Abstract
Introduction
It is recommended that non-operative treatment of knee osteoarthritis (KOA) should be individually tailored and include multiple treatment modalities. Despite these recommendations, no one has yet investigated the efficacy of combining several non-surgical treatment modalities in a randomised controlled study. The purpose of this randomised controlled study is to examine if an optimised, combined non-surgical treatment programme results in greater improvements in pain, function and quality of life in comparison with usual care in patients with KOA who are not eligible for total knee arthroplasty (TKA).
Methods and analysis
This study will include 100 consecutive patients from the North Denmark Region not eligible for TKA with radiographic KOA (K-L grade ≥1) and mean pain during the previous week of ≤60 mm (0–100). The participants will be randomised to receive either a 12-week non-surgical treatment programme consisting of patient education, exercise, diet, insoles, paracetamol and/or NSAIDs or usual care (two information leaflets containing information on KOA and advice regarding the above non-surgical treatment). The primary outcome will be the change from baseline to 12 months on the self-report questionnaire Knee Injury and Osteoarthritis Outcome Score (KOOS)4 defined as the average score for the subscale scores for pain, symptoms, activities of daily living and quality of life. Secondary outcomes include the five individual KOOS subscale scores, pain on a 100 mm Visual Analogue Scale, EQ-5D, self-efficacy, pain pressure thresholds, postural control and isometric knee flexion and knee extension strength.
Ethics and dissemination
This study was approved by the local Ethics Committee of The North Denmark Region (N-20110085) and the protocol conforms to the principles of the Declaration of Helsinki. Data collection will be completed by April 2014. Publications will be ready for submission in the summer of 2014.
Trial registration number
This study is registered with http://clinicaltrials.gov (NCT01535001).
Keywords: Rheumatology, Rehabilitation Medicine
Article summary.
Article focus
Does an optimised non-surgical treatment programme result in greater improvements in pain, function and quality of life in comparison with written information on non-surgical treatment options in knee osteoarthritis (KOA)?
Key messages
The results of this study will provide evidence of the efficacy of combining several non-surgical treatment modalities for KOA.
If the optimised non-surgical treatment programme improves pain, function and quality of life, it could highlight the importance of implementing the recommendations in clinical practice.
Strengths and limitations of this study
The recruitment of participants and the multimodal approach resembles contemporary examination and treatment of KOA in Denmark and several other countries.
The semistructured nature of the MEDIC-treatment enables individualisation of the treatment within the possibilities of a randomised controlled trial framework.
The multimodal approach makes it impossible to identify the efficacy of the different treatment modalities alone.
Introduction
Knee osteoarthritis (KOA) is a prevalent degenerative disease that contributes to pain, reduced functional level and poorer quality of life in older adults.1–3 As a consequence, the burden for the society, due to the cost of the interventions and the persistent clinical course, is substantial.4–6 A prevalence of up to 40% in women and 30% in men aged 65–75 years based on radiological diagnoses of KOA has been reported,7 8 while approximately 30–33% of the community-dwelling population older than 65 years have symptomatic KOA.9 10 Given that the number of people with symptomatic KOA has increased substantially during the last 20 years11 and is expected to continue to increase,12 the need to reduce the size of the problem is obvious.
It is recommended that the treatment of KOA include multiple treatment modalities,13 14 and that it should be targeted on the basis of the characteristics of the individual.13 15 This is supported by a previous randomised controlled trial (RCT) suggesting that there may be an additive effect of exercise and weight loss.16
As a result of existing evidence, a combination of patient education, exercise and weight loss are recommended as the first choice of treatment, with insoles and medication as additional treatment modalities.13–15 Exercise16–20 and weight loss16 21 22 have been shown to be effective in reducing pain and improving functional level in patients with KOA. Furthermore, there is evidence that patients with KOA undergoing patient education experience reduced pain and functional disability as well as improved well-being,19 23 24 while insoles have been recommended as part of a multimodal treatment, although the evidence concerning their efficacy is conflicting.14 25–27 Acetaminophen (paracetamol) is recommended as the primary analgaesic13–15 as it reduces pain in KOA,28 29 while short-term NSAIDs are recommended when an addition of a second analgaesic is needed due to insufficient pain control.13 14 However, clinical practice does not always reflect the recommendations30–33 and usual care in patients not eligible for a total knee arthroplasty is often only oral or written information on KOA and advice regarding recommended treatments.
Despite the recommendations of an individualised, multimodal treatment approach, no one has yet investigated the combined efficacy of all the recommended non-surgical treatment modalities in a controlled design. By combining the recommended non-surgical treatment modalities, it might be possible to optimise the treatment effect.
The purpose of this study is to examine whether a 12-week evidence-based non-surgical treatment programme (the MEDIC-treatment) results in greater improvement in quality of life, pain and function compared to usual care (two information leaflets containing information on KOA and advice regarding the recommended treatments) in patients with KOA, who are not eligible for a TKA and have no more than moderate pain.
We hypothesise that the optimised non-surgical treatment will result in significantly greater pain reduction, functional improvement and increase in quality of life than usual care at the 12-month follow-up.
Methods and analysis
Study design
This is a randomised, assessor-blinded, controlled trial of a 12-week multimodal, optimised non-surgical treatment (the MEDIC-treatment) with 12-month follow-up. Measurements will be taken at baseline, and after 12, 26 and 52 weeks. The study will conform to CONSORT guidelines for reporting parallel group randomised trials.34
Participants
Patients with a diagnosis of symptomatic and radiographic KOA considered ineligible for TKA will be included in this study.
We will recruit 100 patients meeting all of the following inclusion criteria:
Referred from primary care to an orthopaedic surgeon in a public hospital in The North Denmark Region for evaluation of the need for TKA;
Considered ineligible for a TKA by the surgeon;
Diagnosed with KOA using standing, weight-bearing knee radiographs (Kellgren-Lawrence score ≥1 on the original scale35 36);
Aged ≥18 years;
KOOS4≤75 (the average score for four of the five Knee Injury and Osteoarthritis Outcome Score subscales covering pain, symptoms, activities of daily living and quality of life).37 38
The exclusion criteria are any of the following:
Previous ipsilateral knee arthroplasty;
Rheumatoid arthritis;
Mean pain the previous week >60 mm on a 100 mm Visual Analogue Scale (VAS);
Possible pregnancy or planning pregnancy;
Inability to comply with the protocol;
Inadequacy in written and spoken Danish.
Procedure
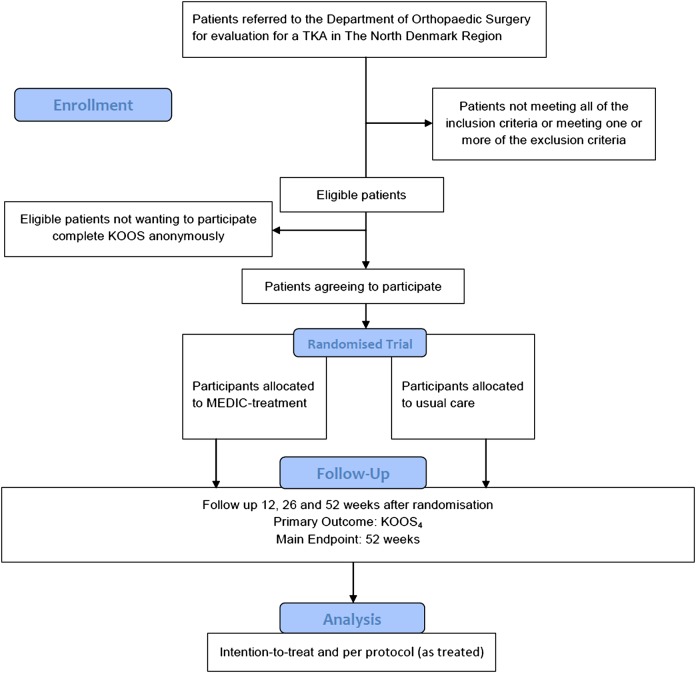
The overall structure of this study is outlined in figure 1. People in need of evaluation for TKA in The North Denmark Region are referred by their general practitioner to an orthopaedic surgeon at the outpatient clinics at Frederikshavn and Farsoe, Department of Orthopaedic Surgery, who specialises in TKAs. A standardised weight-bearing anteroposterior knee x-ray is obtained on arrival.8
Figure 1.
Flowchart.
The orthopaedic surgeon will assess potential participants against inclusion criteria 1–4 and exclusion criteria 1–2 and a research health worker assigned to the project will assess them against inclusion criterion 5 and exclusion criteria 3–6. The research health worker will obtain informed written consent from patients who are eligible and willing to participate after they have received written and verbal information. After the baseline measures are obtained, patients who agree to participate in the RCT will be assigned to one of two treatments: (1) the MEDIC-treatment or (2) usual care.
Participants will be reassessed 3 months after randomisation (12-week follow-up) and again after 6 months (26 weeks) and 12 months (52 weeks). In addition, there will be long-term follow-ups 2, 5 and 10 years after randomisation. All current medication use, co-morbidities and co-interventions will be recorded at all follow-ups.
Patients declining to participate will be asked to fill out the Knee Injury and Osteoarthritis Outcome Score (KOOS) and report age and gender anonymously so as to improve the selection bias analysis.
Randomisation procedure and concealment of allocation
Before the initiation of the trial, the schedule for randomisation will be randomly generated in permuted blocks using a computer. To control for variation in patient characteristics between the two clinics, the randomisation will be stratified according to the clinic (Frederikshavn or Farsoe). The allocation numbers will be put in concealed, opaque C5 envelopes to conceal the outcomes of the randomisation. In blocks of eight, these envelopes will be placed in consecutively numbered opaque larger envelopes (seven larger envelopes in total for each clinic). A staff member, independent of this study, will prepare the envelopes. These will only be accessible by one research assistant at each of the respective clinics. A smaller envelope from the numbered larger envelopes will be opened by the research assistant following the informed consent and completion of the baseline measures, after which the allocation will be revealed to the participant. The smaller envelopes of the second larger envelope will be added, when only two smaller envelopes are left in the first of the numbered larger envelopes. The last two of the smaller envelopes will be added, when there are six smaller envelopes left in the sixth of the seven numbered larger envelopes at each clinic.
Blinding
The outcome assessor will be blinded to group allocation, unaffiliated with the treatment sites and not involved in providing the interventions. The participants, the project physiotherapist and the project dietician delivering the interventions cannot be blinded. The statistician performing the statistical analyses will be blinded.
Interventions
MEDIC-treatment
The MEDIC-treatment consists of five different interventions. Following the clinical guidelines, patient education, exercise and weight loss are the three core elements, while insoles and pharmacological treatment will be included when meeting objective test criteria and if considered needed by the treating clinician.13–15
Participants allocated to the MEDIC-treatment will start the intervention right away. The MEDIC-treatment will take place in Aalborg. Both the project physiotherapist and the project dietician will be the same.
Patient education
The aim of the patient education is to help the participant to take responsibility for and actively engage in the treatment and management of their disease. The patient education consists of two sessions with a duration of 60 min each focusing on the diagnosis, the aetiology, symptoms, risk factors and treatment of KOA. In addition, the participants will receive a DVD containing the information provided during the patient education. Both sessions will be held by the project physiotherapist.
Exercise
The exercises will consist of the NEuroMuscular EXercise training programme for patients with osteoarthritis of the knee or hip (NEMEX-TJR).39 The NEMEX-TJR is based on neuromuscular principles and has been found feasible in patients with hip or knee OA.39 The exercise will be completed two times each week during the 12-week intervention period. Each exercise session will have a duration of 60 min.
After the intervention period the exercise will gradually shift towards home-based individual exercise, since the combination of class-based and individual home-based exercise has been shown to reduce pain more than home-based exercise alone.40
Diet
Participants with a body mass index (BMI)≥25 at baseline will be referred to a dietician for a 12-week dietary weight loss programme. The aim of the intervention is to reduce the body weight by at least 5% and retain the weight loss throughout the project period.22 Participants referred to the weight loss programme will have four dietary sessions.
Insoles
The participants will receive an individually fitted full-length Formthotics System insole with medial arch support (Foot Science International, Christchurch, New Zealand). Depending on hip-knee-foot alignment a lateral wedge will be added to the insole. The project physiotherapist will assess knee alignment using the single limb mini squat previously found to be a valid and reliable tool when investigating mediolateral motion of the knee in clinical settings.41
Medicine
Paracetamol 1 g four times daily, ibuprofen 400 mg three times daily and pantoprazol 20 mg daily will be prescribed for use during the intervention period. The prescription will be renewed every 3 weeks in order to supervise the use of, and indications for, medication. The participants will be instructed to contact the research physiotherapist if they experience pain relief, which make them question continuation of the prescription.
A more thorough description of the five elements of the MEDIC-treatment and the delivery of it is published in the study protocol for another study on KOA.42
Strategies to improve adherence
Following the intervention period, the participants will be encouraged to continue the MEDIC-treatment at home. To improve adherence there will be a transition period of 8 weeks. During the transition period all participants will alternate between class exercise home exercises. Those enrolled in the weight loss programme will be given two additional 30-min telephone sessions with the project dietician. In addition, the project physiotherapist will contact the participants by telephone eight times in the period between the transition period and the 12-month follow-up.16 43 44
Usual care
Participants allocated to usual care will be given two standardised information leaflets after the randomisation (participants allocated to MEDIC-treatment will also be given the information leaflets). The first leaflet contains general information on where in the North Denmark Region it is possible to get help changing one's lifestyle and advice on how to do it. The second leaflet holds brief information on what KOA is, symptoms of KOA and a brief overview of the current treatment options as well as some self-help tools related to KOA.
Discontinuation of allocated treatment
Participants experiencing worsening of symptoms will be reassessed by the orthopaedic surgeon assessing them at the inclusion stage. Pre-defined criteria to be considered eligible for TKA are a score for quality of life and/or for pain equal to or below 25 on the KOOS and agreement between the participant and the orthopaedic surgeon that a TKA is necessary. The reason for each discontinuation will be registered.
Baseline data
The radiographic severity of KOA will be assessed from the baseline x-ray using the Kellgren and Lawrence grading system.35 Furthermore, the following will be obtained by questionnaire: gender, age, nationality, height, alcohol intake, smoking habits, duration of KOA symptoms, previous injuries, treatment and use of medication regarding the affected knee, co-morbidities, physical activity and exercise levels, preferred treatment, previous arthroplasties, living arrangement, satisfaction with self-management of pain, education level and employment status, income, home help and the short version of the Hip/Knee Osteoarthritis Decision Quality Instrument (HK-DQI).45 After the randomisation, the participants will be asked to rate their belief in the effect of their received treatment on pain, function and quality of life.
Primary outcome measure
The primary outcome will be the change from baseline to 12 months in KOOS4, with scores ranging from 0 (worst) to 100 (best; table 1).
Table 1.
Study measures to be collected
| Instrument for data collection | Collection points | |
|---|---|---|
| Primary outcome measure | ||
| KOOS4, average score of four of the KOOS subscale scores | KOOS subscales pain, symptoms, ADL and QOL | 0, 12, 26 and 52 weeks |
| Secondary outcome measures | ||
| Pain, symptoms, ADL, Sport & Rec and QOL | KOOS | 0, 12, 26 and 52 weeks |
| Health outcome | EQ-5D-3L | 0, 12, 26 and 52 weeks |
| Self-efficacy for improving pain, function and QOL | 100 mm VAS | 0, 12, 26 and 52 weeks |
| Pain intensity in various situations | 100 mm VAS | 0, 12, 26 and 52 weeks |
| Pain location | Paper-based pain mannequin | 0, 12, 26 and 52 weeks |
| Functional performance | Timed up and go | 0, 12, 26 and 52 weeks |
| Functional performance | 20 m walk test | 0, 12, 26 and 52 weeks |
| Weight | Scale (seca 813) | 0, 12, 26 and 52 weeks |
| Maximum isometric knee muscle strength in flexion and extension | Handheld dynamometer (Powertrack II Commander) | 0, 12, 26 and 52 weeks |
| Pain reactions | Handheld algometer (Algometer Type II)—pain pressure thresholds at six sites (four sites in the peripatellar region, m. tibialis anterior, m. extensor carpi radialis longus) | 0, 12, 26 and 52 weeks |
| Postural balance | Force platform (Metitur Good Balance) | 0, 12, 26 and 52 weeks |
| Other measures | ||
| Compliance with exercise | Treatment records, log-book | Continuously |
| Use of medication | Questionnaire | 0, 12, 26 and 52 weeks |
| Compliance with diet, insoles and patient education | A five-point Likert scale (ranging from ‘never’ to ‘all the time’) | 0, 12, 26 and 52 weeks |
| Satisfaction | A five-point Likert scale (ranging from very dissatisfied to very satisfied) | 0, 12, 26 and 52 weeks |
| Adverse events | Treatment records and questionnaire | Continuously |
| Health and non-healthcare costs | Questionnaire | 0, 12, 26 and 52 weeks |
ADL, activities of daily living; QOL, quality of life; Sport & Rec, sports and recreational activities.
Secondary outcome measures
A number of other patient-reported outcome measures will be taken (table 1): The five individual subscales of KOOS (the fifth scale being difficulty in sports and recreational activities),37 38 the EQ-5D-3L,46 and pain intensity measured on a 100 mm VAS with terminal descriptors of ‘no pain’ and ‘worst pain possible’ in the following situations: at rest, after 30 min. of walking, and worst pain and least pain in the previous 24 h. The participants will be asked to shade regions on a region-divided body chart where they have had pain during the previous 24 h. Furthermore, self-efficacy in relation to reduction in pain and increase in function and quality of life using a 100 mm VAS with terminal descriptors of ‘very unsure’ and ‘very sure’ will be used in this study.
Several objective measures will be assessed (table 1). The outcome assessor will be the same as in another trial involving KOA with the same objective measure42—someone who has undergone a period of supervised training in the use of the objective measures to optimise the reliability of the measurements. As measures of the functional performance of the participants, the Timed Up and Go47 and 20 mr walk test48 will be taken in this study. In addition, percentage change in weight from baseline to follow-up will be assessed. The measurement of weight will be performed barefooted on the same scales (seca 813, seca gmbh & co. kg., Hamburg, Germany) and at the same time of day.
Maximum isometric muscle strength will be measured in knee flexion and knee extension bilaterally in a make test using a handheld dynamometer (HHD), the Powertrack II Commander (JTech Medical Industries, Salt Lake City, Utah, USA). The procedure of this objective measure has been presented previously42 for both knee extension and knee flexion. The participant will be given a 30 s rest between each measurement.
To assess pressure pain thresholds (PPTs), a hand-held pressure algometer (Algometer Type II, Somedic AB, Hoerby, Sweden) with a 1 cm2 probe will be used. The probe will be placed perpendicular to the skin and force applied at a constant rate of 30 kPa/s until the participant defines the pressure as pain and presses a button. PPTs will be assessed bilaterally at four sites in relation to bony landmarks in the peripatellar region: (1) 3 cm medial to the midpoint of the medial edge of the patella, (2) 2 cm proximal to the superior edge of the patella, (3) 3 cm lateral to the midpoint of the lateral edge of the patella and (4) at the centre of the patella. Furthermore, PPTs will be assessed at two control sites: (5) one on m. tibialis anterior (5 cm distal to the tibial tuberosity) and (6) one on m. extensor carpi radialis longus (5 cm distal to the lateral epicondyle of the humerus; figure 2). Before starting the measurement, the test is performed once or more on the dorsal aspects of the hand to make sure that the participant has understood the test procedure. A PPT will be obtained twice from each site and the mean of the two measurements will be used in the statistical analysis.49 50 The participant will be asked about the location and type of their knee pain using the interviewer-administered questionnaire Knee Pain Map, which has been found to be reliable for this purpose.51
Figure 2.
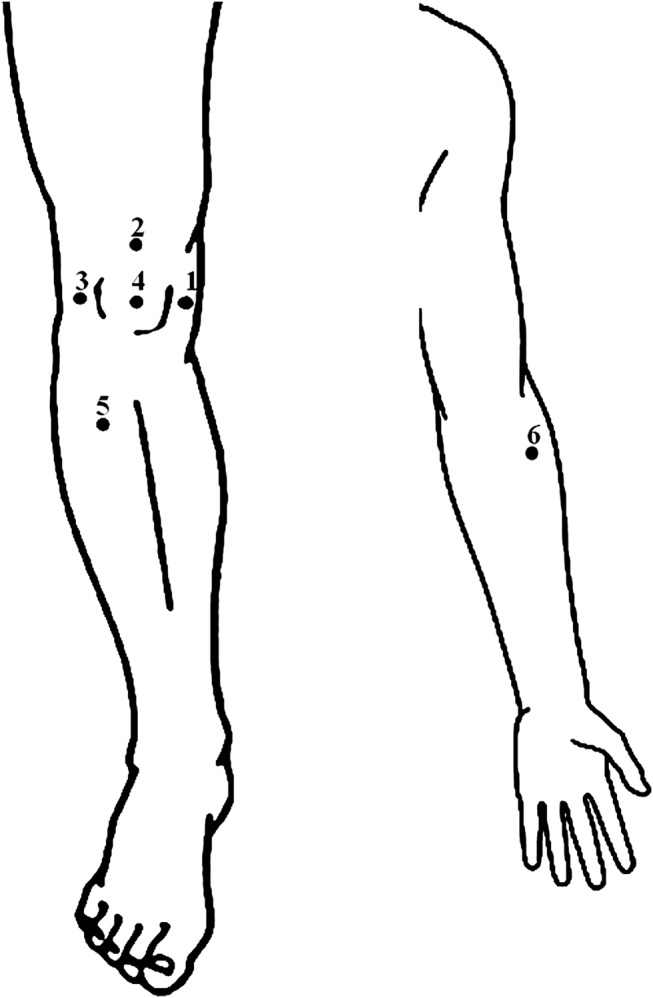
Pressure pain thresholds measurement sites.
The test setup for both isometric muscle strength and PPTs will be investigated in a test–retest reliability study on 20 participants.
Postural balance will be assessed using an instrumented force platform (Good Balance, Metitur Oy, Jyvaskyla, Finland), measuring the centre of pressure (COP) excursion body sway of the participants (100Hz). Participants will be asked to stand barefooted in a comfortable position with their feet positioned side-by-side (about a shoulder width apart). Further, they will be given the standardised cue ‘Stand as still as possible’ with their arms folded across their chest while focusing their eyes on a visual target positioned 3 m away while being tested. Four different sensory conditions will be applied to explore the contribution of different conditions to the postural control in these patients: (1) standing on a firm surface with eyes open, (2) standing on a firm surface with eyes closed, (3) standing on a soft surface (foam) with eyes open and (4) standing on a soft surface (foam) with eyes closed. Each condition will last 1 min and be repeated three times in a random order. During all measurements, an experienced experimenter will be standing next to the patient in case they lose their balance. Between each trial, participants will have the option of a rest if needed. Bipedal static COP measures have previously been proven to be a reliable tool for investigating postural balance.52
Other measures
A number of other measures will be obtained in this study (table 1). In the group allocated to MEDIC-treatment, compliance with exercise will be monitored by the physiotherapist during the intervention period as the total number of exercise sessions completed out of the planned 24 sessions (2 sessions a week for 12 weeks). Good compliance is defined as participation in 75% or more of the exercise sessions, medium compliance as participation in 50–74% of the sessions and poor compliance as participation in less than 50% of the sessions. The participants in the group allocated to the MEDIC-treatment will be requested to record their weekly exercise until the long-term follow-up 2 years after randomisation to investigate the long-term compliance. Use of medication in the group allocated to the MEDIC-treatment will be recorded in a medication diary, which will be examined as part of the follow-up. At each follow-up, all participants will be asked to report their compliance with what they have learned in this study using a five-point scale (never, every month, every week, everyday, all the time). All participants will also be asked to rate their satisfaction with the treatment to date on a five-point Likert scale at each follow-up.
Adverse and seriously adverse events will be registered in two ways and divided into index knee or sites other than index knee. The project physiotherapist will record any adverse events that the participant experiences or tells them about. At all follow-ups, the assessor will use open-probe questioning to assess adverse events in all participants.
Information on direct healthcare costs and direct non-healthcare costs will be collected retrospectively and at all follow-ups. Direct healthcare costs will include cost of the MEDIC-treatment and compliance with the treatment. These elements will be valued using published Danish prices for medical costs. Direct non-healthcare costs will include sick pay (if relevant), change in home help, number of days lost from work and shorter working hours.
Sample size
It is expected that the group allocated to MEDIC-treatment will improve 10 points more than the group allocated to usual care based on the primary outcome KOOS4 at the main endpoint after 12 months. With a common between-subject SD of 14, sample size calculations show that 41 participants in each group are required to detect a statistical difference (power of 90% and significance level at 0.05 (two-sided)). Therefore, a total of 100 participants will be included to allow for crossovers and missing data (drop-out rate will be set to 20%). The minimal clinically important difference between patients having optimised non-surgical treatment in patients not considered eligible for TKR is not known. Some studies have applied an improvement of 15% as a cut-off to determine number needed to treat (NNT).53 We will closely follow the ongoing discussion within this area and apply a cut-off supported by current knowledge at the time of analysis.
Statistical analysis
The primary outcome measure will be the KOOS4 score at the 12-month follow-up. The statistical analysis will follow an intention-to-treat approach and be based on a Generalised Estimating Equations regression model for the KOOS4 score at all follow-ups to take the repeated measurements on the patients into account. The following aspects will be incorporated in the model: the effects of treatment, follow-up time, treatment-by-follow-up time interaction, and KOOS4 score at baseline. Secondary analyses will assess heterogeneity between sites and a within-group analysis will be done to investigate if treatment compliance is associated with the change in KOOS4. Furthermore, an analysis of NNT will be performed. NNT estimates the number of people who would need to go through the MEDIC-treatment for one person to have a clinically meaningful improvement in KOOS4 from baseline to the follow-ups.
Ethics and dissemination
Ethical considerations
The protocol is designed to conform to the principles of the Declaration of Helsinki and has been approved by the local Ethics Committee of The North Denmark Region (N-20110085). The participants in this study will be allocated to either usual care or the MEDIC-treatment, which means that the treatment they receive will be either equivalent to, or superior to, the treatment that they would receive if they did not participate in this study.
Timelines and dissemination plans
Approval from The Danish Data Protection Agency was given in January 2012 while ethics approval was obtained from The North Denmark Region in February 2012. Recruitment and training of the involved project physiotherapist and dietician were undertaken in July and August 2011 and recruitment of participants started in April 2012.
All participants are expected to have completed the 12-month follow-up by April 2014. The statistical analysis will start immediately after the data monitoring is completed. Publications will be ready for submission in the summer of 2014.
Conclusions
The lack of evidence regarding the efficacy of the currently recommended multimodal non-surgical treatment approach to knee osteoarthritis (KOA) indicates a strong need for thoroughly designed clinical trials. Therefore, we have designed this study as a randomised controlled trial to investigate if a 12-week optimised, multimodal non-surgical treatment is more efficacious than written information on non-surgical treatment options in patients with KOA not eligible for a total knee arthroplasty. Since it is the first study combining these recommended treatments in a randomised controlled study, the results will provide evidence about the efficacy of the combination of non-surgical treatment modalities for KOA.
Supplementary Material
Acknowledgments
We would like to thank The Danish Rheumatism Association and The Association of Danish Physiotherapists Research Fund for their support.
Footnotes
Contributions: STS is leading the co-ordination of the trial. STS, EMR, MBL, MSR, LAN, OS and SR assisted with the protocol design and procured the project funding. STS wrote this manuscript. All authors participated in the trial design, provided feedback on drafts of this paper and read and approved the final manuscript.
Funding: This trial is partially funded by The Danish Rheumatism Association and The Association of Danish Physiotherapists Research Fund. The funders do not have any role in this study other than to provide funding.
Competing interests: None.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365:965–73 [DOI] [PubMed] [Google Scholar]
- 3.Mantyselka P, Kumpusalo E, Ahonen R, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain 2001;89:175–80 [DOI] [PubMed] [Google Scholar]
- 4.Healy WL, Iorio R, Ko J, et al. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am 2002;84-A:348–53 [DOI] [PubMed] [Google Scholar]
- 5.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotlarz H, Gunnarsson CL, Fang H, et al. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum 2009;60:3546–53 [DOI] [PubMed] [Google Scholar]
- 7.van Saase JL, van Romunde LK, Cats A, et al. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis 1989;48:271–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laxafoss E, Jacobsen S, Gosvig KK, et al. Case definitions of knee osteoarthritis in 4,151 unselected subjects: relevance for epidemiological studies: the Copenhagen Osteoarthritis Study. Skeletal Radiol 2010;39:859–66 [DOI] [PubMed] [Google Scholar]
- 9.Dawson J, Linsell L, Zondervan K, et al. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology (Oxford) 2004;43:497–504 [DOI] [PubMed] [Google Scholar]
- 10.Mannoni A, Briganti MP, Di Bari M, et al. Epidemiological profile of symptomatic osteoarthritis in older adults: a population based study in Dicomano, Italy. Ann Rheum Dis 2003;62:576–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen US, Zhang Y, Zhu Y, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med 2011;155:725–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt HL, Katz JN, Reichmann WM, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60–64-year-old US adults. Osteoarthritis Cartilage 2011;19:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;16:137–62 [DOI] [PubMed] [Google Scholar]
- 15.National Collaborating Centre for Chronic Conditions (UK) 2008. Osteoarthritis: National clinical guideline for care and management in adults.
- 16.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 2004;50:1501–10 [DOI] [PubMed] [Google Scholar]
- 17.Jamtvedt G, Dahm KT, Christie A, et al. Physical therapy interventions for patients with osteoarthritis of the knee: an overview of systematic reviews. Phys Ther 2008;88:123–36 [DOI] [PubMed] [Google Scholar]
- 18.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 2008;(4):CD004376. [DOI] [PubMed] [Google Scholar]
- 19.Devos-Comby L, Cronan T, Roesch SC. Do exercise and self-management interventions benefit patients with osteoarthritis of the knee? A metaanalytic review. J Rheumatol 2006;33:744–56 [PubMed] [Google Scholar]
- 20.Roddy E, Zhang W, Doherty M. Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann Rheum Dis 2005;64:544–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messier SP. Obesity and osteoarthritis: disease genesis and nonpharmacologic weight management. Rheum Dis Clin North Am 2008;34:713–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen R, Bartels EM, Astrup A, et al. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2007;66:433–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Superio-Cabuslay E, Ward MM, Lorig KR. Patient education interventions in osteoarthritis and rheumatoid arthritis: a meta-analytic comparison with nonsteroidal antiinflammatory drug treatment. Arthritis Care Res 1996;9:292–301 [DOI] [PubMed] [Google Scholar]
- 24.Warsi A, LaValley MP, Wang PS, et al. Arthritis self-management education programs: a meta-analysis of the effect on pain and disability. Arthritis Rheum 2003;48:2207–13 [DOI] [PubMed] [Google Scholar]
- 25.Brouwer RW, Jakma TS, Verhagen AP, et al. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev 2005;(1):CD004020. [DOI] [PubMed] [Google Scholar]
- 26.Hinman RS, Bennell KL. Advances in insoles and shoes for knee osteoarthritis. Curr Opin Rheumatol 2009;21:164–70 [DOI] [PubMed] [Google Scholar]
- 27.Skou ST, Hojgaard L, Simonsen O. Custom made insoles have a positive effect on pain, function and quality of life in patients with medial knee osteoarthritis. J Am Podiatr Med Assoc In press [DOI] [PubMed] [Google Scholar]
- 28.Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev 2006;(1):CD004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;18:476–99 [DOI] [PubMed] [Google Scholar]
- 30.DeHaan MN, Guzman J, Bayley MT, et al. Knee osteoarthritis clinical practice guidelines—how are we doing? J Rheumatol 2007;34:2099–105 [PubMed] [Google Scholar]
- 31.Jordan KM, Sawyer S, Coakley P, et al. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology (Oxford) 2004;43:381–4 [DOI] [PubMed] [Google Scholar]
- 32.Hunter DJ, Neogi T, Hochberg MC. Quality of osteoarthritis management and the need for reform in the US. Arthritis Care Res (Hoboken) 2011;63:31–8 [DOI] [PubMed] [Google Scholar]
- 33.Snijders GF, den Broeder AA, van Riel PL, et al. Evidence-based tailored conservative treatment of knee and hip osteoarthritis: between knowing and doing. Scand J Rheumatol 2011;40:225–31 [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kellgren JH, Jeffrey MR, Ball J. The epidemiology of chronic rheumatism. Atlas of standard radiographs of arthritis. Oxford, UK: Blackwell Scientific Publications, 1963 [Google Scholar]
- 37.Roos EM, Roos HP, Lohmander LS, et al. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 1998;28:88–96 [DOI] [PubMed] [Google Scholar]
- 38.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes 2003;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ageberg E, Link A, Roos EM. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord 2010;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy CJ, Mills PM, Pullen R, et al. Supplementing a home exercise programme with a class-based exercise programme is more effective than home exercise alone in the treatment of knee osteoarthritis. Rheumatology (Oxford) 2004;43:880–6 [DOI] [PubMed] [Google Scholar]
- 41.Ageberg E, Bennell KL, Hunt MA, et al. Validity and inter-rater reliability of medio-lateral knee motion observed during a single-limb mini squat. BMC Musculoskelet Disord 2010;11:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skou ST, Roos EM, Laursen MB, et al. Total knee replacement plus physical and medical therapy or treatment with physical and medical therapy alone: a randomised controlled trial in patients with knee osteoarthritis (the MEDIC-study). BMC Musculoskelet Disord 2012;13:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pisters MF, Veenhof C, Schellevis FG, et al. Exercise adherence improving long-term patient outcome in patients with osteoarthritis of the hip and/or knee. Arthritis Care Res (Hoboken) 2010;62:1087–94 [DOI] [PubMed] [Google Scholar]
- 44.Pisters MF, Veenhof C, van Meeteren NL, et al. Long-term effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review. Arthritis Rheum 2007;57:1245–53 [DOI] [PubMed] [Google Scholar]
- 45.Sepucha KR, Stacey D, Clay CF, et al. Decision quality instrument for treatment of hip and knee osteoarthritis: a psychometric evaluation. BMC Musculoskelet Disord 2011;12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szende A, Williams A. Measuring self-reported population health: an international perspective based on EQ-5D. Budapest: SpringMed Publishing, 2004 [PubMed] [Google Scholar]
- 47.Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8 [DOI] [PubMed] [Google Scholar]
- 48.White DK, Zhang Y, Niu J, et al. Do worsening knee radiographs mean greater chances of severe functional limitation? Arthritis Care Res (Hoboken) 2010;62:1433–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81 [DOI] [PubMed] [Google Scholar]
- 50.Skou ST, Graven-Nielsen T, Lengsoe L, et al. Relating clinical measures of pain with experimentally assessed pain mechanisms in patients with knee osteoarthritis. Scand J Pain In press. [DOI] [PubMed] [Google Scholar]
- 51.Thompson LR, Boudreau R, Hannon MJ, et al. The knee pain map: reliability of a method to identify knee pain location and pattern. Arthritis Rheum 2009;61:725–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruhe A, Fejer R, Walker B. The test-retest reliability of centre of pressure measures in bipedal static task conditions–a systematic review of the literature. Gait Posture 2010;32:436–45 [DOI] [PubMed] [Google Scholar]
- 53.Hurley MV, Walsh NE, Mitchell H, et al. Long-term outcomes and costs of an integrated rehabilitation program for chronic knee pain: a pragmatic, cluster randomized, controlled trial. Arthritis Care Res (Hoboken) 2012;64:238–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.