Abstract
T cells play an indispensable role in immune defense against infectious agents, but can also be pathogenic. These T cells develop in the thymus, are exported into the periphery as naïve cells and participate in immune responses. Upon recognition of antigen, they are activated and differentiate into effector and memory T cells. While effector T cells carry out the function of the immune response, memory T cells can last up to the life time of the individual, and are activated by subsequent antigenic exposure. Throughout this life cycle, the T cell uses the same receptor for antigen, the T cell Receptor, a complex multi-subunit receptor. Recognition of antigen presented by peptide/MHC complexes on antigen presenting cells unleashes signaling pathways that control T cell activation at each stage. In this review, we discuss the signals regulated by the T cell receptor in naïve and effector/memory T cells.
Introduction
T cells are critical regulators of the mammalian immune system and express a very unique receptor that is exquisitely specific for antigen, but conventional T cells only recognize peptide antigens presented by Major Histocompatibility complex proteins (MHC) I or II presented by antigen presenting cells (APCs) (Anderson et al., 1996a). T cells undergo different stages of maturation, from antigen driven development in the thymus, to the response of naïve T cells to specific antigen in the periphery during an immune response to generate effector and memory T cells, and the response of the latter cells during antigen re-exposure. Throughout this process, they use the same TcR for signaling antigen recognition, with different outcomes for each stage of the T cell’s life (Anderson et al., 1996a). This review will discuss those early signaling pathways used by the TcR upon recognition of antigen in naïve and effector/memory T cells.
Functions
The TcR is a complex receptor with 5–6 proteins, two receptor subunits that recognize antigen (αβ or γδ), and 3–4 proteins that signal (ε, δ, γ and ζ homodimers or ζ/η heterodimers, the CD3 complex)(van der Merwe and Dushek, 2011). In the thymus, developing T cells undergo maturation and express one of the two types of TcRs, either αβ or γδ. αβ TcR-bearing T cells represent greater than 95% of all peripheral T cells and significantly more is known about its function, and this review will address this receptor, although γδ TcRs may use similar signaling pathways (Hayes et al., 2010). Immature T cell precursors rearrange gene segments within the TcR locus, placing unique V region segments upstream of the α and β chains (to generate αβ T cells), or the γ and δ chains (to generate γδ T cells). This results in T cells bearing between 2x106 and 2.5x108 unique TcRs in the periphery of mouse and humans respectively (Casrouge et al., 2000, Robins et al., 2009). While these αβ proteins recognize antigen, they have a very short cytoplasmic tails and so are thought to be unable to signal on their own. Instead, they use the associated common CD3 signaling chains for this purpose. The CD3 chains contain one (ε, δ, γ) or three (ζ) Immunoreceptor Tyrosine based activation motifs (ITAMs). The combination of 4 ITAMs in ε, δ, γ, δ/ε chains and 6 in the ζ homodimers make up a total of 10 of these motifs that connect to signaling proteins inside the T cell (Guy and Vignali, 2009, Wucherpfennig et al., 2010). In the thymus, the TcR interacts with MHC proteins carrying self-antigen resulting in positive or negative selection (Kisielow et al., 1988, Anderson et al., 1996b). If this process is not well controlled, auto reactive T cells will be allowed to leave the thymus and may cause autoimmune disease (von Boehmer and Melchers, 2010). This selective event is controlled by the strength of TcR signal; strong signals lead result in negative selection and apoptosis, while weak signals result in survival and export to the periphery (Hogquist et al., 1994, Sebzda et al., 1996). In the periphery by contrast, weak TcR signals are required for maintenance of these cells, while strong signals generate an immune response (Ernst et al., 1999, Viret et al., 1999).
Cascades and Key Molecules
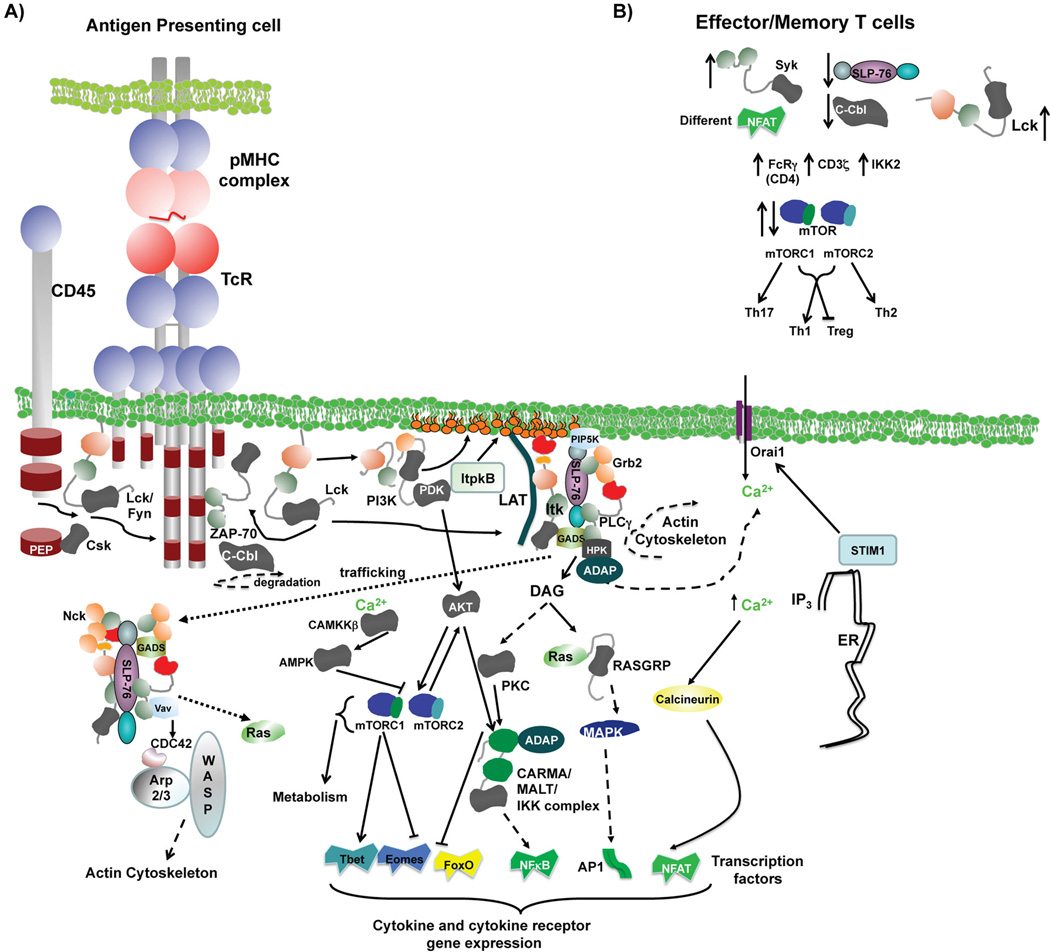
TcR interaction with MHC/peptide complexes on APCs results in clustering of the TcR, and it has been suggested that a single peptide/MHC molecule can serially trigger up to 200 TcRs for productive activation (Valitutti et al., 1995). The participation of co-receptors such as CD4 (on T helper cells) and CD8 (on cytotoxic T cells) are critical for binding to MHC molecules (CD4 to MHC II and CD8 to MHC I), and provide the TcR with the Src kinase Lck, which is associated with these co-receptors (van der Merwe and Dushek, 2011). Lck, and the related Src kinase Fyn initiate tyrosine phosphorylation of the CD3 ITAMs (Smith-Garvin et al., 2009a)(Fig. 1). Early signaling is accompanied by recruitment of the TcR into lipid rafts and a higher order structure referred to as a cSMAC. This is surrounded by a peripheral or pSMAC or ring of other cell surface proteins such as the integrins (also called distal or dSMAC) (Grakoui et al., 1999). The function of this cSMAC is controversial, but is thought to be involved in distinguishing the TcR signal (Alarcon et al., 2011).
Figure 1. Signaling pathways used by the TcR in naïve and effector/memory T cell activation.
A) Signaling pathways used by the TcR to activate T cells. Dashed lines indicate indirect interactions or connections. B) Changes in the indicated signaling molecules in the activation of effector/memory T cells compared to naïve T cells. Further details are found in the text.
Early phosphorylation of the ITAMs leads to recruitment of ZAP-70, a Syk-family non-receptor tyrosine kinase. ZAP-70 is tyrosine phosphorylated and activated by Lck, going on to phosphorylate the adaptor protein LAT (Fig. 1). Lck is negatively regulated by the balanced actions of the kinase Csk, and the phosphatases CD45 and PEP (Rhee and Veillette, 2012). ZAP-70 is regulated by the E3-ubiquitin ligase c-Cbl, which targets it for proteasomal degradation and attenuates its signal (Rao et al., 2002). The phosphorylation of LAT initiates the assembly of a ‘proximal signaling complex’ that includes LAT, which binds the adapters Grb2 and GADs. These two proteins bind to the adaptor SLP-76 (Smith-Garvin et al., 2009a). SLP-76 is phosphorylated by ZAP-70, allowing it to bind to the guanine nucleotide exchange factor Vav1 and the Tec kinases Itk and Txk/Rlk. Itk is recruited to the signaling complex via its PH domain interacting with phosphatidylinositol (3,4,5) triphosphate (PIP3), generated at the plasma membrane by the enzyme PI3K, activated by Lck and regulated by ItpkB (Huang et al., 2007, August and Ragin, 2012). Itk is also activated by direct tyrosine phosphorylation by Lck (Gibson. et al., 1996). PLCγ is also brought to the complex through its interaction with LAT and SLP-76 and is activated by tyrosine phosphorylation via both ZAP-70 and Itk (Smith-Garvin et al., 2009a, Schwartzberg et al., 2005). Lipids phosphorylated by activated PI3K also recruit and activate the kinase PDK, which further activates AKT/PKB via (Fayard et al., 2010). AKT/PKB is an important node that regulates multiple downstream pathways, including the activation of the transcription factors NFκB and those of the FoxO family, and in the activation of the mTOR pathway (Sheppard et al., 2012, Song et al., 2008). PI3K regulated pathways are counteracted by the lipid phosphatase PTEN (Song et al., 2012).
TcR-induced increases in intracellular calcium and the activation of MAPK pathways are critical in the activation of T cells. Phosphorylated PLCγ catalyzes the breakdown of PI4,5P to second messengers- IP3 and DAG. Tec kinases also enhance this process by recruiting the enzyme PIP5K to the membrane, which is able to replenish PI4,5P at the site of action (Saito et al., 2003). IP3 binds to the IP3 receptor in the membrane of ER, resulting in the release of Ca2+ from the ER. This depletion of ER Ca2+ stores results in the opening of Ca2+ channels in the plasma membrane, triggered through the sensor STIM1 communicating with CRAC channels in the plasma membrane, including the Orai1 channels (reviewed in (Hogan et al., 2010)). The resulting Ca2+ influx leads to the activation and nuclear translocation of NFAT through calcineurin and calmodulin (Smith-Garvin et al., 2009b). DAG on the other hand activates PKCθ as well as the RAS guanine nucleotide releasing protein, RASGRP. The CARMA proteins are also recruited to ADAP (which also interacts with SLP-76), and are phosphorylated by PKCθ, along with AKT, leading to activation of the NFκB transcription factor via the PKCθ/CARMA/MALT/Bcl10/NEMO/IKK pathway (Wegener et al., 2006, Narayan et al., 2006, Blonska and Lin, 2011). RASGRP on the other hand, initiates the MAPK pathway by activating Ras, leading to Raf and ERK/MAPK. Ras can also activate JNK and p38 MAPKs downstream of the TcR (Smith-Garvin et al., 2009a). The activation of other small G-proteins by TcR signals occur in part by the guanine nucleotide exchange factor Vav, which can activate CDC42 and Rac, both of which are upstream of MAPKs JNK and p38. CDC42 and Rac, along with the actin regulators Arp2/3 and WASP, are also important for regulating actin polymerization downstream of the TcR (for review see (Andreotti et al., 2010)). This TcR regulation of F-actin and retrograde actin flow also sustains the phosphorylation of PLCγ and the release of intracellular Ca2+ store (Babich et al., 2012). Antigen induced TcR clusters generates SLP-76 containing signaling complexes, which emanate from activated TcRs (Seminario and Bunnell, 2008). These SLP-76 complexes forms unique microclusters within the cell that is separate from the TcR clusters, and includes LAT, Grb-2 and GADS, along with kinases ZAP-70, Itk and HPK, and other signaling mediators Vav and PLCγ (Seminario and Bunnell, 2008). These microclusters are important for activation of downstream pathways critical for activating the T cell (Seminario and Bunnell, 2008).
Activation of the mTOR pathway is another critical event during T cell activation, controlling differentiation of multiple T cell lineages (Waickman and Powell, 2012). This pathway, composed of two complexes, mTORC1 and mTORC2, is suggested to lie downstream of the kinase AKT, although exactly how mTOR is activated downstream of the TcR remains to be determined, since AKT also lies downstream of mTORC2 (Sarbassov et al., 2006, Sarbassov et al., 2005). It is likely that mTOR activation occurs via the inactivation of a negative regulator of AMPK by CAMKKβ, acting downstream of calcium signals (Anderson et al., 2008). mTOR can also be activated by nutrient sensing and cytokine signals (Russell et al., 2011), and the mTOR complexes regulate a wide range of processes including autophagy, metabolism and transcription factor expression (such as T-bet, Eomesodermin and FoxO) and function (for review see (Chi, 2012)).
Distal TcR signaling events
The early biochemical signals from the TcR leads to the activation of a number of transcription factors that play critical roles in regulating T cell responses. These transcription factors sense the activation of the MAPK pathway (such as AP1 and Egr family members), Ca2+ increase and accompanying PKC activation (the NFAT and NFκB family members) (Smith-Garvin et al., 2009a). Increases in intracellular Ca2+ results in the activation of calcineurin, which leads to activation of NFAT and enhances NFκB activation (Hogan et al., 2010). Thus early TcR induced gene expression, including cytokines and their receptors, is largely driven by transcription by AP1, Egr, NFAT and NFκB family members (Fig. 1, (Smith-Garvin et al., 2009a)). In addition, dependent on whether the T cell is CD4+ or CD8+, effector cell specific transcription factors such as T-bet (CD4+ Th1 cells, effector CD8+ T cells), Eomesodermin (Memory CD8+ T cells), GATA3 (CD4+ Th2 cells), Rorγt (CD4+ Th17 cells), FoxO family (naïve and effector CD4+ and CD8+ T cells), and Foxp3 (CD4+ regulatory T cells), play critical roles in the function of subsequent responses (Waickman and Powell, 2012, Chi, 2012).
TcR signaling in naïve versus effector and memory T cells
During the generation of an immune response, naïve T cells utilize TcR signals, along with other environment cues/signals, including cytokines and chemokine signals, to differentiate into effector T cells and memory T cells, the latter being responsible for responses to subsequent antigenic exposure (Farber, 2009, Curtsinger et al., 1998). Manipulation of these signals can alter the activation and development of effectors versus long-term memory T cells (Araki et al., 2009, Rao et al., 2010, Waickman and Powell, 2012). Indeed, the strength of the initial TcR signal in naïve T cells may dictate the subsequent generation of effector and memory T cell populations (Williams et al., 2008). Activation of naive T cells differs from activation of effector and memory T cells, but less is known about these differences in CD4+ T cells compared to CD8+ T cells. We will discuss differences in both populations based on what is currently understood, although in some cases, effector and memory T cells have not been distinguished and so we conflate discussion of the two types. In some cases, differences in activation may be due to differential expression of signaling molecules and transcription factor family members, while in other cases, the sensitivity of the TcR is increased, such that they are less reliant on some signaling pathways for their activation, or for example due to changes in avidity of the receptor in CD8+ T cells (Hussain et al., 2002, Farber, 2009, Fahmy et al., 2001). While tyrosine phosphorylation of the CD3 complex is similar to that seen in naïve T cells, memory CD4+ and CD8+ T cells seem to have more extensive lipid rafts and phosphoprotein content, and the TcR in these cells may be more efficient at inducing tyrosine phosphorylation and/or activation of proteins such as LAT, ERK, JNK, and p38 (Kersh et al., 2003). Effector CD4+ T cells show reduced expression of c-cbl and higher basal levels of overall tyrosine phosphorylation (Krishnan et al., 2001, Brembilla et al., 2008), and in human but not mouse, CD4+ effectors express an altered CD3 signaling complex with the FcRγchain replacing the CD3-ζ chain, allowing recruitment of Syk rather than ZAP-70 (Krishnan et al., 2003). Syk has been demonstrated to be more efficient at phosphorylating downstream targets than ZAP-70 (Oliver et al., 1994). Murine memory CD4+ T cells also utilize Syk instead of ZAP-70 (Farber et al., 1997). SLP-76 expression and phosphorylation also differs between naïve and effector/memory CD4+ T cells, with reduced expression in memory cells, although it is still required for effective function of these cells (Bushar et al., 2010). SLP-76 and LAT are however, hyper phosphorylated in response to TcR signals in effector CD4+ T cells, with accompanying increases in ERK activation (Bushar et al., 2010). Naïve and memory CD4+ T cells also differ in their ability to rapidly activate NFAT, perhaps due to differences in expression of family members of this transcription factor (Dienz et al., 2007). In addition, activation of the NFκB pathway also differs between naïve and memory CD4+ T cells, with the upstream kinase IKK2 being dispensable for activation of naïve cells, but required for activation of memory T cells (Schmidt-Supprian et al., 2003). mTOR complexes also regulate the activation and/or differentiation of CD4+ effector Th cells in a differential fashion. mTORC1 and 2 are both required for the development of Th1 cells, while mTORC2 but not 1 is required for development of Th2 cells, and mTORC1 but not 2 for the development of Th17 cells. By contrast, neither mTORC1 nor 2 is required for the development of T regulatory cells (Waickman and Powell, 2012). However, it is not clear whether the upstream source of activation of the mTOR complexes is the initial or continuing TcR signal, or subsequent cytokine signals driving these cells. In addition, it is not clear whether these mTOR complexes are required (or not) for the subsequent activation of these cells once they have differentiated.
In CD8+ T cells, Lck seems to be dispensable for the activation of effector/memory T cells (Tewari et al., 2006). In addition, while the expression of ZAP-70 is not different between effector/memory and naïve CD8+ T cells, its activity is required for CD8+ effector/memory T cell activation (Bachmann et al., 1999, Slifka and Whitton, 2001, Kaech et al., 2002). mTOR complexes regulate the switch from catabolic to anabolic metabolism in effector CD8+ T cells, and the switch back to catabolic metabolism in memory CD8+ T cells (Pearce et al., 2009, Tamas et al., 2006, Peter et al., 2010, Gwinn et al., 2008). However while mTOR pathways plays a critical role in the activation of naïve CD8+ T cells to effector cells, and negatively regulate the development of CD8+ memory T cells, it is not clear which mTOR complexes are involved (Chi, 2012). In addition, as in CD4+ T cells, it is not clear which upstream source of activation of the mTOR complexes is critical for these processes, nor whether these mTOR complexes are required (or not) for the subsequent activation of these cells once they have differentiated. Nevertheless regulators of mTOR complexes such as Rapamycin could play critical roles in the activation of naïve T cells and subsequent development to and effector and memory T cells.
Associated pathologies and therapeutic implications
T cells regulate the development of vaccine induced immune memory, as well as autoimmune and other pathologies including transplant rejection, lupus, asthma and other airway inflammatory diseases, diabetes. T cells are therefore targets of a number of drugs that target the immune response, including enhancing vaccine efficacy, but also in preventing transplant rejection and other autoimmune diseases. The monoclonal antibodies against the CD3 proteins (e.g. Muromonab-CD3, Orthoclone OKT3©) has also been used effectively in the clinic to reduce T cell activation (most likely by depleting T cells or inducing immune tolerance) and reduce transplant rejection and type I diabetes (Isaacs, 2007). Two of the first significant immunosupressants, Tacrolimus (FK506, Prograf©, Advagraf© or Protopic©) and Cyclosporin A (Neoral© or Sandimmune©), target calcineurin, regulating NFAT activation (Martinez-Martinez and Redondo, 2004). These drugs are potent immunosuppressants used in the clinic to decrease the incidence of transplant rejection (Kahan, 2008). In addition, the immunosuppressant Rapamycin (Sirolimus, Rapamune©), targets mTOR, which reduces T cell proliferation (Sigal and Dumont, 1992). However, more recent exciting findings suggest that Rapamycin may be able to manipulate CD8+ T cell responses, enhancing long term development of CD8+ memory T cells, In addition, Rapamycin may be able to alter the development of CD4+ T helper subsets (Th1/2/17/Treg) (Waickman and Powell, 2012, Chi, 2012). More recent efforts have focused on targeted ZAP-70, Itk, PI3K, and MAPK (Hirabayashi et al., 2009, Lo, 2010, Norman, 2011, Chung, 2011, Trujillo, 2011). Expression of some of these targets such as ZAP-70, Lck, Fyn, and Itk are T cell specific or selective and so are much more likely to have specific effects on T cells without affecting other cell types.
The TcR is one of the most important receptors in the immune system, and signal critical events in the life of a T cell in control of the immune response. These pathways represent a rich array of targets for manipulating T cell activation and differentiation, and thus immune function in disease.
Signaling Network Facts.
T cell Receptor (TcR) – the antigen specific receptor carried by all T cells. TcRs recognize antigenic peptides only when presented by peptide/Major Histocompatibility (MHC) protein complexes found on antigen presenting cells.
The antigen specific component of the TcR is generated by rearrangements of the TcR genomic locus in each developing T cell to generate a unique TcR in association with the common CD3 signaling chains.
The TcR signals differently dependent on the differentiation state of the T cells, naïve vs. effector/memory.
Targeting TcR signaling pathways are beneficial in a number of autoimmune diseases and in transplant rejection.
Acknowledgements
We thank members of the August lab for discussions. This work was supported by grants from the National Institutes of Health (AI051626, AI065566 and AI073955 to AA) and a post-doctoral fellowship from the USDA (FH). We apologize that due to space constraints, we are unable to discuss and cite other critical papers in this area.
Abbreviations Used
- APC
Antigen Presenting Cell
- AP1
Activated Protein 1
- ADAP
adhesion and degranulation-promoting adaptor protein
- AMPK
AMP activated kinase
- AKT
RAC-alpha serine/threonine-protein kinase/Protein kinase B
- CAMKKβ
Calcium /calmodulin activated kinase kinase beta
- c-cbl
cellular Casitas B-lineage Lymphoma
- CARMA
Caspase recruitment domain (CARD)- and membrane-associated guanylate kinase-like domain-containing protein
- cSMAC
Central Supramolecular Activation Cluster
- CRAC
Calcium release activated calcium current
- DAG
diacylglycerol
- Egr
Early growth response gene
- ER
endoplasmic reticulum
- FoxO
Forkhead protein O
- FcRγ
Fc receptor gamma chain
- HPK
Hematopoietic cell kinase
- IKK
Inhibitor of nuclear factor kappa-B kinase
- Itk
Interleukin-2 inducible T cell kinase
- IP3
inositol 1,4,5-trisphosphate
- ItpkB
IP3 3-kinase B
- MHC
Major Histocompatibility Complex
- mTOR
murine Target of Rapamycin
- mTORC
murine Target of Rapamycin Complex
- NFAT
Nuclear Factor of Activated T cell
- NFκB
Nuclear Factor kappa B
- NEMO
NFκB essential modulator
- PH
Pleckstrin Homology
- PLCγ
Phospholipase C-γ
- PKCθ
Protein Kinase C theta
- PDK
3-phosphoinositide-dependent protein kinase
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PI3K
phosphatidylinositol-3-kinase
- PIP5K
1-phosphatidylinositol-5-kinase
- PEP
PEST domain enriched tyrosine phosphatase
- PTEN
Phosphatase and Tensin homolog
- pSMAC
peripheral Supramolecular Activation Cluster
- RORγt
retinoic Acid related orphan receptor gamma T
- SH2
Src Homology domain 2
- SH3
Src Homology Domain 3
- SLP-76
SH3 containing Lymphocyte protein
- STIM1
Stromal Interaction molecule 1
- Tec
Tyrosine kinase expressed in hepatocellular carcinoma
- Txk/Rlk
Tyrosine protein kinase/Resting lymphocyte kinase
- TcR
T cell Receptor
- ZAP-70
Zeta–chain-associated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- Alarcon B, Mestre D, Martinez-Martin N. The immunological synapse: a cause or consequence of T-cell receptor triggering? Immunology. 2011;133:420–425. doi: 10.1111/j.1365-2567.2011.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Moore N, Owen J, Jenkinson EJ. Cellular interactions in thymocyte development. Annu. Rev. Immunol. 1996a;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- Anderson G, Moore NC, Owen JJ, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996b;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Andreotti AH, Schwartzberg PL, Joseph RE, Berg LJ. T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harbor perspectives in biology. 2010;2:a002287. doi: 10.1101/cshperspect.a002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- August A, Ragin MJ. Regulation of T-cell responses and disease by tec kinase Itk. Int Rev Immunol. 2012;31:155–165. doi: 10.3109/08830185.2012.668981. [DOI] [PubMed] [Google Scholar]
- Babich A, Li S, O'Connor RS, Milone MC, Freedman BD, Burkhardt JK. F-actin polymerization and retrograde flow drive sustained PLCgamma1 signaling during T cell activation. J Cell Biol. 2012;197:775–787. doi: 10.1083/jcb.201201018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Gallimore A, Linkert S, Cerundolo V, Lanzavecchia A, Kopf M, Viola A. Developmental regulation of Lck targeting to the CD8 coreceptor controls signaling in naive and memory T cells. J Exp Med. 1999;189:1521–1530. doi: 10.1084/jem.189.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonska M, Lin X. NF-kappaB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55–70. doi: 10.1038/cr.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembilla NC, Weber J, Rimoldi D, Pradervand S, Schutz F, Pantaleo G, Ruegg C, Quadroni M, Harshman K, Doucey MA. c-Cbl expression levels regulate the functional responses of human central and effector memory CD4 T cells. Blood. 2008;112:652–660. doi: 10.1182/blood-2008-01-134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proc Natl Acad Sci U S A. 2010;107:827–831. doi: 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KF. p38 mitogen-activated protein kinase pathways in asthma and COPD. Chest. 2011;139:1470–1479. doi: 10.1378/chest.10-1914. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C-) to TCR/CD8 signaling in response to antigen. J Immunol. 1998;160:3236–3243. [PubMed] [Google Scholar]
- Dienz O, Eaton SM, Krahl TJ, Diehl S, Charland C, Dodge J, Swain SL, Budd RC, Haynes L, Rincon M. Accumulation of NFAT mediates IL-2 expression in memory, but not naive, CD4+ T cells. Proc Natl Acad Sci U S A. 2007;104:7175–7180. doi: 10.1073/pnas.0610442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 2001;14:135–143. [PubMed] [Google Scholar]
- Farber DL. Biochemical signaling pathways for memory T cell recall. Semin Immunol. 2009;21:84–91. doi: 10.1016/j.smim.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DL, Acuto O, Bottomly K. Differential T cell receptor-mediated signaling in naive and memory CD4 T cells. Eur J Immunol. 1997;27:2094–2101. doi: 10.1002/eji.1830270838. [DOI] [PubMed] [Google Scholar]
- Fayard E, Moncayo G, Hemmings BA, Hollander GA. Phosphatidylinositol 3-kinase signaling in thymocytes: the need for stringent control. Sci Signal. 2010;3:re5. doi: 10.1126/scisignal.3135re5. [DOI] [PubMed] [Google Scholar]
- Gibson S, August A, Branch D, Dupont B, Mills G. Functional LCK Is required for optimal CD28-mediated activation of the TEC family tyrosine kinase EMT/ITK. J Biol Chem. 1996;271:7079–7083. doi: 10.1074/jbc.271.12.7079. [DOI] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The Immunological Synapse: A Molecular Machine Controlling T Cell Activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Guy CS, Vignali DA. Organization of proximal signal initiation at the TCR:CD3 complex. Immunol Rev. 2009;232:7–21. doi: 10.1111/j.1600-065X.2009.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Laird R, Love P. Beyond alphabeta/gammadelta lineage commitment: TCR signal strength regulates gammadelta T cell maturation and effector fate. Semin Immunol. 2010;22:247–251. doi: 10.1016/j.smim.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi A, Mukaiyama H, Kobayashi H, Shiohara H, Nakayama S, Ozawa M, Miyazawa K, Misawa K, Ohnota H, Isaji M. Structure-activity relationship studies of 5-benzylaminoimidazo[1,2-c]pyrimidine-8-carboxamide derivatives as potent, highly selective ZAP-70 kinase inhibitors. Bioorg Med Chem. 2009;17:284–294. doi: 10.1016/j.bmc.2008.10.070. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Huang Y, Grasis J, Miller A, Xu R, Soonthornvacharin S, Andreotti A, Tsoukas C, Cooke M, Sauer K. Positive regulation of Itk PH domain function by soluble IP4. Science. 2007;316:886–889. doi: 10.1126/science.1138684. Epub 2007 Apr 2005. [DOI] [PubMed] [Google Scholar]
- Hussain SF, Anderson CF, Farber DL. Differential SLP-76 expression and TCR-mediated signaling in effector and memory CD4 T cells. J Immunol. 2002;168:1557–1565. doi: 10.4049/jimmunol.168.4.1557. [DOI] [PubMed] [Google Scholar]
- Isaacs JD. T cell immunomodulation--the Holy Grail of therapeutic tolerance. Curr Opin Pharmacol. 2007;7:418–425. doi: 10.1016/j.coph.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kahan BD. Fifteen years of clinical studies and clinical practice in renal transplantation: reviewing outcomes with de novo use of sirolimus in combination with cyclosporine. Transplant Proc. 2008;40:S17–S20. doi: 10.1016/j.transproceed.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Kersh EN, Kaech SM, Onami TM, Moran M, Wherry EJ, Miceli MC, Ahmed R. TCR signal transduction in antigen-specific memory CD8 T cells. J Immunol. 2003;170:5455–5463. doi: 10.4049/jimmunol.170.11.5455. [DOI] [PubMed] [Google Scholar]
- Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Warke VG, Nambiar MP, Tsokos GC, Farber DL. The FcR gamma subunit and Syk kinase replace the CD3 zeta-chain and ZAP-70 kinase in the TCR signaling complex of human effector CD4 T cells. J Immunol. 2003;170:4189–4195. doi: 10.4049/jimmunol.170.8.4189. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Warke VG, Nambiar MP, Wong HK, Tsokos GC, Farber DL. Generation and biochemical analysis of human effector CD4 T cells: alterations in tyrosine phosphorylation and loss of CD3zeta expression. Blood. 2001;97:3851–3859. doi: 10.1182/blood.v97.12.3851. [DOI] [PubMed] [Google Scholar]
- Lo HY. Itk inhibitors: a patent review. Expert Opin Ther Pat. 2010;20:459–469. doi: 10.1517/13543771003674409. [DOI] [PubMed] [Google Scholar]
- Martinez-Martinez S, Redondo JM. Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem. 2004;11:997–1007. doi: 10.2174/0929867043455576. [DOI] [PubMed] [Google Scholar]
- Narayan P, Holt B, Tosti R, Kane LP. CARMA1 is required for Aktmediated NF-kappaB activation in T cells. Mol Cell Biol. 2006;26:2327–2336. doi: 10.1128/MCB.26.6.2327-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman P. Selective PI3Kdelta inhibitors, a review of the patent literature. Expert Opin Ther Pat. 2011;21:1773–1790. doi: 10.1517/13543776.2011.629606. [DOI] [PubMed] [Google Scholar]
- Oliver JM, Burg DL, Wilson BS, McLaughlin JL, Geahlen RL. Inhibition of mast cell Fc epsilon R1-mediated signaling and effector function by the Syk-selective inhibitor, piceatannol. J Biol Chem. 1994;269:29697–29703. [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter C, Waldmann H, Cobbold SP. mTOR signalling and metabolic regulation of T cell differentiation. Curr Opin Immunol. 2010;22:655–661. doi: 10.1016/j.coi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Rao N, Dodge I, Band H. The Cbl family of ubiquitin ligases: critical negative regulators of tyrosine kinase signaling in the immune system. J Leukoc Biol. 2002;71:753–763. [PubMed] [Google Scholar]
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RC, Fang C, Guan KL. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011;138:3343–3356. doi: 10.1242/dev.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Tolias K, Saci A, Koon H, Humphries L, Scharenberg A, Rawlings D, Kinet J, Carpenter C. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–678. doi: 10.1016/s1074-7613(03)00297-8. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israel A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P, Finkelstein L, Readinger J. Tec-family kinases: regulators of T-helper-cell differentiation. Nature Reviews Immunology. 2005;5:284–295. doi: 10.1038/nri1591. [DOI] [PubMed] [Google Scholar]
- Sebzda E, Kundig TM, Thomson CT, Aoki K, Mak SY, Mayer JP, Zamborelli T, Nathenson SG, Ohashi PS. Mature T cell reactivity altered by peptide agonist that induces positive selection. J Exp Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminario MC, Bunnell SC. Signal initiation in T-cell receptor microclusters. Immunol Rev. 2008;221:90–106. doi: 10.1111/j.1600-065X.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog. 2012;17:69–95. doi: 10.1615/critrevoncog.v17.i1.60. [DOI] [PubMed] [Google Scholar]
- Sigal NH, Dumont FJ. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin J, Koretzky G, Jordan M. T cell activation. Annu Rev Immunol. 2009a;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009b;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Lei FT, Xiong X, Haque R. Intracellular signals of T cell costimulation. Cell Mol Immunol. 2008;5:239–247. doi: 10.1038/cmi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K, Walent J, Svaren J, Zamoyska R, Suresh M. Differential requirement for Lck during primary and memory CD8+ T cell responses. Proc Natl Acad Sci U S A. 2006;103:16388–16393. doi: 10.1073/pnas.0602565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo JI. MEK inhibitors: a patent review 2008–2010. Expert Opin Ther Pat. 2011;21:1045–1069. doi: 10.1517/13543776.2011.577068. [DOI] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- van der Merwe P, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol. 2011;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- Viret C, Wong FS, Janeway CA., Jr Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- Waickman AT, Powell JD. Mammalian target of rapamycin integrates diverse inputs to guide the outcome of antigen recognition in T cells. J Immunol. 2012;188:4721–4729. doi: 10.4049/jimmunol.1103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener E, Oeckinghaus A, Papadopoulou N, Lavitas L, Schmidt-Supprian M, Ferch U, Mak TW, Ruland J, Heissmeyer V, Krappmann D. Essential role for IkappaB kinase beta in remodeling Carma1-Bcl10-Malt1 complexes upon T cell activation. Mol Cell. 2006;23:13–23. doi: 10.1016/j.molcel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Gagnon E, Call MJ, Huseby ES, Call ME. Structural biology of the T-cell receptor: insights into receptor assembly, ligand recognition, and initiation of signaling. Cold Spring Harb Perspect Biol. 2010;2:a005140. doi: 10.1101/cshperspect.a005140. [DOI] [PMC free article] [PubMed] [Google Scholar]