Abstract
Recent behavioral genetic research has found evidence for a gene-by-environment interaction on cognitive ability: Individual differences in cognitive ability among children raised in socioeconomically advantaged homes are primarily due to genes, whereas environmental factors are more influential for children from disadvantaged homes. We investigated the developmental origins of this interaction in a sample of 750 pairs of twins measured at 10 months and 2 years of age on the Bayley Short Form test of infant mental ability. A gene-by-environment interaction was evident for the longitudinal change in mental ability over the study period. At 10 months, genes accounted for negligible variance in mental ability across all socioeconomic strata. Rather, genetic variance emerged over development, with larger genetic influences emerging for those infants being raised in higher SES homes. By 2 years, genes accounted for nearly 50% of the variance in mental ability for children raised in high socioeconomic status homes but they continued to have negligible influences on mental ability for children raised in low socioeconomic status homes.
Keywords: Gene-by-Environment Interaction (GxE), Socioeconomic Status, Infancy, Cognitive Development, Intelligence, Behavioral Genetics
Socioeconomic status has robust relations with children’s cognitive ability and academic performance (McLoyd, 1998; Noble, McCandliss, & Farah, 2007; White, 1982), and socioeconomic disparities widen over the course of child development (Heckman, 2006; Tucker-Drob, 2010). Theoretical work in behavioral genetics has hypothesized that one major pathway through which socioeconomic disparities emerge may be an interaction between cumulative environmental disadvantage and genes: In more advantaged homes, children have the opportunity to evoke and select environmental experiences that allow them to maximize their genetic “potential” for cognitive ability, whereas this process is stifled in disadvantaged homes (Bronfenbrenner & Ceci, 1994; Dickens & Flynn, 2001; Scarr & McCartney, 1983). Evidence supporting this hypothesis comes from recent empirical work indicating that the heritability of cognitive ability, while consistently estimated to be upwards of 50% in the general population (McGue, 1997), is positively moderated by family socioeconomic status (SES). To illustrate, Turkheimer, Haley, Waldron, D’Onofrio, and Gottesman (2003) found that the heritability of cognitive ability in seven-year-old twins was only 10% in low SES families but was 72% in high SES families. This gene-by-SES interaction has been found across much of the lifespan, from middle childhood to middle adulthood (Harden, Turkheimer, & Loehlin, 2007; Kremen et al., 2005; Rowe, Jacobson, & Van den Oord, 1999), although some less conclusive findings have also been reported (Asbury, Wachs, & Plomin, 2005; van der Sluis et al., 2008).
This line of research suggests a substantial role of the environment in the expression of genetic variance in cognitive ability over child development. However, it is not yet clear when in childhood this gene-by-SES effect begins to emerge. The youngest children for whom a gene-by-SES interaction on cognitive ability has been reported were seven-year-olds (Turkheimer et al., 2003), but it is possible that socioeconomic disparities in the realization of genetic potential begin much earlier in life. SES is, of course, associated with parents’ ability to provide high-quality educational resources to their children, but socioeconomic disparities in children’s life experiences precede the beginning of formal schooling (Bradley, Corwyn, McAdoo, & Coll, 2001). For example, lower SES parents spend less time with their children (Guryan, Hurst, & Kearney, 2008), are less able to allocate time spent with children in accordance with their children’s developmental needs (Kalil, Ryan, & Corey, 2010), and are less sensitive in responding to their children’s signals, such that their children are less likely to “experience that their social initiatives are successful in establishing a reciprocal interchange with the mother” (De Wolff & Ijzendoorn, 1997, p. 571; Bradley & Corwyn, 2002). Such proximal processes between child and parent are particularly important during infancy, when children cannot actively seek out interactions that fit their needs, and must instead elicit experiences from their parents. Given the breadth of differences between the early experiences of children in high versus low SES homes, socioeconomic disparities in the realization of children’s genetic potentials for cognitive development may begin as early as infancy.
The present study tested this hypothesis using longitudinal data on infant twins (age 10 months and 2 years) from the Early Childhood Longitudinal Study – Birth Cohort (ECLS-B). Data are presented from three statistical models. The first model examined socioeconomic disparities in phenotypic (i.e., observed) mental ability. Consistent with previous research (e.g., Tucker-Drob, 2010), we predicted that SES would be positively correlated with developmental gains in mental ability, such that the disparity in mental ability between low SES and high SES children would increase from 10 months to 2 years. The second model examined the population-average contribution of genes and environmental factors to mental ability at 10-months and to change in mental ability from 10 months to 2 years. We predicted, again based on previous research (Davis, Haworth, & Plomin, 2009; Fulker, DeFries, & Plomin, 1988), that there would be genetic influences on change in mental ability, such that heritability would be higher at age 2 years. Finally, the third model tested whether SES interacts with genetic and environmental contributions to mental ability at 10 months and to change in mental ability from 10 months to 2 years. We predicted that SES would moderate the genetic contribution to change in mental ability, such that the increasing heritability of mental ability in infancy would be most evident for children from high SES families. In sum, we predicted that by late infancy, there would be significant socioeconomic disparities not only in average levels of mental ability, but in the heritable variation in mental ability.
Method
Participants
Participants were approximately 7501 pairs of twins drawn from the Early Childhood Longitudinal Survey – Birth Cohort (ECLS-B). These were all the twins in the dataset for whom zygosity information was collected, out of 800 twin pairs in total. Children were sampled from a range of locations, ethnicities, and incomes, designed to be representative of the population of American children born in 2001. The first wave of ECLS-B data was collected on children at approximately 10 months (mean = 10.4 mo; range = 7.2 to 19.5 mo), and the second wave of data was collected at approximately 2 years (mean = 24.4 mo; range = 20.9 to 33.1 mo). At each wave, children participated in several direct assessments of their abilities and behavior; 98% of our sample had cognitive assessment data at 10 months, and 94% had cognitive assessment data at 2 years. Sixty-one percent of children were White, 16% were African-American, 16% were Hispanic, 2% were Asian, less than 1% were Pacific Islander, American Indian, or Alaska Native, and 4% were of mixed race. Controlling for race and the interaction of race with genetic and environmental factors did not change the pattern of results reported below.
Zygosity
To assess the twins’ zygosity, trained observers responded to six questions about the similarity of same-sex twins with regards to hair color, hair texture, complexion, facial appearance, and ear lobe shape. Previous research has found such physical ratings by both trained observers and by twins’ parents to be highly predictive (i.e. greater than 90%) of objectively determined zygosity (Forget-Dubois et al., 2003; Goldsmith, 1991; Price, Freeman, Craig, Petrill, Ebersole, & Plomin, 2000). Observers’ responses to each feature were coded as 1 (“no difference”), 2 (“slight difference”) or 3 (“clear difference”). We summed the scores for each twin pair, resulting in a bimodal distribution of scores ranging from 6 to 18. Based on the shape of this distribution, twin pairs whose scores fell in the 6–8 range were classified as monozygotic (MZ). All other twin pairs were classified as dizygotic (DZ). Approximately 25% of twin pairs were classified as MZ, 35% as same-sex DZ, and 40% were opposite-sex DZ. To check the validity of these ratings, we computed a composite based on parents’ responses to the same questions, and dichotomized the scores in the same way. Correlating the two dichotomized distributions resulted in φ = .80. Here we report the results of analyses based on the zygosity determined from observers’ similarity ratings. Analyses based on the zygosity determined from parent ratings of their twins’ similarity produced the same pattern of results, as did analyses in which twins of with physical similarity ratings of 7, 8, and 9 were excluded.
Measures
Mental ability
At each of the two time points, an experimenter administered the Bayley Short Form – Research Edition (BSF-R; for details, see Andreassen & Fletcher, 2007), which is a shortened form of the Bayley Scales of Infant Development, Second Edition (Bayley, 1993). The BSF-R has a mental scale (composed of 29 items at 10 months and 33 items at 2 years) and a motor scale (composed of 35 items at 10 months and 32 items at 2 years). Both scales have been extensively validated using Item Response Theory for measurement invariance, unidimensionality, and discriminant validity relative to one another (Andreassen & Fletcher, 2007). Moreover, each has been placed on a “vertical” metric that is appropriate for assessing developmental change across time. The present study used scores from the mental ability scale only. Example items from this scale include pulling a string to ring a bell, putting 3 cubes in a cup, repeating vowel-consonant combinations, matching pictures, and sorting pegs by color. At the first data collection wave, the mean score was 71.14 (SD = 9.09, range = 33.84 – 112.58), and the reliability was .81. At the second data collection wave the mean score was 122.57 (SD = 10.80, range = 92.61 – 159.04), and the reliability was .88. The correlation between MZ twins was .80 at the first wave, and .76 at the second wave. The correlation between DZ twins was .77 at the first wave, and .68 at the second wave. All correlations were significant at p < .001.
Socioeconomic status
SES was computed based on parental survey data at the first assessment occasion. The SES variable was a composite of five variables: paternal and maternal education, paternal and maternal occupation, and family income (Hollingshead, 1975), each of which was standardized in the larger ECLS-B sample to a mean of 0 and a standard deviation of 1. In the larger ECLS-B sample, the SES composite had a mean of -.05 and a standard deviation of .86; in the twin subsample, its mean was .13 and its standard deviation was .87. SES ranged from −2.13 to 2.12. The correlation between SES and BSF-R Mental Scale scores was .05 (n.s.) at the first wave, and .32 (p < .001) at the second wave. A histogram of the distribution of SES is contained in the online supplement to this article.
Analytical Methods
We fit three structural equation models using full information maximum likelihood estimation in MPlus statistical software (Muthén and Muthén, 1998–2010).
Model 1: Main effects of SES on change in mental ability
To begin, we estimated a model for phenotypic change in mental ability. Model 1 specified two separate factors: a factor representing initial BSF-R performance at 10 months (y0), and a factor representing the change in performance from 10 months to 2 years (Δ). Both the initial performance factor and the change factor were regressed onto SES. This initial model is written as:
BSF-R10m,t,p = y0t,p,
BSF-R2y,t,p = y0t,p + Δ,t,p,
y0 = s0 · SESp + u0,t,p, and
Δ,t,p = sΔ · SESp + uΔ,t,p,
where t = twin, p = twin pair, s = the regression of the factor on SES, u = a residual term representing the variation in the factor unaccounted for by SES, the subscript 0 refers to the 10-month testing occasion, and the subscript Δ refers to the change from 10 months to 2 years.
Model 2: Genetic and environmental influences on change in mental ability
Next we used a biometric model for twin data to identify the relative contributions of genes and environment on infant mental ability at the 10-month testing occasion and on the developmental change from 10 months to 2 years. Each latent factor described above (initial level and change in mental ability) was modeled as a linear combination of three standardized (z-scored) biometric components: an additive genetic component (A), a shared environmental component (C, i.e., all environmental influences that make twins similar), and a nonshared environmental component (E, i.e., all environmental influences that make twins less similar, plus measurement error). Consistent with genetic theory, the correlation between temporally corresponding A components in the first and second members of each twin pair were fixed to 1.0 in MZ twin pairs and to 0.5 in DZ twin pairs (DZ pairs, like regular siblings, share approximately 50% of their genetic variance).2 The temporally corresponding C components were, by definition, perfectly correlated across all twin pairs, whereas the temporally corresponding E components were, by definition, uncorrelated across twins. All regression coefficients in the model were constrained to be the same for both twins in the model, and for both MZ and DZ twins. SES was included as a covariate to partial out the main effect of SES on mental ability scores. Thus, mental ability at 10-months and 2-years were predicted as follows:
BSF-R10m,t,p = y0t,p,
BSF-R2y,t,p = y0t,p + Δ,t,p,
y0,t,p = s0 · SESp + (a0) · A0,t,p + (c0) · C0,t,p + (e0) · E0,t,p, and
Δ,t,p = sΔ · SESp + (aΔ) · AΔ,t,p + (cΔ) · CΔ,t,p+ (eΔ) · EΔ,t,p .
The effects of the additive genetic, shared environmental, and nonshared environmental components on initial level and change in mental ability are represented with the a, c, and e regression coefficients, respectively.
Model 3: Socioeconomic differences in genetic and environmental influences on change in mental ability
In our final model, the effects of A, C, and E were allowed to vary as functions of SES (Purcell, 2002). That is, each regression path was modeled as a combination of a main effect (a, c, and e) and an interaction with SES (a’, c’, and e’). This model is written as:
BSF-R10m,t,p = y0t,p,
BSF-R2y,t,p = y0t,p + Δ,t,p,
y0,t,p = s0 · SESp + (a0+ a’0 · SESp) · A0,t,p + (c0+ c’0 · SESp) · C0,t,p + (e0 + e’0 · SESp) · E0,t,p, and
Δ,t,p = sΔ · SESp + (aΔ+ a’Δ · SESp) · AΔ,t,p + (cΔ+ c’Δ · SESp) · CΔ,t,p + (eΔ + e’Δ · SESp) · EΔ,t,p
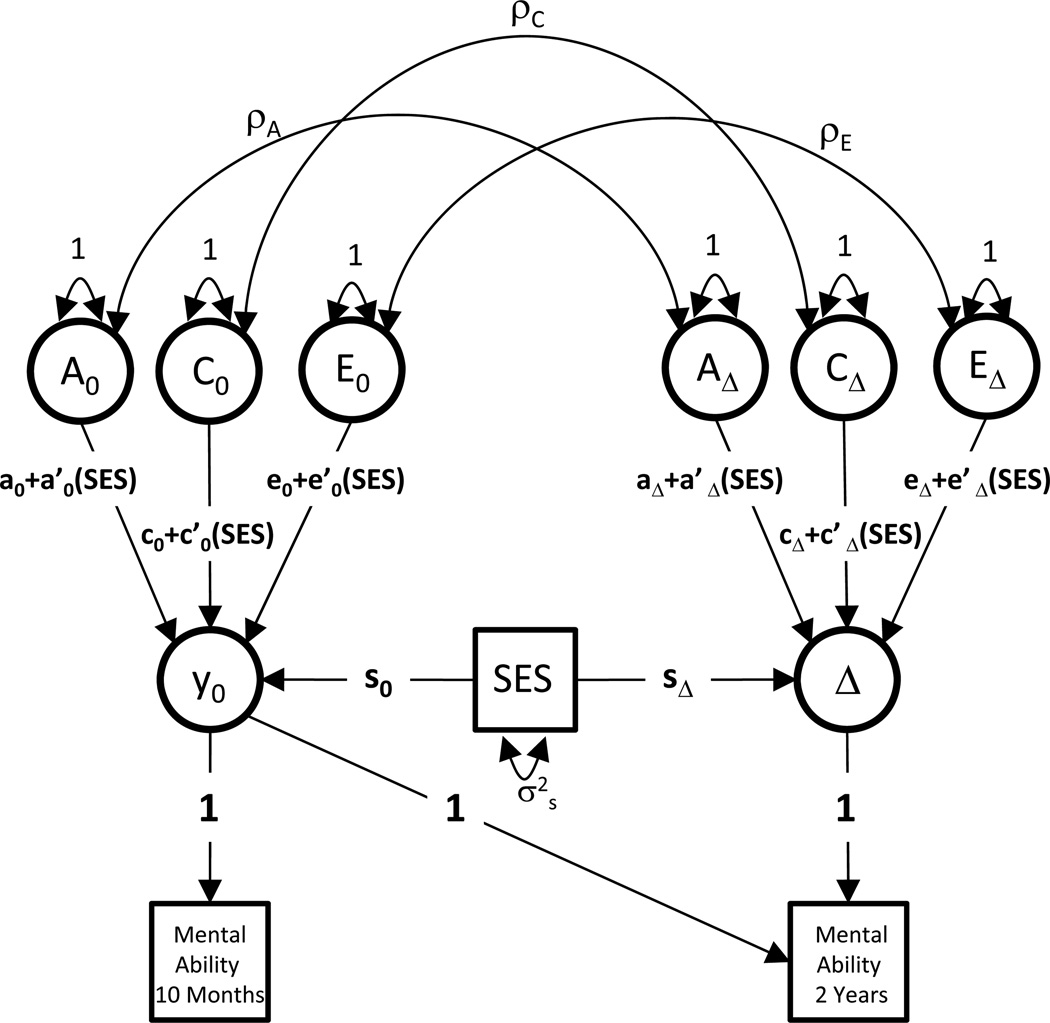
Model 3 is depicted as a path diagram in Figure 1. Note that when the interaction parameters (a’, c’, and e’) are fixed to 0, Model 3 is identical to Model 2.
Figure 1.
Path diagram of the behavior genetic model (Model 3) fit to Bayley mental ability scores at 10 months and 2 years. This diagram represents one half of the model, i.e., one twin in each pair. A, C, and E are latent factors corresponding to additive genetic influences, environmental influences shared among twins (i.e. shared environment), and unique environmental influences (i.e. nonshared environment), respectively. The 0 subscripts denote the baseline wave of measurement (age 10 months), and the Δ subscripts denote the change between baseline and follow-up (age 2 years). Bayley scores at the second time point are modeled as a function of baseline scores plus change scores.
Results
Model 1: Main Effects of SES on Mental Development
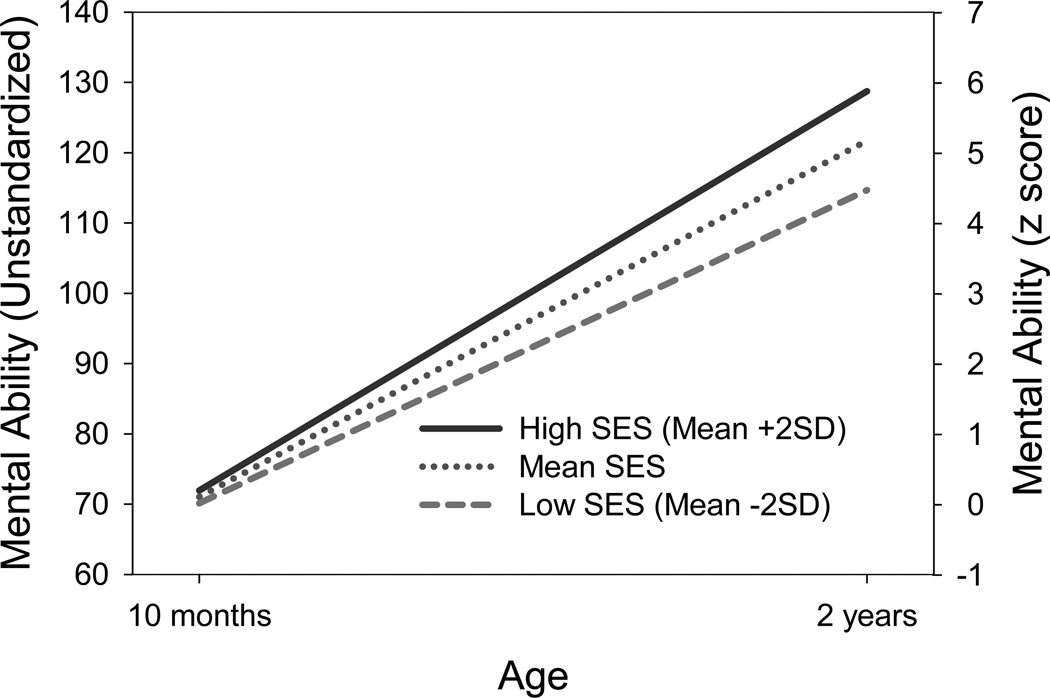
Before reporting the results from behavioral genetic models, we call attention to the three major results from the phenotypic analyses (see Table 1). First, at 10 months, SES was not related to mental ability. Second, as would be expected, mental ability increased dramatically from 10 months to 2 years. The average change was 50.85 points, which corresponds to about 5 SD units relative to the variation observed at 10 months. Third, there was a statistically significant relation between SES and the magnitude of this change. A difference in one standard deviation of SES was associated with about 3.6 points more developmental gain from 10 months to 2 years. Although this effect may appear to be small relative to the overall magnitude of gain over the study period, it is a moderate effect relative to the individual differences in mental ability at 10 months: 3.6 points of gain is over one-third of a standard deviation of baseline mental ability. Overall, the phenotypic results indicate that socioeconomic disparities in mental ability emerge in early development and are evident by 2 years, with higher SES children experiencing more rapid developmental gains in mental ability. This is illustrated in Figure 2, which plots longitudinal trends in mental ability for low, average, and high levels of SES.
Table 1.
Parameter Estimates for Model 1.
| Model 1 |
||
|---|---|---|
| Parameter | Estimate | 95% C. I. |
| s0 | .53 | (−.19, 1.26) |
| sΔ | 3.56 | (2.63, 4.50) |
| μ0 | 71.06 | (70.43, 71.69) |
| μΔ | 50.85 | (50.04, 51.66) |
| σ2u0 | 82.14 | (74.56, 89.73) |
| σ2uΔ | 142.90 | (130.29, 155,52) |
| σu0, uΔ | −60.35 | (−52.43, −68.28) |
| −2LL | 21419.60 | |
| Free parameters | 13 | |
Note: The subscript 0 represents the baseline (10 month) occasion of data collection. The subscript Δ represents the change from baseline (10 month) to follow-up (2 year) occasions of data collection. s = regression on SES. μ = mean. σ2 = variance. σ = covariance. u = variation unaccounted for by SES. Parameters in bold are significant at p<.05.
Figure 2.
Age trends in mental ability scores for low, mean, and high levels of socioeconomic status (SES). Based on parameter estimates from Model 1. The Y axis on the right is in the original units of the BSF-R. The Y axis on the left has been scaled relative to mean and standard deviation of mental ability at 10 months.
Model 2: Genetic and Environmental Influences on Mental Development
Model 2 estimated the relative contributions of genes, shared environment, and nonshared environment on infants’ initial level of mental ability and on developmental change in mental ability from 10 months to 2 years. Parameter estimates from Model 2 are presented in the left columns of Table 2. Note that adding biometric components to Model 1 did not change the model’s fit. The model test statistic (for both Model 1 and 2) was χ2 (23) = 21.77, p = .53, indicating excellent fit.
Table 2.
Parameter Estimates for Model 2 and Model 3.
| Model 2 |
Model 3 |
|||
|---|---|---|---|---|
| Parameter | Estimate | (95% C. I.) | Estimate | (95% C. I.) |
| a0 | 1.34 | (−1.76, 4.44) | .98 | (−2.73, 4.68) |
| a'0 | .90 | (−1.05, 2.84) | ||
| c0 | 7.91 | (7.32, 8.5) | 7.97 | (7.41, 8.52) |
| c'0 | −.49 | (−1.05, .06) | ||
| e0 | 4.22 | (3.80, 4.63) | 4.25 | (3.9, 4.61) |
| e'0 | −.37 | (−.78, .04) | ||
| aΔ | 5.69 | (3.82, 7.55) | 5.18 | (.13, 10.23) |
| a'Δ | 2.28 | (.2, 4.37) | ||
| cΔ | 8.38 | (7.25, 9.50) | 8.49 | (7.34, 9.63) |
| c'Δ | −1.16 | (−2.26, −.07) | ||
| eΔ | 6.36 | (5.72, 7.00) | 6.28 | (5.67, 6.89) |
| e'Δ | −.34 | (−.91, .24) | ||
| ρA | −.67 | (−1.73, .40) | −.69 | (−1.89, .51) |
| ρC | −.59 | (.50, .69) | −.60 | (−.74, −.45) |
| ρE | −.60 | (.50, .69) | −.60 | (−.74, −.45) |
| s0 | .53 | (−.19, 1.26) | .54 | (−.19, 1.27) |
| sΔ | 3.56 | (2.63, 4.50) | 3.59 | (2.64, 4.54) |
| μ0 | 71.06 | (70.43, 71.69) | 71.06 | (70.42, 71.70) |
| μΔ | 50.85 | (50.04, 51.66) | 50.85 | (50.03, 51,66) |
| −2LL | 21419.60 | 21449.08 | ||
| Free parameters | 13 | 19 | ||
| χ2Δ(df) | — | 29.48 (6) | ||
| p | — | <.001 | ||
Note: The subscript 0 represents the baseline (10 month) occasion of data collection. The subscript Δ represents the change from baseline (10 month) to follow-up (2 year) occasions of data collection. a = regression on genetic factor. c = regression on shared environment factor. e = regression on nonshared environment factor. a' = regression on gene by SES interaction. c' = regression on shared environment by SES interaction. e' = regression on nonshared environment by SES interaction. ρ = factor intercorrelation. s = regression on SES. μ = mean. Parameters in bold are significant at p<.05.
Based on the parameters of Model 2, we computed heritability coefficients for mental ability at 10 months and at 2 years, as well as the heritability of change in mental ability. These heritability coefficients represent the variability accounted for by the additive genetic component (A) as a proportion of the total variability accounted for by all three of the biometric components (A, C, and E). The heritability of mental ability at 10 months was 2% (p = .67), whereas the heritability of the change in mental ability from 10 months to 2 years was 23% (p = .003), and the heritability of mental ability at 2 years was 23% (p = .001). Thus the effect of genes on mental ability increases over infant development.
Model 3: Socioeconomic Differences in Genetic and Environmental Influences on Mental Development
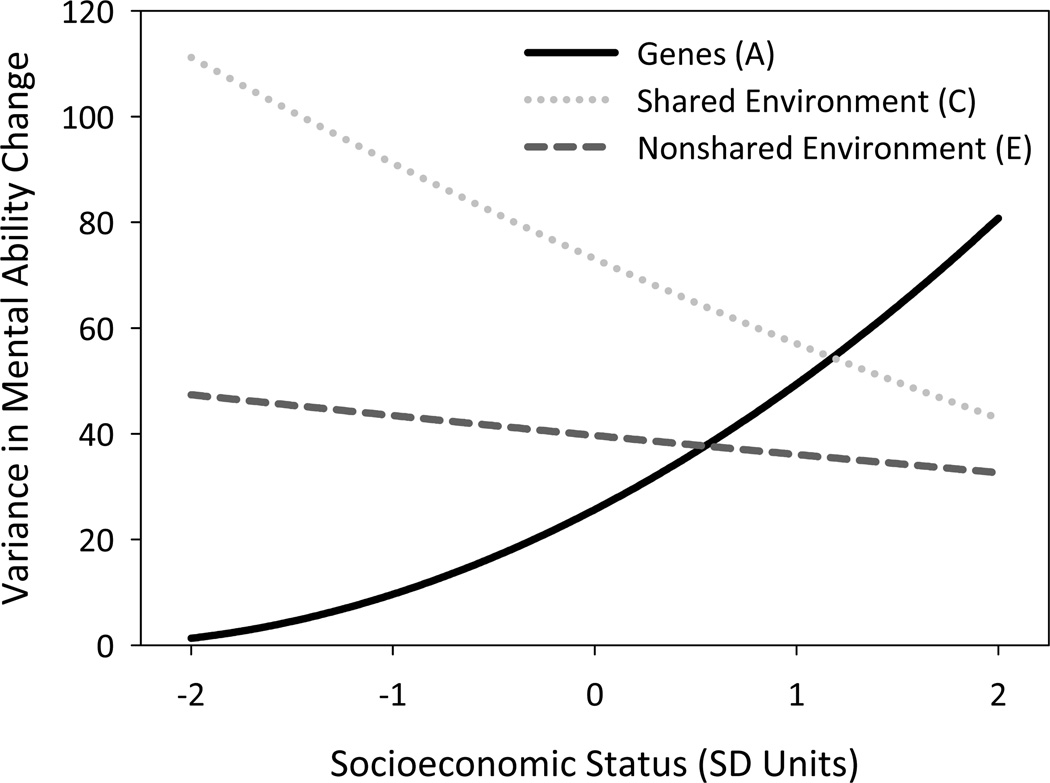
Model 3 tested whether the contribution of additive genetic, shared environmental, and nonshared environmental influences on change in mental ability were moderated by SES. Parameter estimates from Model 3 are shown in the right columns of Table 2. The pattern of significant main effects of A, C, and E was virtually identical to results obtained from Model 2. The fit of Model 3 was significantly better than that of Model 2 (p < .001). None of the interactions between SES and the effects of A, C, and E on mental ability at 10 months (a'0, c'0, e'0, respectively) was significant. For developmental change in mental ability, however, there were significant interactions between SES and both genes (a'Δ) and shared environment (c'Δ). Figure 3 illustrates these interaction effects by plotting the variance in change in mental ability from 10 months to 2 years accounted for by genetic, shared environmental, and nonshared environmental influences, as functions of SES. For children of low SES, almost all change is due to shared home environment, and genes appear to play a negligible role in the development of mental ability. For children of high SES, the opposite is true.
Figure 3.
Variance in longitudinal change in Bayley mental ability scores accounted for by genes (A), shared environment (C), and nonshared environment (E) according to SES. Based on parameter estimates from Model 3.
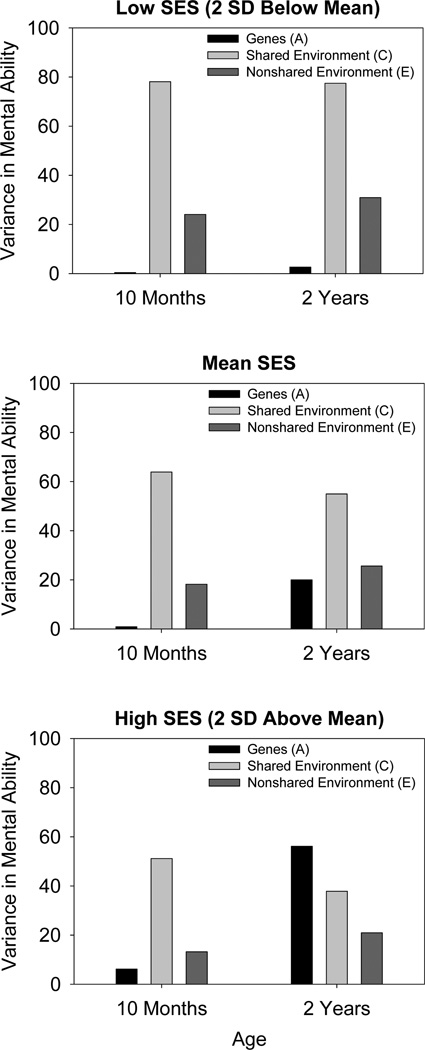
To further illustrate these findings, Figure 4 displays the Model 3-implied amounts of variance in mental ability at the 10-month and 2-year testing occasions that were accounted for by genes, shared environment, and nonshared environment at three levels of SES: −2 SD below the mean SES, mean SES, and 2 SD above the mean SES. This figure clearly shows the three-way interaction between age, SES, and the genetic influences on infant mental ability. Note the black bars, corresponding to the effects of genes. At 10 months, there is very little genetic variance in mental ability at any level of SES. At two years, however, the socioeconomic discrepancies in genetic influences on mental ability are quite large. For low-SES infants, genes play virtually no greater role at 2 years than at 10 months, whereas for high-SES infants, genes account for nearly 50% of the variability in mental ability.
Figure 4.
Variance in Bayley mental ability scores at each testing occasion accounted for by genes (A), shared environment (C), and nonshared environment (E) at low, mean, and high levels of socioeconomic status (SES). Derived from parameter estimates from Model 3.
Discussion
This article reports three main findings. First, socioeconomic disparities in mental ability emerged over development: SES was unrelated to mental ability at 10 months, but was related to change in mental ability from 10 months to 2 years, such that by 2 years each standard deviation of SES was associated with approximately one third of a standard deviation of mental ability. Second, at the population level, genes began to play a role in the development of mental ability between 10 months and 2 years. Third, the extent to which genes influenced mental development differed according to SES, such that by two years of age, genetic influences on mental ability were larger for children being raised in higher SES homes.
These findings are consistent with the hypothesis that the emergence of genetic variation in complex behavioral phenotypes depends on reciprocal interactions between the child and his or her environment (Bronfenbrenner & Ceci, 1994; Dickens & Flynn, 2001; Scarr & McCartney, 1993). According to this perspective, poor socioeconomic contexts constrain children’s opportunities to engage with supportive environments that foster cognitive growth, resulting in suppression of genetic influences on mental ability. In particular, during infancy, socioeconomic disadvantage is likely to impair an infant’s ability to elicit responsive and developmentally appropriate stimulation from caregivers (i.e., evocative processes). However, later in childhood, the role of socioeconomic status likely shifts, such that socioeconomic disadvantage restricts genetic variation in cognitive ability by limiting opportunities for individuals to actively seek out educational and social experiences that are congruent with their own genetically-influenced interests and motivations (Scarr & McCartney, 1983). We should emphasize that this specific mechanism may not generalize to all psychological outcomes, and that there are likely to be some outcomes, e.g. Attention Deficit Hyperactivity Disorder, that are actually more heritable in higher risk social environments (Pennington et al., 2009). Additionally, although socioeconomic status is often conceived of as a purely environmental variable, differences in the frequencies of specific genetic polymorphisms may also exist between different socioeconomic groups.
These findings highlight the importance of early life experience on cognitive development. Even though current evidence suggests that the predictive validity of infant mental ability for later cognitive ability is only moderate (Bornstein & Sigman, 1986; Rose & Feldman, 1995) and that children maintain a great deal of neurological and behavioral plasticity well past infancy (Garlick, 2002; Brehmer, Li, Müller, von Oertzen, & Lindenberger, 2007), the current findings build on a growing body of literature that highlights the importance of early life experiences for cognitive development (Nelson, Zeanah, Fox, Marshall, Smyke, & Guthrie, 2007). Bornstein & Signman (1986), for example, have strongly argued against the perspective “that infancy might play little or no role in determining the eventual cognitive performance of the child and, therefore, that individuals could sustain neglect in infancy if remediation were later made available” (p. 269). Heckman (2006) has recently taken an economic perspective on this topic. He has argued that early prophylactic interventions for disadvantaged children produce much higher rates of return on what he terms “human skill formation” than later remedial interventions for older children and adults. Based on this perspective, Heckman (2006) has concluded that “at current levels of funding, we overinvest in most schooling and post-schooling programs and underinvest in preschool programs for disadvantaged persons” (p. 1901).
This article makes an important contribution to the literature by establishing the developmental timing of gene-by-SES effects on mental ability. However, future research will be necessary to address a number of remaining issues. First, as in previous studies, the current study only examined the moderation of genetic influences by an omnibus index of socioeconomic status. In order to translate the current findings into useful recommendations for policy and intervention, it will be important for future research to examine the specific aspects of SES that contribute to the gene-by-SES effect, ranging from contextual aspects of neighborhoods, schools, and homes, to more proximal aspects of caregiver behavior. Second, it will be important to further investigate the developmental patterns of gene-environment correlation that have been hypothesized to underlie gene-by-SES effects on mental ability, and to identify specific child and caregiver characteristics that become matched to one another over time. Third, it will be important to identify the neurobiological foundations of gene-by-SES effects on mental ability. There is evidence that genetic differences in cognitive ability are strongly related to genetic differences in brain volume and cortical thickness (Posthuma, De Geus, Baare, Pol, Kahn, & Boomsma, 2002; Toga & Thomspon, 2005); and that the population-level heritability of brain regions that have been linked with mental ability increases with childhood age (Lenroot & Giedd, 2008). This suggests that the gene-by-SES interaction on mental ability in early childhood may be mediated by a gene-by-SES interaction on measures of regional brain volumes.
Supplementary Material
Acknowledgements
The Population Research Center at the University of Texas at Austin is supported by a center grant from the National Institute of Child Health and Human Development (R24 HD042849). The analyses presented here use data from the ECLS-B data file, which can be requested at no charge through the U.S. Department of Education National Center for Education Statistics website at http://nces.ed.gov/ecls/Birth.asp.
Footnotes
Sample sizes are rounded to the nearest 50, in accordance with ECLS-B data security regulations.
The assumed values will be incorrect under conditions of assortative mating or when twin pair zygosity is misclassified. Loehlin, Harden, & Turkheimer (2009) have demonstrated that, although varying the assumed genetic correlation between DZ twins may alter the magnitude of the estimated main effects of genes and environments, it does not appreciably alter the magnitude or significance level of the gene-by-environment interaction, which is of primary interest in the current paper. We confirmed that this was the case in the current analyses, by fitting models in which the MZ and DZ correlations deviated from 1 down to .85 and from .5 up to .65, respectively.
References
- Andreassen C, Fletcher P. Early Childhood Longitudinal Study, Birth Cohort (ECLS–B) Psychometric Report for the 2-Year Data Collection (NCES 2007–084). National Center for Education Statistics, Institute of Education Sciences) Washington, DC: U.S. Department of Education; 2007. [Google Scholar]
- Asbury K, Wachs TD, Plomin R. Environmental moderators of genetic influence on verbal and nonverbal abilities in early childhood. Intelligence. 2005;33:643–661. [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. Second Edition. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Bornstein MH, Sigman MD. Continuity in mental development from infancy. Child Development. 1986;57:251–274. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brehmer Y, Li S-C, Müller V, Oertzen T von, Lindenberger U. Memory plasticity across the life span: Uncovering children's latent potential. Developmental Psychology. 2007;43:465–478. doi: 10.1037/0012-1649.43.2.465. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: A bioecological model. Psychological Review. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Davis OSP, Haworth CMA, Plomin R. Dramatic increases in heritability of cognitive development from early to middle childhood: An 8-year longitudinal study of 8,700 pairs of twins. Psychological Science. 2009;20:1301–1308. doi: 10.1111/j.1467-9280.2009.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens WT, Flynn JR. Heritability estimates versus large environmental effects: The IQ paradox resolved. Psychological Review. 2001;108:346–369. doi: 10.1037/0033-295x.108.2.346. [DOI] [PubMed] [Google Scholar]
- Forget-Dubios N, Pérusse D, Turecki G, Girard A, Billette JM, Rouleau G, Boicin M, Malo J, Tremblay RE. Diagnosing zygosity in infant twins: physical similarity, genotyping, and chorionicity. Twin Research and Human Genetics. 2003;6:479–485. doi: 10.1375/136905203322686464. [DOI] [PubMed] [Google Scholar]
- Fulker DW, DeFries JC, Plomin R. Genetic influence on general mental ability increases between infancy and middle childhood. Nature. 1988;336:767–769. doi: 10.1038/336767a0. [DOI] [PubMed] [Google Scholar]
- Garlick D. Understanding the nature of the general factor of intelligence: the role of individual differences in neural plasticity as an explanatory mechanism. Psychological Review. 2002;109:116–136. doi: 10.1037/0033-295x.109.1.116. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. A zygosity questionnaire for young twins: A research note. Behavior Genetics. 1991;21:257–269. doi: 10.1007/BF01065819. [DOI] [PubMed] [Google Scholar]
- Guryan J, Hurst E, Kearney MS. Parental education and parental time with children. Journal of Economic Perspectives. 2008;22:23–46. [Google Scholar]
- Harden KP, Turkheimer E, Loehlin JC. Genotype by environment interaction in adolescents’ cognitive aptitude. Behavior Genetics. 2007;37:273–283. doi: 10.1007/s10519-006-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312:1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Kalil A, Ryan R, Corey M. Diverging destinies: Maternal education and investments in children. Manuscript submitted for publication. 2010 doi: 10.1007/s13524-012-0129-5. Retrieved on 5/15/2010 from http://www.popcenter.umd.edu/research/sponsored-events/atus-conf-workshop-2009/papers-atus-2009/01-kalil-etal.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Jacobson KC, Xian H, Eisen SA, Waterman B, Toomey R, Neale MC, Tsuang MT, Lyons MJ. Heritability of word recognition in middle-aged men varies as a function of parental education. Behavior Genetics. 2005;35:417–433. doi: 10.1007/s10519-004-3876-2. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: Initial findings from neuroimaging study of pediatric twins. Development and Psychopathology. 2008;20:1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehlin JC, Harden KP, Turkheimer E. The effects of assumptions about parental assortative mating and genotype-income correlation on estimates of genotype-environment interaction in the National Merit Twin Study. Behavior Genetics. 2009;39:165–169. doi: 10.1007/s10519-008-9253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M. The democracy of the genes. Nature. 1997;388:417–418. doi: 10.1038/41199. [DOI] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén L. MPlus user’s guide. Sixth Edition. Los Angeles, CA: Muthén and Muthén; 1998–2010. [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Pennington BF, et al. Gene × environment interactions in reading disability and attention-deficit/hyperactivity disorder. Developmental Psychology. 2009;45:77–89. doi: 10.1037/a0014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJC, Baare WFC, Pol HEH, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Research. 2000;3:129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF. Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Developmental Psychology. 1995;31:685–696. [Google Scholar]
- Rowe DC, Jacobson KC, Van den Oord EJCG. Genetic and environmental influences on vocabulary IQ: Parental education level as moderator. Child Development. 1999;70:1151–1162. doi: 10.1111/1467-8624.00084. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype → environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annual Review of Neuroscience. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM. How many developmental pathways underlie socioeconomic disparities in children’s cognition and achievement? Manuscript Submitted for Publication. 2010 [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman I. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- van der Sluis S, Willemsen G, de Geus EJ, Boomsma DI, Posthuma D. Gene-environment interaction in adults’ IQ scores: measures of past and present environment. Behavior Genetics. 2008;38:348–360. doi: 10.1007/s10519-008-9212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KR. The relation between socioeconomic status and academic achievement. Psychological Bulletin. 1982;91:461–481. [Google Scholar]
- De Wolff MS, van Ijzendoorn MH. Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Development. 1997;68:571–591. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.