Abstract
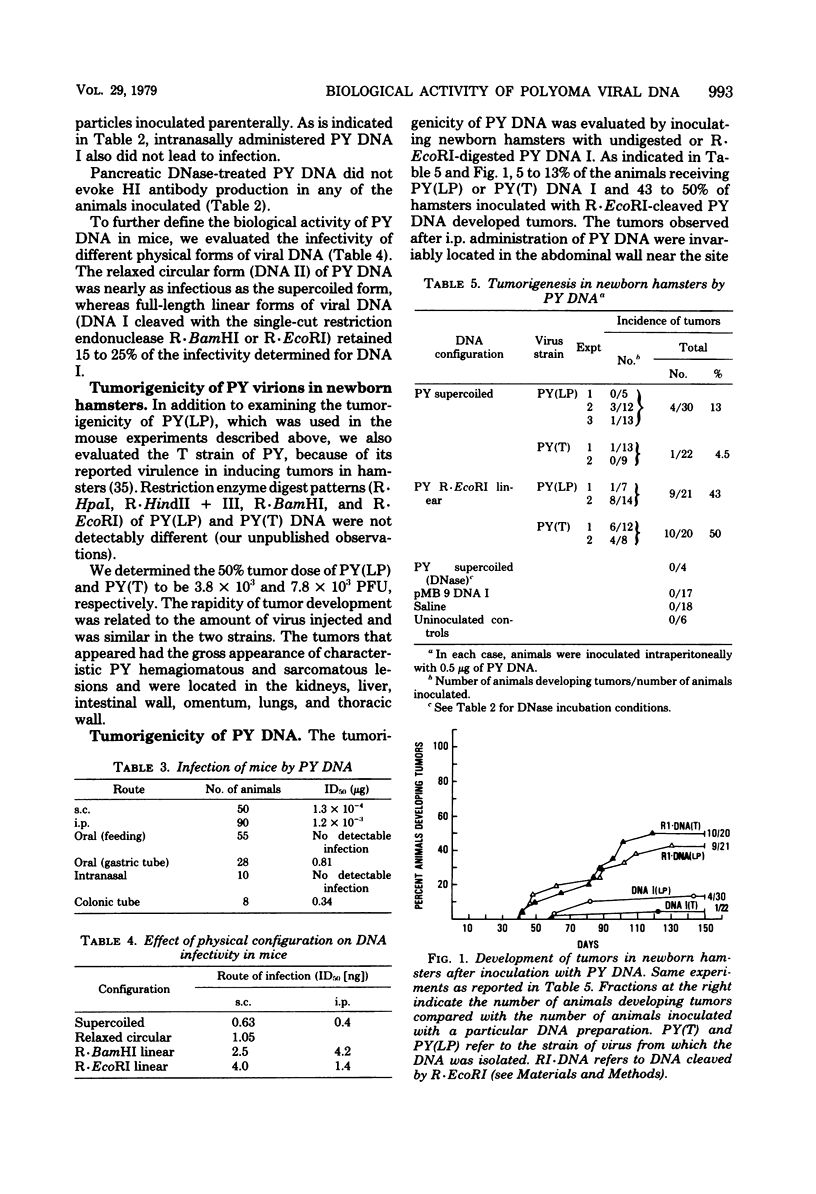
The biological activity of polyoma viral DNA was evaluated in mice and hamsters. Viral DNA administered parenterally is about 4 to 5 logs less efficient than polyoma virions in establishing infection in mice. Supercoiled viral DNA was infectious for mice after parenteral administration, giving mean infective doses of 10(-3) to 10(-4) microgram. However, animals fed microgram quantities of polyoma DNA I did not become infected. Linearization of viral DNA with R.EcoRI or R.BamHI, which are single-cut enzymes cleaving in the early and late regions of the genome, respectively, reduced the infectivity for mice approximately fivefold. Approximately 10% of newborn hamsters inoculated intraperitoneally with polyoma DNA I developed tumors. In contrast, the same amount of viral DNA which had been cleaved in the early region with R.EcoRI induced tumors in 50% of inoculated hamsters.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATANASIU P., ORTH G., REBIERE J. P., BOIRON M., PAOLETTI C. [Production of tumors in the hamster by inoculation of desoxyribonucleic acid extracted from tissue cultures infected with polyoma virus]. C R Hebd Seances Acad Sci. 1962 Jun 13;254:4228–4230. [PubMed] [Google Scholar]
- Abrahams P. J., Mulder C., Van De Voorde A., Warnaar S. O., van der Eb A. J. Transformation of primary rat kidney cells by fragments of simian virus 40 DNA. J Virol. 1975 Oct;16(4):818–823. doi: 10.1128/jvi.16.4.818-823.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams P. J., Van der Eb A. J. In vitro transformation of rat and mouse cells by DNA from simian virus 40. J Virol. 1975 Jul;16(1):206–209. doi: 10.1128/jvi.16.1.206-209.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOIRON M., THOMAS M., CHENAILLE P. A BIOLOGICAL PROPERTY OF DEOXYRIBONUCLEIC ACID EXTRACTED FROM BOVINE PAPILLOMA VIRUS. Virology. 1965 May;26:150–153. doi: 10.1016/0042-6822(65)90037-1. [DOI] [PubMed] [Google Scholar]
- Black P. H., Rowe W. P. Increase of malignant potential of BHK-21 cells by SV40 DNA without persistent new antigen. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1126–1133. doi: 10.1073/pnas.54.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett J. P., Harrington J. A. Simian adenovirus SA7 DNA: chemical, physical, and biological studies. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1023–1029. doi: 10.1073/pnas.60.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIDERHOLM H., STENKVIST B., PONTEN J., WESSLEN T. TRANSFORMATION OF BOVINE CELLS IN VITRO AFTER INOCULATION OF SIMIAN VIRUS 40 OR ITS NUCLEIC ACID. Exp Cell Res. 1965 Feb;37:452–459. doi: 10.1016/0014-4827(65)90192-8. [DOI] [PubMed] [Google Scholar]
- Dimayorca G. A., Eddy B. E., Stewart S. E., Hunter W. S., Friend C., Bendich A. ISOLATION OF INFECTIOUS DEOXYRIBONUCLEIC ACID FROM SE POLYOMA-INFECTED TISSUE CULTURES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1805–1808. doi: 10.1073/pnas.45.12.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Sompayrac L., Fluck M., Benjamin T. Localization of gene functions in polyoma virus DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4169–4173. doi: 10.1073/pnas.73.11.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Daniel M. D., Hunt R. D., Werner J., Falk L. A., Mulder C. Tumour induction with DNA of oncogenic primate herpesviruses. Nature. 1978 Jul 6;274(5666):57–59. doi: 10.1038/274057a0. [DOI] [PubMed] [Google Scholar]
- GERBER P. An infectious deoxyribonucleic acid derived from vacuolating virus (SV40). Virology. 1962 Jan;16:96–97. doi: 10.1016/0042-6822(62)90209-x. [DOI] [PubMed] [Google Scholar]
- GOLDNER H., GIRARDI A. J., LARSON V. M., HILLEMAN M. R. INTERRUPTION OF SV-40 VIRUS TUMORIGENESIS USING IRRADIATED HOMOLOGOUS TUMOR ANTIGEN. Proc Soc Exp Biol Med. 1964 Dec;117:851–857. doi: 10.3181/00379727-117-29717. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEL K. Resistance of polyoma virus immune animals to transplanted polyoma tumors. Proc Soc Exp Biol Med. 1961 Apr;106:722–725. doi: 10.3181/00379727-106-26453. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Mullarkey M. F., Takemoto K. K., Martin M. A. Characterization of human papovavirus BK DNA. J Virol. 1975 Jan;15(1):173–181. doi: 10.1128/jvi.15.1.173-181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO Y. A tumor-producing factor extracted by phenol from papillomatous tissue (Shope) of cottontail rabbits. Virology. 1960 Dec;12:596–601. doi: 10.1016/0042-6822(60)90182-3. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Byrne J. C., Martin M. A. Biologic activity of oligomeric forms of SV40 DNA. Virology. 1978 Jun 15;87(2):239–246. doi: 10.1016/0042-6822(78)90129-0. [DOI] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- Mayne N., Burnett J. P., Butler L. K. Tumour induction by simian adenovirus SA7 DNA fragments. Nat New Biol. 1971 Aug 11;232(2):182–183. doi: 10.1038/newbio232182a0. [DOI] [PubMed] [Google Scholar]
- McCULLOCH E. A., HOWATSON A. F., SIMINOVITCH L., AXELRAD A. A., HAM A. W. A cytopathogenic agent from a mammary tumour in a C3H mouse that produces tumours in Swiss mice and hamsters. Nature. 1959 May 30;183(4674):1535–1536. doi: 10.1038/1831535a0. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Murakami W. T., Fine R., Harrington M. R., Sassan Z. B. Properties and amino acid composition of polyoma virus purified by zonal ultracentrifugation. J Mol Biol. 1968 Aug 28;36(1):153–166. doi: 10.1016/0022-2836(68)90226-x. [DOI] [PubMed] [Google Scholar]
- ORTH G., ATANASIU P., BOIRON M., REBIERE J. P., PAOLETTI C. INFECTIOUS AND ONCOGENIC EFFECT OF DNA EXTRACTED FROM CELLS INFECTED WITH POLYOMA VIRUS. Proc Soc Exp Biol Med. 1964 Apr;115:1090–1095. doi: 10.3181/00379727-115-29124. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Growth curves of polyoma virus in mice and hamsters. Natl Cancer Inst Monogr. 1960 Sep;4:189–209. [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Studies of mouse polyoma virus infection. 1. Procedures for quantitation and detection of virus. J Exp Med. 1959 Apr 1;109(4):379–391. doi: 10.1084/jem.109.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P. The epidemiology of mouse polyoma virus infection. Bacteriol Rev. 1961 Mar;25:18–31. doi: 10.1128/br.25.1.18-31.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Studies on cells rendered neoplastic by polyoma virus: the problem of the presence of virus-related materials. Virology. 1962 Jan;16:41–51. doi: 10.1016/0042-6822(62)90200-3. [DOI] [PubMed] [Google Scholar]
- Warden D., Thorne H. V. The infectivity of polyoma virus DNA for mouse embryo cells in the presence of diethylaminoethyl-dextran. J Gen Virol. 1968 Dec;3(3):371–377. doi: 10.1099/0022-1317-3-3-371. [DOI] [PubMed] [Google Scholar]


