic balances (Mettler-Toledo, LLC, Columbus, OH) allowed for continuous food intake monitoring. The calorimetry cages were contained within temperature-controlled incubator cabinets (Powers Scientific, Pipersville, PA), which were set to 28 ± 1°C. Total and ambulatory activity was detected with 16 infrared beam arrays (Oxymax/CLAMS, Columbus Instruments) with a beam spacing of 1 cm along the length of the chambers. Respiratory gases were measured with an integrated zirconia oxide sensor and single-beam nondispersed infrared CO2 sensor. Gas sensors were calibrated at the beginning of each experiment with a span gas containing known concentrations of O2 and CO2 with a balance of N2 (PraxAir, Tacoma WA). O2 consumption (V̇o2) and CO2 (V̇co2) production were measured for each rat for 1-min at 20-min intervals. Incurrent air reference values were determined after measuring every four cages. RQ was calculated as the ratio of CO2 production over O2 consumption. Energy expenditure was calculated by Columbus Instrument's software using the equation: V̇o2 × (3.815 × 1.232 RQ) and was expressed in units of kilocalories per hour. [Note: The Columbus system does not use the Weir equation, namely, kcal/h = 60 × (0.003941 × V̇o2 + 0.001106 × V̇co2) (55)]. Total and ambulatory activity was determined simultaneously with the collection of the calorimetry data. Consecutive adjacent infrared beam breaks in the y-axes, i.e., the length of the cage, were scored as an activity count, and a tally was recorded every 20 min. Data acquisition, instrument control, and data processing were coordinated by Oxymax software (v4.59, Oxymax/CLAMS, Columbus Instruments).
LCM
The Arcturus AutoPix LCM system (Molecular Devices, Sunnyvale, CA) was used as previously described (43) to collect bilateral samples containing the region of ARC and DMH on 1 section/slide. Six separate slide-mounted cryostat sections (10 μm) of ARC (bregma −3.48 to −2.04) (43) and DMH (bregma −3.60 to −2.80) (43) were selected at 200-μm intervals, dehydrated in ethanol and xylene, and then air dried (12). Sections mounted on adjacent slides were stained with Cresyl violet to confirm anatomical boundaries for the ARC and DMH prior to LCM (10). Hypothalamic sections were imaged in the AutoPix, and the ARC and DMH were outlined on a monitor using the mouse-controlled cursor. The AutoPix then transferred the circumscribed ARC or DMH tissue to a plastic substrate for analysis. All microdissected ARC or DMH samples from a single brain were pooled for RNA extraction and PCR analysis.
Real-Time PCR
RNA extracted from the pooled samples of ARC and DMH were analyzed using the Arcturus Picopure RNA isolation kit (Molecular Devices) followed by reverse transcription into cDNA using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Quantitative analysis for relative levels of mRNA in the RNA extracts was measured in triplicate by real-time PCR on a Prism 7000 sequence detection system (Applied Biosystems) and normalized to the cycle threshold value of GAPDH mRNA in each sample. Primers and probes were designed using Primer Express (version 2.0.0) from Taqman (Applied Biosystems). The primer sequences used for RT-PCR were rat GAPDH forward primer, 5′-GCCAGCCTCGTCTCATAGACA-3′; rat GAPDH reverse primer, 5′-GTCCGATACGGCCAAATCC-3′; and rat GAPDH probe primer, VIC-5′-ATGGTGAAGGTCGGTGTG-3′. The probe and primers for rat Cck1r (catalog no. Rn00562164_m1) were acquired from Applied Biosystems.
Study Protocols
Study 1A. absence of CCK1R in ARC and DMH of Cck1r−/− rats.
To confirm that CCK1R are not expressed in the brain of the Cck1r−/− rats, we used LCM to selectively examine CCK1R mRNA in both the ARC and the DMH from ad libitum-fed Cck1r−/− and Cck1r+/+ rats (n = 4–6/group).
Study 1B. lack of feeding response to the CCK1R antagonist, devazepide in Cck1r−/− rats.
As an additional negative control to confirm the absence of CCK1R in an in vivo model, we also examined the effects of devazepide to stimulate food intake in Cck1r−/− rats relative to Cck1r+/+ rats (9). On the day of the study, 0.5-h-fasted Cck1r−/− (n = 9/group) and Cck1r+/+ rats (n = 12/group) received an IP injection of either devazepide (1 mg/kg) or vehicle 0.5 h prior to dark-cycle onset. Food was returned immediately prior to dark-cycle onset and measured 0.5, 2, and 24 h later.
Study 2. examination of meal patterns in Cck1r−/− and Cck1r+/+ rats.
To determine the impact of CCK1R deficiency on meal patterns in Cck1r−/− (n = 8/group) and Cck1r+/+ rats (n = 8/group), computerized recordings of meal patterns were measured across a 2-day light and 3-day dark period (68 h), while in the CLAMS metabolic units. Meal size and meal frequency were examined in both Cck1r−/− and Cck1r+/+ rats fed powdered chow and averaged across the dark and light period separately. The criterion for meal initiation was a food intake of at least 0.1 g in one bout, and meal termination was an absence of ingestion for at least 10 min. Total food intake was also summed across the 68-h period.
Study 3. examination of body composition, energy expenditure, total spontaneous activity, and RQ in Cck1r−/− and Cck1r+/+ rats.
To determine the impact of CCK1R deficiency on energy expenditure, total spontaneous activity, and RQ (fuel utilization) in F344 rats, computerized recordings were measured across 68 h. Body composition was assessed prior to study onset. All measurements were completed in Cck1r−/− (n = 8/group) and Cck1r+/+ rats (n = 8/group) fed powdered chow and averaged across the dark and light periods separately.
Study 4A. evaluation of predisposition to DIO in Cck1r−/− and Cck1r+/+ rats.
To determine whether the animals with defective CCK1R signaling were more susceptible to DIO, we measured the ability of Cck1r−/− and Cck1r+/+ rats to become obese following ad libitum feeding on a HFD (n = 10–12/group) relative to chow (n = 10–11/group) over an 18-wk period. Body weight was measured weekly, and body composition was measured at ∼4-wk intervals.
Study 4B: evaluation of food intake in Cck1r−/− and Cck1r+/+ rats.
Daily food intake (g) was measured once per week at ∼4-wk intervals throughout the 18-wk period.
Study 4C. evaluation of blood glucose and serum leptin levels in Cck1r−/− and Cck1r+/+ rats.
BLOOD GLUCOSE.
Blood glucose was measured by tail vein in 4-h fasted chow and HFD-fed animals using the AlphaTRAK blood glucose monitoring system (Abbott Laboratories, Abbott Park, IL).
SERUM LEPTIN:
Trunk blood was collected in chilled EDTA-treated tubes [BD Microtainer Tubes with K2E (K2EDTA), Becton Dickinson, Franklin Lakes, NJ] following CO2 euthanasia. Whole blood was centrifuged at 6,000 rpm for 1.5 min at 4°C, serum removed, aliquoted, and stored at −80°C for subsequent analysis. Serum leptin was measured in duplicate samples after a dilution (1:1) with assay buffer by rat leptin ELISA (EZRL-83K, EMD; Millipore, Billerica, MA) (39).
Study 5. examination of responsiveness to peripheral and central administration of MTII in Cck1r−/− and Cck1r+/+ rats.
On the day of the study, 6-h-fasted animals received an intraperitoneal injection of either MTII (1 or 3 mg/kg) or vehicle (saline) 1 h prior to dark cycle onset. Animals were used as their own control and each received injections at 48–72-h intervals. A separate cohort of animals prepared with 3V cannulas received 3V injections of MTII (0.05 or 0.1 nmol/μl) or vehicle (saline) using an identical paradigm. Food was returned immediately prior to dark cycle onset and measured 0.5, 2, 4, and 18 h later.
Statistical Analysis
All results are expressed as means ± SE. Comparisons between multiple groups as involving between-subjects designs were made using one- or two-way ANOVA as appropriate followed by Tukey's honestly significant difference test, as a post hoc test. Comparisons involving within-subjects designs were made using a one-way repeated-measures ANOVA followed by Tukey's honestly significant difference test as a post hoc test. Analyses were performed using the statistical program SYSTAT version 11.0 (Systat Software, San Jose, CA). To adjust for the influence of body size variation on total energy expenditure (11), group comparisons involving this outcome were adjusted for lean body mass using multiple-regression analysis as recommended by Kaiyala and colleagues (21, 22). This was performed using the linear mixed-model procedure in SPSS Statistics version 20 (IBM, Somers, NY). Analyses were done within the photoperiod and encompassed three light and three dark cycles as repeated measures with a compound symmetry covariance structure. Differences were considered significant when P < 0.05, 2-tailed.
RESULTS
Study 1A. Lack of Expression of CCK1R in Cck1r−/− Rats
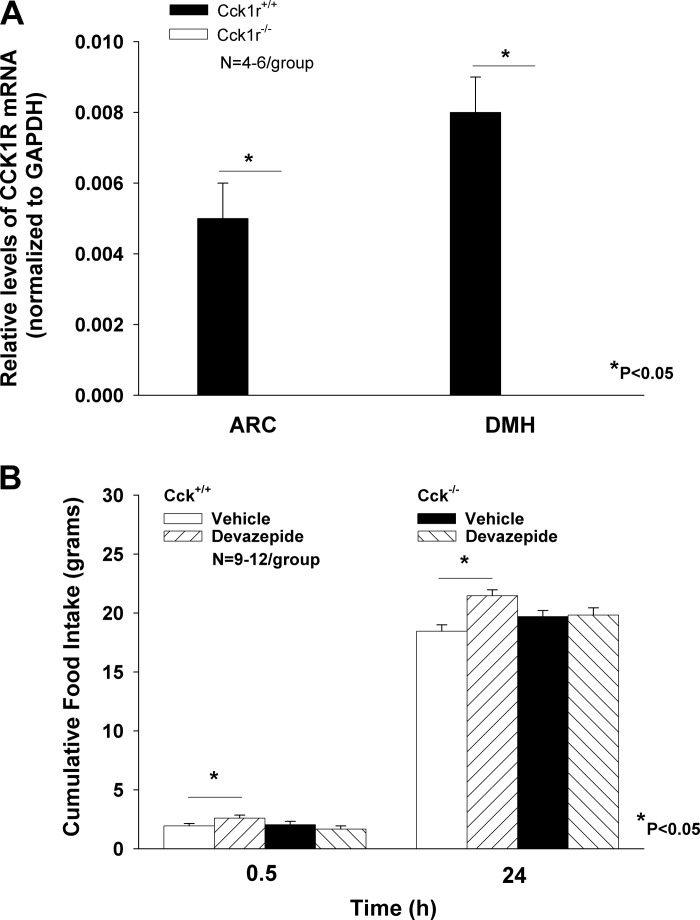
As expected, the absence of CCK1R in the ARC (P = 0.003) and DMH (P = 0.005) of Cck1r−/− was confirmed in ad libitum-fed Cck1r−/− relative to Cck1r+/+ rats (Fig. 1A).
Fig. 1.
In vitro and in vivo verification that CCK1r−/− rats lack CCK1R. CCK1R was not detectable in the arcuate nucleus (ARC) or dorsomedial hypothalamus (DMH) in Cck1r−/− rats (A), whereas Cck1r were expressed, as expected, in both the ARC and DMH of Cck1r+/+ rats. Devazepide, as expected, failed to stimulate food intake in Cck1r−/− rats but was effective in stimulating food intake in Cck1r+/+ rats [data in Cck1r+/+ rats (9)]) (B).
Study 1B. Lack of Feeding Response to the CCK1R Antagonist, Devazepide, in Cck1r−/− Rats
The CCK1R antagonist, devazepide, failed to stimulate food intake in the Cck1r−/− at 0.5 (P = NS) and 24 h (P = NS) (Fig. 1B). Its stimulatory effects in Cck1r+/+ rats were reported previously (9). The lack of response to CCK-8 (8 nmol/kg ip) on food intake in Cck1r−/− was confirmed previously (9), as well as in the current study (data not shown).
Study 2. Examination of Meal Patterns in Cck1r−/− and Cck1r+/+ Rats
Meal patterns were different between genotypes throughout the light-dark cycle. The average meal size across the dark period was greater in the Cck1r−/− rats relative to the Cck1r+/+ rats (Table 1) (P = 0.008). This coincided with increased intake in the Cck1r−/− rats relative to the Cck1r+/+ rats (Table 1) during the dark (P = 0.009). During the light cycle, the average meal size was reduced in the Cck1r−/− rats relative to the Cck1r+/+ rats (P = 0.0004) (Table 1). This also coincided with reduced intake in the Cck1r−/− rats relative to the Cck1r+/+ rats during this period (P = 0.005). Total daily food intake remained unchanged between Cck1r−/− rats relative to the Cck1r+/+ rats (P = NS). There was not a significant change in meal frequency across the light (P = NS) or dark cycles in Cck1r−/− rats relative to Cck1r+/+ rats (P = 0.07) (Table 1).
Table 1.
Examination of meal patterns in Cck1r+/+ and Cck1r−/− rats
| Light Cycle | Dark Cycle | Total | |
|---|---|---|---|
| Total intake, g | |||
| CCK1r+/+ | 8.4 ± 0.5* | 23 ± 1.2* | 31.4 ± 1.0 |
| CCK1r−/− | 4.6 ± 0.7 | 28 ± 0.9 | 32.1 ± 1.1 |
| Meal size, g | |||
| CCK1r+/+ | 0.23 ± 0.01* | 0.64 ± 0.03* | |
| CCK1r−/− | 0.13 ± 0.02 | 0.77 ± 0.02 | |
| Meal frequency | |||
| CCK1r+/+ | 10.1 ± 0.9 | 24.5 ± 3.7 | |
| CCK1r−/− | 10.0 ± 1.4 | 17.0 ± 1.0 |
P < 0.05 CCK1r+/+ vs. CCK1r−/−. n =8/group.
Study 3. Examination of Body Composition, Energy Expenditure, Total Spontaneous Activity, and RQ in Cck1r−/− and Cck1r+/+ Rats
Body composition.
Body weight in the Cck1r−/− animals was slightly higher than that of the Cck1r+/+ rats (407 ± 2.7 g vs. 392 ± 5.4 g; P = 0.03), and this corresponded to a significant increase in lean body mass (lean mass: 284.5 ± 3.3 vs. 266.4 ± 3.8 g; P = 0.003) and percent lean body mass (69.9 ± 0.6 vs. 67.9 ± 0.5 g; P = 0.02) in the Cck1r−/− animals relative to the Cck1r+/+ rats. There were no significant differences with respect to total fat mass (67.1 ± 3.5 vs. 69.5 ± 3.4 g; P = NS) or percent fat mass (fat mass: 16.5 ± 0.8 vs. 17.7 ± 0.8 g; P = NS).
Energy expenditure.
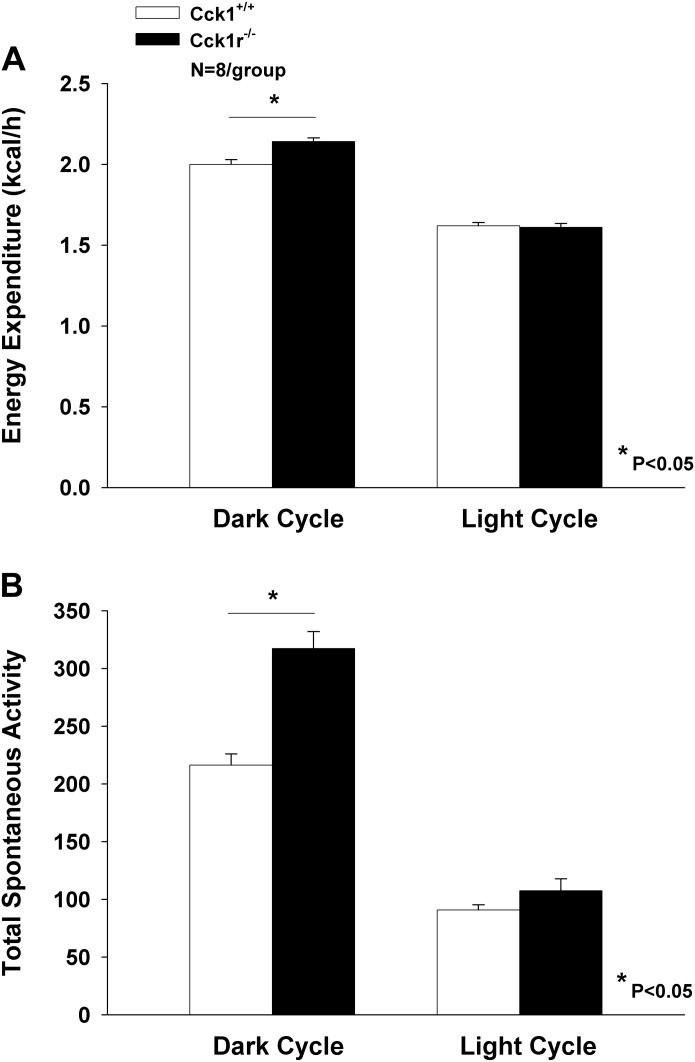
Unadjusted light cycle energy expenditure was similar between groups (Cck1r−/− = 1.61 ± 0.024; Cck1r+/+= 1.62 ± 0.02 kcal/h; P = 0.81) (Fig. 2A). Unadjusted dark-cycle energy expenditure was significantly higher in Cck1r−/− compared with Cck1r+/+ animals (2.14 ± 0.024 vs. 2.00 ± 0.029 kcal/h; P = 0.004) (Fig. 2A).
Fig. 2.
Examination of energy expenditure and total spontaneous activity in Cck1r+/+ and Cck1r−/− rats. Cck1r−/− rats showed between 6.5 (adjusted for lean body mass) to 8.1% (unadjusted for lean body mass) in energy expenditure throughout the dark period relative to Cck1r+/+ rats, but there was no change in energy expenditure during the light period (A). Cck1r−/− rats showed nearly a 48% increase in total spontaneous activity evident throughout the dark period relative to Cck1r+/+ rats, but there was no change in total spontaneous activity during the light period (B).
Because body mass and composition differed between groups, we also examined energy expenditure adjusted for measures of body size. Body mass-adjusted light cycle energy expenditure as estimated using repeated-measures analysis of covariance with body mass and genotype as predictor variables was similar between groups (1.60 ± 0.026 vs. 1.63 ± 0.026 kcal/h for Cck1r−/− vs. Cck1r+/+, respectively; P = 0.47). By contrast, body mass-adjusted dark-cycle energy expenditure was significantly higher in Cck1r−/− compared with Cck1r+/+ animals (2.12 ± 0.032 vs. 2.012 ± 0.032 kcal/h, respectively; P = 0.044). Energy expenditure adjusted for body mass minus QMR-estimated fat mass (2.13 ± 0.034 vs. 2.00 ± 0.034 kcal/h; P = 0.034) and for QMR-estimated lean body mass (2.12 ± 0.035 vs. 2.02 ± 0.035 kcal/h; P = 0.10) exhibited the same patterns. It should be noted that for dark-cycle energy expenditure, the slopes of energy expenditure on total body mass (but not on QMR-estimated lean body mass or on total body mass minus QMR-estimated fat mass) differed significantly between groups, with good evidence for a steeper dependence in the Cck1r−/− group (P = 0.002).
Accordingly, we also examined a regression model that included as predictor variables body mass, genotype, and the genotype × body mass interaction term. This model indicated that energy expenditure adjusted to the mean body mass of the Cck1r−/− rats was reliably higher compared with Cck1r+/+ rats (2.14 ± 0.028 vs. 2.00 ± 0.020 kcal/h, respectively; P = 0.002).
Total spontaneous activity.
Cck1r−/− rats showed nearly a 48% increase in total spontaneous activity evident throughout the dark period (317.4 ± 14.6) relative to Cck1r+/+ rats (216.3 ± 9.7) (P = 0.0001). There was no change in total spontaneous activity between the Cck1r−/− (107.6 ± 10.2) and Cck1r+/+ rats (90.8 ± 4.5) during the light period (P = NS) (Fig. 2B). When activity was included as a covariate in regression models to assess for group differences in dark-cycle energy expenditure, activity emerged as a significant determinant of this outcome. When both genotype and lean body mass were included as predictor variables, activity was significant (P = 0.028). Specifically, inclusion of activity in regression models examining energy expenditure in relation to body size and genotype identified activity as a significant and independent positive determinant of energy expenditure (P = 0.028), while abolishing evidence for differences in energy expenditure between groups (P = NS). Moreover, activity was indeed significantly higher in the Cck1r−/− knockout rats compared with Cck1r+/+ wild-type rats.
RQ.
RQ tended to be lower during the light cycle in the Cck1r−/− rats (0.85 ± 0.005) relative to Cck1r+/+ rats (0.87 ± 0.004) (P = 0.058) with no significant difference occurring during the dark cycle (0.85 ± 0.006; Cck1r−/− vs. 0.86 ± 0.004; Cck1r+/+) (P = NS).
Study 4A. Evaluation of Predisposition to DIO in Cck1r−/− and Cck1r+/+ Rats
At study onset, there were no differences with respect to body weight between chow-fed and HFD-fed Cck1r−/− (P = NS) or Cck1r+/+ rats (P = NS), but the body weight of both chow-fed (P = 0.005) and HFD-fed Cck1r−/− (P = 0.005) rats exceeded that of the Cck1r+/+ rats. Therefore, we explored the changes in body weight, % body fat, and % lean mass following 18 wk of exposure to chow or HFD.
Change in body weight over 18 wk.
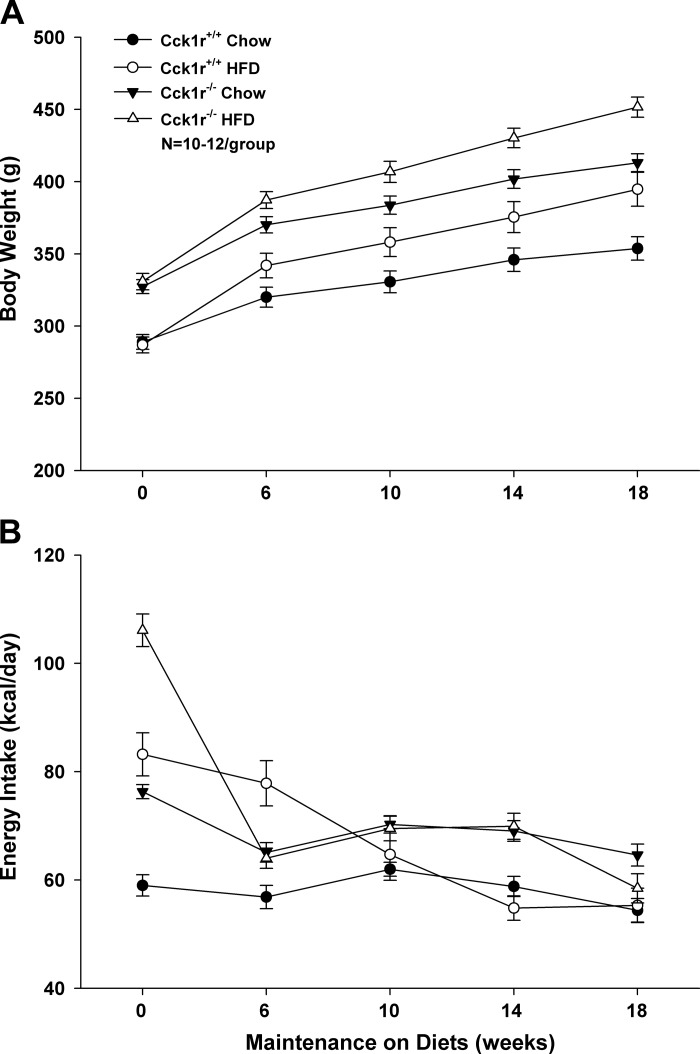
HFD intake increased body weight gain over 18 wk in both Cck1r−/− (P = 0.005) and Cck1r+/+ rats (P = 0.005; Fig. 3A) and resulted in an increased % fat in both HFD-fed Cck1r−/− (24.4 ± 0.4) and Cck1r+/+ rats (21.9 ± 0.9) relative to chow fed Cck1r−/− (15.2 ± 0.3; P = 0.005) and wild-type Cck1r+/+ (13.3 ± 0.4; P = 0.005). There was a greater increase in body weight gain in chow-fed Cck1r−/− rats relative to Cck1r+/+ rats (P = 0.02). There was a significant effect of strain (P = 0.002) and diet (P = 0.005) but no interactive effect between strain and diet (P = NS) on body weight gain. The change in body weight was reflected by increases in both % body fat and % lean mass over this period of time.
Fig. 3.
Evaluation of body weight gain and energy intake (kcal/day) in Cck1r+/+ and Cck1r−/− rats. HFD intake increased body weight gain over 18 wk in both Cck1r−/− and Cck1r+/+ rats (see Fig. 4A) and resulted in an increased % fat in both HFD-fed Cck1r−/− and Cck1r+/+ rats relative to chow fed Cck1r−/− and wild-type Cck1r+/+. Differences in body weight gain between chow-fed and HFD-fed Cck1r−/− rats, as well as chow-fed and HFD-fed Cck1r+/+ rats were highly significant (A). At study onset, HFD-fed Cck1r+/+ rats had greater energy intake relative to chow-fed Cck1r+/+ and, this was also true for HFD-fed Cck1r−/− rats relative to chow-fed Cck1r−/−. Chow-fed and HFD fed Cck1r−/− rats had greater energy intake relative to chow-fed and HFD fed Cck1r+/+ rats, respectively. Differences between HFD-fed Cck1r−/− and Cck1r+/+ rats were evident over weeks 0, 6, and 14. Differences with respect to chow and HFD intake in Cck1r+/+ rats were evident at 0 and 6 wk, whereas differences between diets in the Cck1r−/− rats were evident only at week 0 (B).
Change in fat mass over 18 wk.
As expected, there was also a greater increase in % body fat gain in HFD-fed Cck1r−/− and Cck1r+/+ rats relative to chow-fed Cck1r−/− (P = 0.005) and Cck1r+/+ rats (P = 0.005). There was a significant effect of strain (P = 0.016) and diet (P = 0.005) on change in % fat mass, but there was no interactive effect between strain and diet (P = NS).
Change in lean mass over 18 wk.
Both HFD-fed Cck1r−/− and Cck1r+/+ rats gained a greater % lean mass relative to chow-fed Cck1r−/− (P = 0.005) and Cck1r+/+ rats (P = 0.005), but there were no significant differences between Cck1r−/− and Cck1r+/+ rats fed either chow (P = NS) or HFD (P = NS; data not shown). There was no significant effect of strain (P = NS), a significant effect of diet (P = 0.005), but no interactive effect between strain and diet (P = NS).
Study 4B: Evaluation of Daily Energy Intake (kcal/day) in Cck1r−/− and Cck1r+/+ Rats
At study onset, HFD-fed Cck1r+/+ rats had greater energy intake relative to chow-fed Cck1r+/+ rats (P = 0.005), and this was also true for as HFD-fed Cck1r−/− rats relative to chow-fed Cck1r−/− (P = 0.005). Chow-fed and HFD-fed Cck1r−/− rats had greater energy intake relative to chow-fed (P = 0.005) and HFD-fed Cck1r+/+ rats (P = 0.005), respectively (Fig. 3B). These differences persisted between chow-fed Cck1r−/− and Cck1r+/+ rats throughout the study. HFD-fed Cck1r−/− rats had greater energy intake relative to Cck1r+/+ rats at weeks 0 (P = 0.005) and 14 (P = 0.005), yet consumed less energy relative to Cck1r+/+ rats at week 6 (P = 0.003). HFD-fed Cck1r+/+ rats consumed greater energy intake relative to chow-fed Cck1r+/+ rats at weeks 0 (P = 0.005) and 6 (P = 0.005), whereas HFD-fed Cck1r−/− rats consumed more energy than chow-fed Cck1r−/− rats only at week 0 (P = 0.005). There was a significant effect of strain with the exception of week 6 (P = NS), and there was a significant effect of diet with the exception of week 10 (P = NS), 14 (P = NS), and 18 (P = NS). There was no interactive effect between strain and diet throughout the extent of the study (P = NS), with the exception of week 6 (P = 0.005).
Study 4C. Evaluation of Blood Glucose and Serum Leptin in Cck1r−/− and Cck1r+/+ Rats
Both HFD-fed Cck1r−/− (122 ± 3.0 mg/dl) and Cck1r+/+ rats (113.7 ± 3.3 mg/dl) exhibited higher blood glucose levels relative to chow-fed Cck1r−/− (106.2 ± 3.1 mg/dl; P = 0.017) and Cck1r+/+ (99.6 ± 3.8 mg/dl; P = 0.029). There were no significant differences in glucose levels in the HFD-fed Cck1r−/− (122 ± 3.0 mg/dl) relative to the wild-type Cck1r+/+ rats (P = NS), nor were there significant differences found between chow-fed genotypes (P = NS). There was a trend or a significant effect of strain (P = 0.076) and diet (P = 0.005), but there was no interactive effect between strain and diet (P = NS). As expected, HFD-fed Cck1r−/− (23.2 ± 3.0 ng/ml) exhibited higher serum leptin levels relative to chow-fed Cck1r−/− (5.7 ± 0.4 ng/ml; P = 0.002), with a trend toward increased leptin levels in the HFD-fed Cck1r+/+ rats (16.7 ± 3.8 ng/ml) relative to chow-fed Cck1r+/+ (6.6 ± 1.2 ng/ml; P = 0.076). No significant differences in leptin levels were found between HFD-fed Cck1r−/− and wild-type Cck1r+/+ rats (P = NS), or between chow-fed genotypes (P = NS). There was a significant effect of diet (P = 0.005) but not strain (P = NS) and no interactive effect between strain and diet (P = NS).
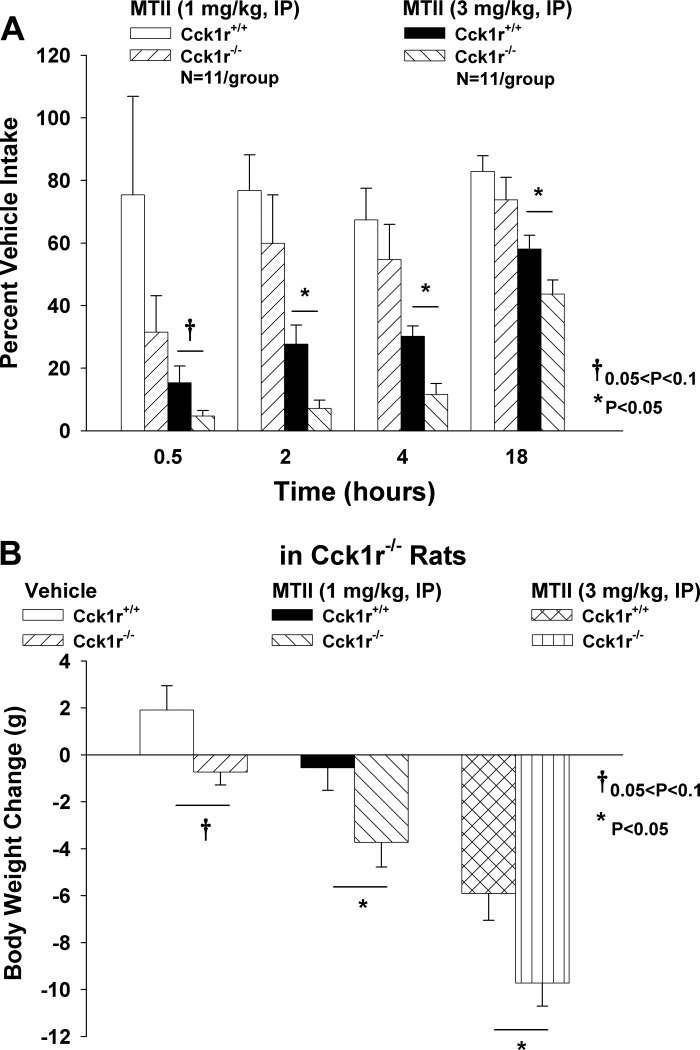
Study 5A. Examination of Responsiveness to Peripheral Administration of MTII in Cck1r−/− and Cck1r+/+ Rats
To determine whether there is increased effectiveness of MC3/MC4R stimulation to reduce both food intake and body weight in the Cck1r−/− rats relative to the Cck1r+/+ rats, we measured the effects of systemic (intraperitoneal) administration of MTII to reduce food intake and body weight in both genotypes. There were no differences in food intake between Cck1r−/− and F344.Cck1r+/+ rats at any time point following vehicle injections (P = NS). There were no significant differences in % vehicle intake (1 mg/kg) between Cck1r−/− and F344.Cck1r+/+ rats at 0.5 (P = NS), 2 (P = NS), 4 (P = NS), and 18 h (P = NS; Fig. 4A). However, there was either a trend or a significant difference between % vehicle intake following administration of the higher dose of MTII (3 mg/kg) at 0.5 (P = 0.08), 2 (P = 0.006), 4 (P = 0.001), and 18 h (P = 0.034). These effects were accompanied by a significant reduction in body weight at 1 (P = 0.021) and 3 mg/kg (P = 0.005) in the Cck1r−/−, whereas the effects on body weight in the Cck1r+/+ rats were only evident at the high dose (P = 0.001). Comparisons between body weight loss in both genotypes revealed MTII to be more effective at reducing body weight in the Cck1r−/− at both 1 (P = 0.038) and 3 mg/kg (P = 0.02) relative to the Cck1r+/+ rats (Fig. 4B).
Fig. 4.
Effects of peripheral administration of MTII on food intake and body weight in Cck1r+/+ and Cck1r−/− rats. There was either a near-significant or significant difference between % vehicle intakes following administration of MTII (3 mg/kg) at 0.5 (P = 0.08), 2, 4, and 18 h (P < 0.05) (A). Comparisons between body weight loss in both genotypes revealed MTII to be more effective at reducing body weight in the Cck1r−/− at both 1 (P < 0.05) and 3 mg/kg (P < 0.05) relative to the Cck1r+/+ rats (B).
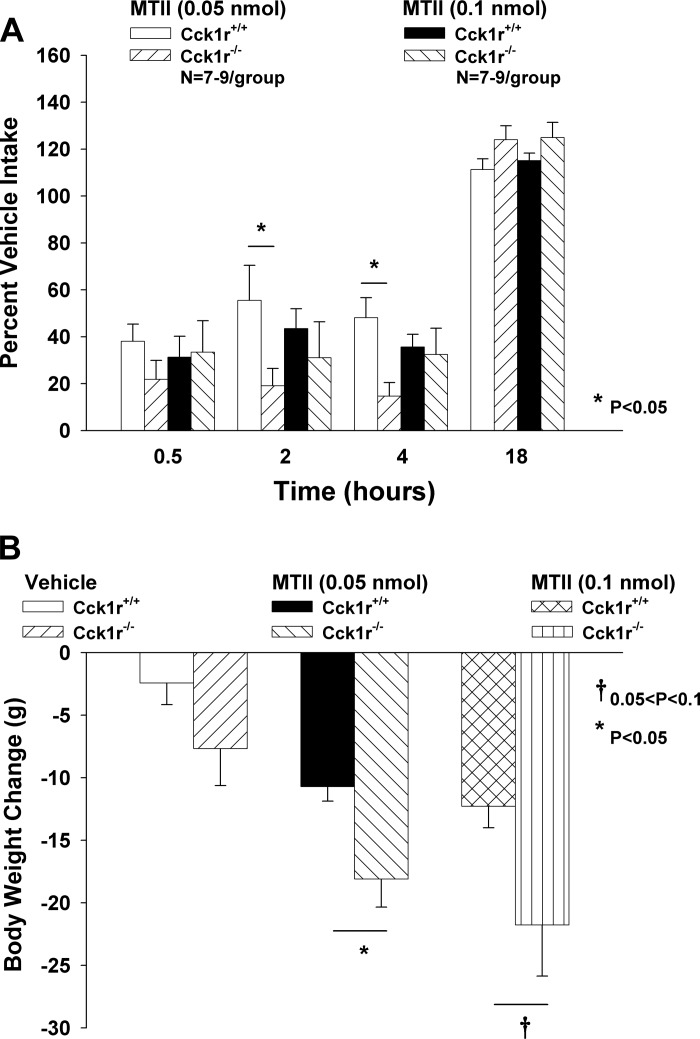
Study 5B. Examination of Responsiveness to Central Administration of MTII in Cck1r−/− and Cck1r+/+ Rats
To confirm whether the increased effectiveness of MC3/MC4R stimulation in Cck1r−/− rats could be attributed to action at CNS MC3/MC4Rs, we measured the effects of 3V administration of MTII to reduce food intake and body weight in both genotypes. There were no differences in food intake between Cck1r−/− and F344.Cck1r+/+ rats at any time point following vehicle injections (P = NS). There was either a trend or a significant difference between % vehicle intakes following administration of the lower dose of MTII (0.05 nmol) at 2 (P = 0.034) and 4 (P = 0.005), but not at 18 h (P = 0.1) (Fig. 5A). These effects were accompanied by a significant reduction in body weight at 0.05 (P = 0.015) and 1 nmol (P = 0.004) in Cck1r+/+ rats, with a trend or significant effect of MTII to reduce body weight at 0.05 (P = 0.034) and 1 nmol (P = 0.025) in the Cck1r−/− rats. Comparisons between body weight loss in both genotypes revealed that MTII was more effective at reducing body weight in the Cck1r−/− at 0.05 nmol (P = 0.017) with a trend toward significance at 0.1 nmol (P = 0.073) relative to the Cck1r+/+ rats (Fig. 5B).
Fig. 5.
Effects of central administration of MTII on food intake and body weight in Cck1r+/+ and Cck1r−/− rats. There was either a trend or a significant difference between % vehicle intakes following administration of the lower dose of MTII (0.05 nmol) at 2 (P < 0.05) and 4 (P < 0.05), but not at 18 h (P = 0.1) (A). Comparisons between body weight loss in both genotypes revealed that MTII was more effective at reducing body weight in the Cck1r−/− at 0.05 nmol (P < 0.05) with a trend toward significance at 0.1 nmol (P = 0.073) relative to the Cck1r+/+ rats (B).
DISCUSSION
In the present study, we characterized the metabolic profile of the recently described lean Cck1r−/− rat on a Fischer 344 background. With our unique animal model, we hypothesized that the lean Cck1r−/− rats would show increased meal size and energy expenditure relative to their Fischer 344 wild-type counterparts. Cck1r−/− rats consumed larger meals during the dark cycle and smaller meals during the light cycle. These effects were accompanied by increased total spontaneous activity and energy expenditure during the dark cycle, as well as an apparent shift toward increased fat utilization as demonstrated by the reduction in RQ during the light cycle. On the basis of the findings in the OLETF rats (3), we predicted that both Cck1r+/+ and Cck1r−/− rats would show increased weight gain during chronic exposure to a highly palatable, HFD. Indeed, both Cck1r+/+ and Cck1r−/− rats were prone to DIO when maintained on a HFD, which was associated with increased serum leptin levels. We also found an enhanced effect of both central and peripheral administration of MTII to suppress food intake and body weight in Cck1r−/− rats, raising the possibility that increased sensitivity to endogenous melanocortin tone in the Cck1r−/− rats may contribute to the increased energy expenditure and lean phenotype in the Cck1r−/− rats. These results more fully characterize our recently described lean Cck1r−/− rat model and suggest that the lean phenotype likely results from increases in total spontaneous activity and energy expenditure.
The present results are the first demonstration of an increase in both total spontaneous activity and energy expenditure throughout the dark cycle in lean Cck1r−/− rats. OLETF rats, when allowed access to a running wheel are known to show increased running-wheel activity relative to the LETO rats, and this is sufficient to prevent their obesity and hyperphagia (6). Although the CCK−/− mice fed a low-fat diet fail to show increased activity or energy expenditure when assessed throughout the entire day, these mice show enhanced energy expenditure at varying times throughout the light and dark cycle (25), which may also contribute to their lean body weight. While our results must be interpreted cautiously owing to the modest sample sizes in this study, they nonetheless suggest that an effect of Cck1 receptor knockout to increase total spontaneous activity contributed to increased energy expenditure in Cck1r−/− rats. This finding may have important implications to efforts aimed at identifying the neurobiological substrates underlying individual differences in physical activity, a variable of potential importance to obesity resistance. Together, these findings highlight the contribution of both activity and energy expenditure to the lean phenotype of the Cck1r−/− rats and warrant further dissection with respect to energy expenditure and activity during the light and dark cycles in Cck1r−/− rats fed a HFD.
Our data indicate a steeper dependence of energy expenditure on body mass in Cck1r−/− rats compared with Cck1r−/− animals, suggesting that Cck1 receptor knockout intensifies the per-gram effect of body tissue mass on energy expenditure. While this effect could reflect an inherent increase in the tissue metabolic rate of knockout animals, it seems more likely that elevated per-gram and total energy expenditure in the mutant rats reflects an activity phenotype because activity was reliably higher in the knockout compared with control animals. Thus, our data suggest an important and novel role for CCK1R signal transduction in the modulation of activity and activity-related energy expenditure.
Here, we report an increase in food intake and meal size during the dark period in age-matched ad libitum-fed animals. Although these results are consistent with the observations in the lean Cck1r−/− mice (7), these results contrast with our previous findings that show the Cck1r−/− rats to have no obvious aberrations in meal size over the dark period (9) in both 6-h fasted and ad libitum-fed animals when ingesting liquid diet. However, in this previous study, there appeared to be a tendency for ad libitum-fed Cck1r−/− rats to eat larger meals during the first hour of the dark period: Cck1r−/− (5.8 ± 0.8 g) vs. Cck1r+/+ (4.5 ± 0.1 g; P = 0.1). One possible explanation for the differences between studies may be attributed to meal size being derived from number of licks. Together, these findings suggest that the lean phenotype of the Cck1r−/− rats is largely attributed to increased total spontaneous activity leading to increased energy expenditure, rather than a primary effect on food intake or patterns of food intake.
Our finding that Cck1r−/− rats become DIO when maintained on a HFD are consistent with those found in the OLETF rats and Cck1r−/− mice, both of which are susceptible to DIO. Unlike the OLETF rats, both the Cck1r−/− and wild-type mice show nearly identical growth rate and energy intake following chronic exposure to HFD (7). Our results indicate that there tends to be a slight increase in body weight gain and energy intake of the Cck1r−/− rats relative to the Cck1r+/+ rats. This difference is most likely attributed to the increased body weights of the Cck1r−/− rats at study onset. Throughout the study, chow-fed Cck1r−/− rats had greater energy intake relative to chow-fed Cck1r+/+ rats, but HFD-fed Cck1r−/− rats only had greater energy intake relative to Cck1r+/+ rats at weeks 0 and 14 (P < 0.05). The lack of consistency between energy intake and body weight gain makes it a priority in future studies to examine energy expenditure in both lean and DIO Cck1r−/− and Cck1r+/+ rats. Although we did not find a difference between daily intakes of powdered chow between the Cck1r−/− and Cck1r+/+ rats when maintained in the CLAMS units, this particular group of animals was older (180–265 days; 379–420 g) and maintained under thermoneutral conditions relative to the animals at the onset of the DIO study (117–129 days; 287–331 g) making it difficult to draw direct comparisons across studies.
Here, we report that the Cck1r−/− rats tended to have a reduction in RQ during the light cycle. Coupled with the observation that these animals are eating less food during this time, these findings are consistent with the possibility that these animals are oxidizing more lipid rather than carbohydrate. These results appear similar to the reported reductions in RQ in CCK−/− mice prior to onset of the dark cycle (26). Together, these findings indicate that increased oxidation of lipid may also contribute to the lean phenotype apparent in the Cck1r−/− rats.
The finding that the Cck1r−/− rats appear to have increased sensitivity to melanocortin ligands is also consistent with what others have shown in the OLETF rat (5). Downregulation of melanocortin receptors have been reported in the ventromedial hypothalamus, nucleus accumbens shell, and ventral tegmental area (24) of OLETF rats. This may indicate that release of endogenous α-MSH is increased in animal models that lack CCK1R, and this may provide a plausible mechanism to explain the increased energy expenditure in the Cck1r−/− rats. The paraventricular nucleus (PVN) (11) and the nucleus of the solitary tract (42) are areas that receive dense α-MSH innervation from the ARC, and future studies will determine MC3/MC4R expression levels in the PVN in both Cck1r+/+ and Cck1r−/− rats.
Although increased food intake and/or decreased energy expenditure across species is attributed to the absence of the CCK1R, recent data from transgenic mice have linked the CCK2 receptor to the regulation of food intake and body weight. CCK2R-deficient (CCK2R−/−) mice show increased body weight (13) and energy intake (13, 29). Furthermore, the CCK2R ablation is linked with reductions in energy expenditure (13) in both light and dark cycles, and locomotor activity during the dark cycle (13, 53). In contrast to the recent findings in transgenic mice, earlier pharmacological studies have yielded contradictory results. CCK2 antagonists fail to block the effects of CCK-8 to reduce food intake (37) and in some cases, CCK2R agonists do not inhibit food intake as effectively as CCK1R agonists (2, 47) or only inhibit food intake at relatively high doses (2, 47). Moreover, the pharmacological data suggest that the effects of CCK2R agonists to inhibit food intake may be nonspecific, as behaviors such as anxiety in rats (48), foot stomping/vocalization in sheep (16), or panic attacks in humans (32) are elicited in response to CCK2R agonist administration. These contradictory findings make determining the significance of CCK2Rs in the regulation of body weight an important future endeavor.
Perspectives and Significance
The present study more fully characterizes the metabolic and behavioral profile to explain the phenotype in our model of Cck1r−/− rats. Our findings couple the absence of CCK1R to increased energy expenditure and total spontaneous activity and an apparent increase in fat utilization. Our data suggest an important and novel role for CCK1R signal transduction in the modulation of activity and activity-related energy expenditure. Similar to the findings observed in the OLETF model with defective CCK1R signaling, the absence of CCK1R in our model is also associated with a heightened sensitivity to MC3/MC4R stimulation. These findings may translate to an enhanced responsiveness to endogenous MC3/MC4R activation, which may provide a mechanism to explain the increased energy expenditure and total spontaneous activity in our rat model of defective CCK1R signaling. Important future endeavors should examine the extent to which alterations in MC3/MC4R signaling impact the metabolic phenotype in our model of Cck1r−/− rats, and the degree to which the development of DIO in this model reflects reductions in activity and activity-related energy expenditure during the light and dark cycles.
GRANTS
This material is based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (VA), the VA Puget Sound Health Care System Rodent Metabolic and Behavioral Phenotyping Core, the biomedical research core program of the National Institutes of Health (NIH) Diabetes Research Center at the University of Washington, and the American Diabetes Association. The research in our laboratory has been supported by the Department of VA Merit Review Research Program, NIH Grants DK-17047, RO1DK-61516, P30DK017047-31, and P30KD017047-31689, and by the Junior Faculty Award 1-05-JF-32 by the American Diabetes Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Arya R, Duggirala R, Jenkinson CP, Almasy L, Blangero J, O'Connell P, Stern MP. Evidence of a novel quantitative-trait locus for obesity on chromosome 4p in Mexican Americans. Am J Hum Genet 74: 272–282, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asin KE, Gore PA, Jr, Bednarz L, Holladay M, Nadzan AM. Effects of selective CCK receptor agonists on food intake after central or peripheral administration in rats. Brain Res 571: 169–174, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Bi S, Chen J, Behles RR, Hyun J, Kopin AS, Moran TH. Differential body weight and feeding responses to high-fat diets in rats and mice lacking cholecystokinin 1 receptors. Am J Physiol Regul Integr Comp Physiol 293: R55–R63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol 281: R254–R260, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bi S, Moran TH. Actions of CCK in the controls of food intake and body weight: lessons from the CCK-A receptor deficient OLETF rat. Neuropeptides 36: 171–181, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka Long-Evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology 146: 1676–1685, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Bi S, Scott KA, Kopin AS, Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology 145: 3873–3880, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res 993: 30–41, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Blevins JE, Overduin J, Fuller JM, Cummings DE, Matsumoto K, Moralejo DH. Normal feeding and body weight in Fischer 344 rats lacking the cholecystokinin-1 receptor gene. Brain Res 1255: 98–112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blevins JE, Stanley BG, Reidelberger RD. Brain regions where cholecystokinin suppresses feeding in rats. Brain Res 860: 1–10, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7: 179–185, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen DY, Deutsch JA, Gonzalez MF, Gu Y. The induction and suppression of c-fos expression in the rat brain by cholecystokinin and its antagonist L364,718. Neurosci Lett 149: 91–94, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Clerc P, Coll Constans MG, Lulka H, Broussaud S, Guigne C, Leung-Theung-Long S., Perrin C., Knauf C., Carpene C., Penicaud L., Seva C., Burcelin R., Valet P., Fourmy D., Dufresne M. Involvement of cholecystokinin 2 receptor in food intake regulation: hyperphagia and increased fat deposition in cholecystokinin 2 receptor-deficient mice. Endocrinology 148: 1039–1049, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Day HE, McKnight AT, Poat JA, Hughes J. Evidence that cholecystokinin induces immediate early gene expression in the brainstem, hypothalamus and amygdala of the rat by a CCKA receptor mechanism. Neuropharmacology 33: 719–727, 1994 [DOI] [PubMed] [Google Scholar]
- 15. de Krom M, van der Schouw YT, Hendriks J, Ophoff RA, van Gils CH, Stolk RP, Grobbee DE, Adan R. Common genetic variations in CCK, leptin, and leptin receptor genes are associated with specific human eating patterns. Diabetes 56: 276–280, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Della-Fera MA, Baile CA. Cholecystokinin octapeptide: continuous picomole injections into the cerebral ventricles of sheep suppress feeding. Science 206: 471–473, 1979 [DOI] [PubMed] [Google Scholar]
- 17. Fraser KA, Raizada E, Davison JS. Oral-pharyngeal-esophageal and gastric cues contribute to meal-induced c-fos expression. Am J Physiol Regul Integr Comp Physiol 268: R223–R230, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Funakoshi A, Miyasaka K, Jimi A, Kawanai T, Takata Y, Kono A. Little or no expression of the cholecystokinin-A receptor gene in the pancreas of diabetic rats (Otsuka Long-Evans Tokushima Fatty = OLETF rats). Biochem Biophys Res Commun 199: 482–488, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Hill DR, Woodruff GN. Differentiation of central cholecystokinin receptor binding sites using the non-peptide antagonists MK-329 and L-365,260. Brain Res 526: 276–283, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Honda T, Wada E, Battey JF, Wank SA. Differential gene expression of CCK(A) and CCK(B) receptors in the rat brain. Mol Cell Neurosci 4: 143–154, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes 60: 17–23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest 103: 383–391, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindblom J, Schioth HB, Watanobe H, Suda T, Wikberg JE, Bergstrom L. Downregulation of melanocortin receptors in brain areas involved in food intake and reward mechanisms in obese (OLETF) rats. Brain Res 852: 180–185, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Lo CM, King A, Samuelson LC, Kindel TL, Rider T, Jandacek RJ, Raybould HE, Woods SC, Tso P. Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity. Gastroenterology 138: 1997–2005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lo CM, Samuelson LC, Chambers JB, King A, Heiman J, Jandacek RJ, Sakai RR, Benoit SC, Raybould HE, Woods SC, Tso P. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol Regul Integr Comp Physiol 294: R803–R810, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Marchal-Victorion S, Vionnet N, Escrieut C, Dematos F, Dina C, Dufresne M, Vaysse N, Pradayrol L, Froguel P, Fourmy D. Genetic, pharmacological and functional analysis of cholecystokinin-1 and cholecystokinin-2 receptor polymorphism in type 2 diabetes and obese patients. Pharmacogenetics 12: 23–30, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Miller LJ, Holicky EL, Ulrich CD, Wieben ED. Abnormal processing of the human cholecystokinin receptor gene in association with gallstones and obesity. Gastroenterology 109: 1375–1380, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Miyasaka K, Ichikawa M, Ohta M, Kanai S, Yoshida Y, Masuda M, Nagata A, Matsui T, Noda T, Takiguchi S, Takata Y, Kawanami T, Funakoshi A. Energy metabolism and turnover are increased in mice lacking the cholecystokinin-B receptor. J Nutr 132: 739–741, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Miyasaka K, Kanai S, Ohta M, Kawanami T, Kono A, Funakoshi A. Lack of satiety effect of cholecystokinin (CCK) in a new rat model not expressing the CCK-A receptor gene. Neurosci Lett 180: 143–146, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Monnikes H, Lauer G, Arnold R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res 770: 277–288, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Montigny D. Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Arch Gen Psychiatry 46: 511–517, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Moralejo DH, Ogino T, Kose H, Yamada T, Matsumoto K. Genetic verification of the role of CCK-AR in pancreatic proliferation and blood glucose and insulin regulation using a congenic rat carrying CCK-AR null allele. Res Commun Mol Pathol Pharmacol 109: 259–274, 2001 [PubMed] [Google Scholar]
- 34. Moralejo DH, Wei S, Wei K, Weksler-Zangen S, Koike G, Jacob HJ, Hirashima T, Kawano K, Sugiura K, Sasaki Y, Ogino T, Yamada T, Matsumoto K. Identification of quantitative trait loci for non-insulin-dependent diabetes mellitus that interact with body weight in the Otsuka Long-Evans Tokushima Fatty rat. Proc Assoc Am Physicians 110: 545–558, 1998 [PubMed] [Google Scholar]
- 35. Moran TH. Unraveling the obesity of OLETF rats. Physiol Behav 94: 71–78, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moran TH, Ameglio PJ, Peyton HJ, Schwartz GJ, McHugh PR. Blockade of type A, but not type B, CCK receptors postpones satiety in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 265: R620–R624, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Moran TH, Ameglio PJ, Schwartz GJ, McHugh PR. Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am J Physiol Regul Integr Comp Physiol 262: R46–R50, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol Regul Integr Comp Physiol 274: R618–R625, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab 302: E134–E144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ogino T, Wei S, Wei K, Moralejo DH, Kose H, Mizuno A, Shima K, Sasaki Y, Yamada T, Matsumoto K. Genetic evidence for obesity loci involved in the regulation of body fat distribution in obese type 2 diabetes rat, OLETF. Genomics 70: 19–25, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Olson BR, Freilino M, Hoffman GE, Stricker EM, Sved AF, Verbalis JG. c-Fos expression in rat brain and brainstem nuclei in response to treatments that alter food intake and gastric motility. Mol Cell Neurosci 4: 93–106, 1993 [DOI] [PubMed] [Google Scholar]
- 42. Palkovits M, Mezey E, Eskay RL. Pro-opiomelanocortin-derived peptides (ACTH/beta-endorphin/alpha-MSH) in brainstem baroreceptor areas of the rat. Brain Res 436: 323–338, 1987 [DOI] [PubMed] [Google Scholar]
- 43. Paxinos G, Watson C. The rat brain in stereotaxic coordinates (6th ed). Burlington: Academic Press, 2007 [Google Scholar]
- 44. Reidelberger RD, ORourke MF. Potent cholecystokinin antagonist L-364,718 stimulates food intake in rats. Am J Physiol Regul Integr Comp Physiol 257: R1512–R1518, 1989 [DOI] [PubMed] [Google Scholar]
- 45. Reidelberger RD, Varga G, Solomon TE. Effects of selective cholecystokinin antagonists L364,718 and L365,260 on food intake in rats. Peptides 12: 1215–1221, 1991 [DOI] [PubMed] [Google Scholar]
- 46. Rinaman L, Verbalis JG, Stricker EM, Hoffman GE. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cFos following peripheral administration of cholecystokinin. J Comp Neurol 338: 475–490, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Schick RR, Yaksh TL, Go VL. Intracerebroventricular injections of cholecystokinin octapeptide suppress feeding in rats–pharmacological characterization of this action. Regul Pept 14: 277–291, 1986 [DOI] [PubMed] [Google Scholar]
- 48. Singh L, Lewis AS, Field MJ, Hughes J, Woodruff GN. Evidence for an involvement of the brain cholecystokinin B receptor in anxiety. Proc Natl Acad Sci USA 88: 1130–1133, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sugiura K, Miyake T, Taniguchi Y, Yamada T, Moralejo DH, Wei S, Wei K, Sasaki Y, Matsumoto K. Identification of novel non-insulin-dependent diabetes mellitus susceptibility loci in the Otsuka Long-Evans Tokushima fatty rat by MQM-mapping method. Mamm Genome 10: 1126–1131, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Wang L, Martinez V, Barrachina MD, Tache Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res 791: 157–166, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Watanabe TK, Okuno S, Oga K, Mizoguchi-Miyakita A, Tsuji A, Yamasaki Y, Hishigaki H, Kanemoto N, Takagi T, Takahashi E, Irie Y, Nakamura Y, Tanigami A. Genetic dissection of “OLETF,” a rat model for non-insulin-dependent diabetes mellitus: quantitative trait locus analysis of (OLETF × BN) × OLETF. Genomics 58: 233–239, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Wei S, Wei K, Moralejo DH, Ogino T, Koike G, Jacob HJ, Sugiura K, Sasaki Y, Yamada T, Matsumoto K. Mapping and characterization of quantitative trait loci for non-insulin-dependent diabetes mellitus with an improved genetic map in the Otsuka Long-Evans Tokushima fatty rat. Mamm Genome 10: 249–258, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Weiland TJ, Voudouris NJ, Kent S. The role of CCK2 receptors in energy homeostasis: insights from the CCK2 receptor-deficient mouse. Physiol Behav 82: 471–476, 2004 [DOI] [PubMed] [Google Scholar]
- 54. West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol Regul Integr Comp Physiol 246: R776–R787, 1984 [DOI] [PubMed] [Google Scholar]
- 55. Wier JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu G, Yan J, Smith WW, Moran TH, Bi S. Roles of dorsomedial hypothalamic cholecystokinin signaling in the controls of meal patterns and glucose homeostasis. Physiol Behav 105: 234–241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]