Abstract
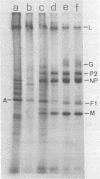
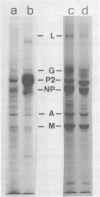
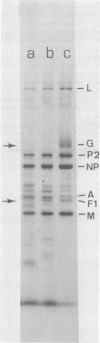
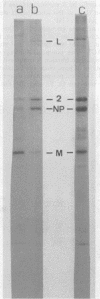
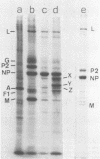
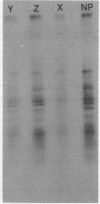
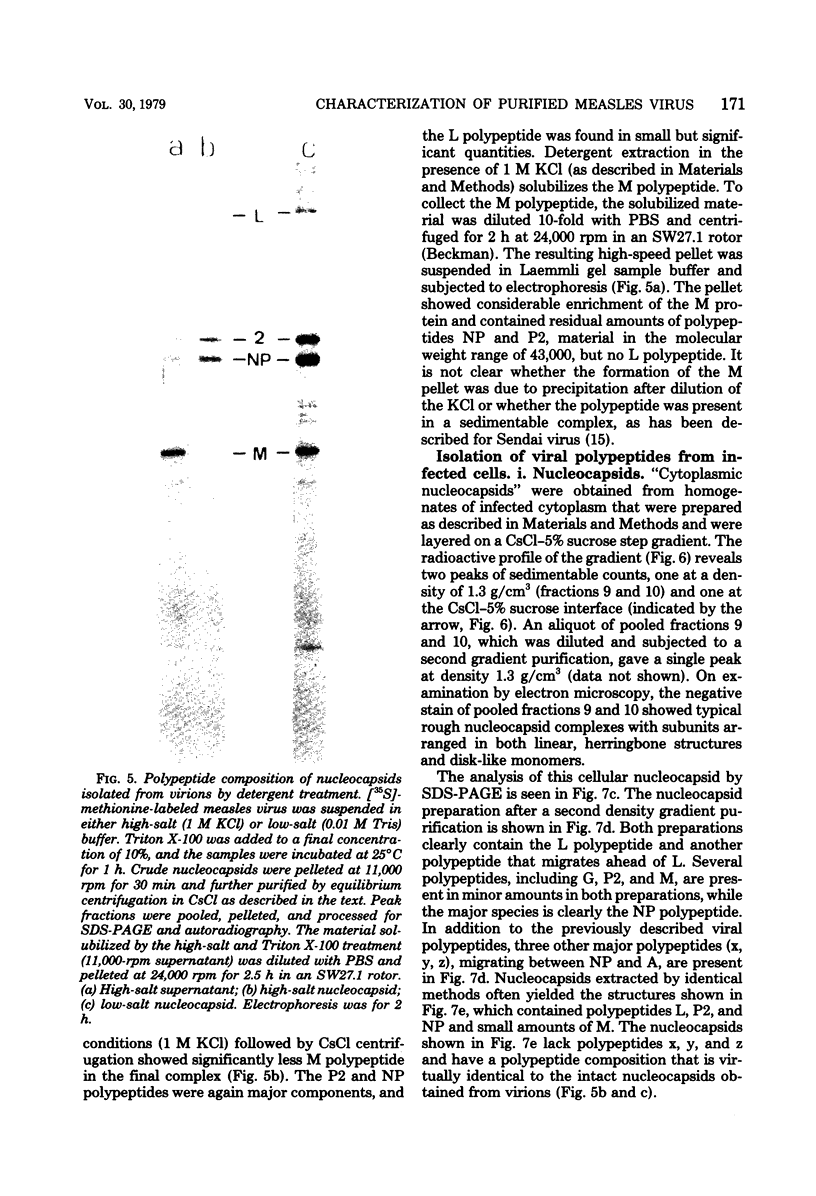
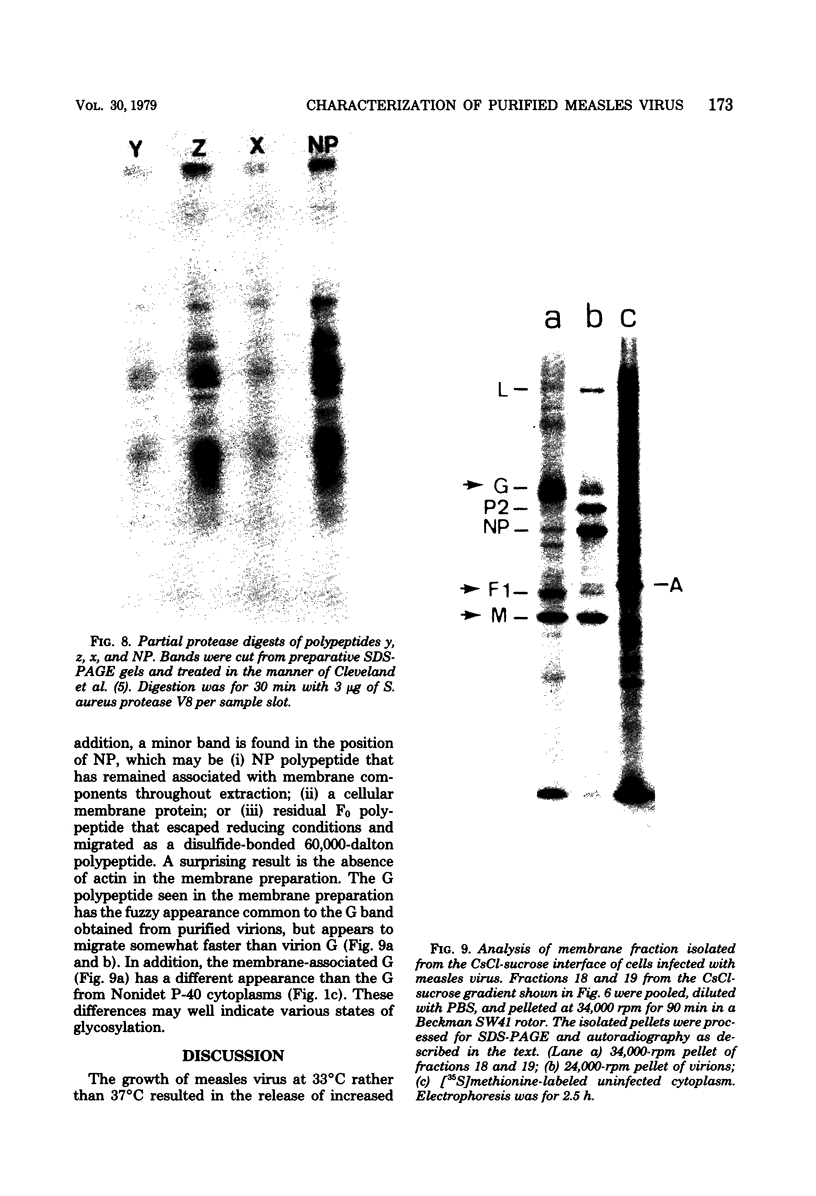
Purified measles virus was obtained from [35S]methionine-labeled cells infected at 33 degrees C and maintained in the absence of fetal calf serum. The pellet that was produced by a single high-speed ultracentrifuge spin of culture medium contained virus of purity sufficient for structural analysis. Purified virions contain seven polypeptides with estimated molecular weights of: L, 200,000; G, 80,000; P2, 70,000; NP, 60,000; A, 43,000; F1, 41,000; and M, 37,000, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions. Treatment of virions with 0.25% trypsin resulted in a less dense particle which lacked polypeptides G and F1. Solubilization of the viral membrane with the detergent Triton X-100 in low-salt buffer resulted in the loss of the G polypeptide, whereas in the presence of 1 M KCl, Triton X-100 also removed most of the M polypeptide. The nucleocapsids (p = 1.3) obtained from virions treated with Triton X-100 and 1 M KCl contained the L, P2, NP, and M polypeptides. Nucleocapsids isolated from the cytoplasm of infected cells were predominantly composed of the NP polypeptide with smaller amounts of either polypeptide P2 or novel polypeptides, related to NP, with estimated molecular weights of 56,000 to 58,000 and 45,000 to 46,000. A significant amount of polypeptide L was always found in association with nucleocapsids isolated either from virions or from the cytoplasm of infected cells. A membrane component containing the viral membrane polypeptides G, F1, and M was also isolated from infected cells. The data presented here thus suggest that L is an integral part of the nucleocapsid complex. In addition, 37,000-molecular-weight polypeptide (M) appears to have the function described for the matrix proteins of other paramyxoviruses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buetti E., Choppin P. W. The transcriptase complex of the paramyxovirus SV5. Virology. 1977 Oct 15;82(2):493–508. doi: 10.1016/0042-6822(77)90021-6. [DOI] [PubMed] [Google Scholar]
- Bussell R. H., Waters D. J., Seals M. K. Measles, canine distemper and respiratory syncytial virions and nucleocapsids. A comparative study of their structure, polypeptide and nucleic acid composition. Med Microbiol Immunol. 1974;160(2-3):105–124. doi: 10.1007/BF02121718. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F. Surface structure of vesicular stomatitis virus. J Gen Virol. 1969 Jul;5(1):1–10. doi: 10.1099/0022-1317-5-1-1. [DOI] [PubMed] [Google Scholar]
- Cathala F., Brown P. The possible viral aetiology of disseminated sclerosis. J Clin Pathol Suppl (R Coll Pathol) 1972;6:141–151. [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Stone H. O. Isolation of a transcriptive complex from Newcastle disease virions. J Virol. 1976 Sep;19(3):1080–1089. doi: 10.1128/jvi.19.3.1080-1089.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc Natl Acad Sci U S A. 1967 Apr;57(4):949–956. doi: 10.1073/pnas.57.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves M. C., Silver S. M., Choppin P. W. Measles virus polypeptides synthesis in infected cells. Virology. 1978 May 1;86(1):254–263. doi: 10.1016/0042-6822(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Kiessling W., ter Meulen V. Membrane proteins of subacute sclerosing panencephalitis and measles viruses. Nature. 1978 Mar 30;272(5652):460–462. doi: 10.1038/272460a0. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. Purification and characterization of measles virus. J Gen Virol. 1973 May;19(2):175–188. doi: 10.1099/0022-1317-19-2-175. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. The biochemical and biological characteristics of the surface components of measles virus. J Gen Virol. 1974 Mar;22(3):363–374. doi: 10.1099/0022-1317-22-3-363. [DOI] [PubMed] [Google Scholar]
- Hardwick J. M., Bussell R. H. Glycoproteins of measles virus under reducing and nonreducing conditions. J Virol. 1978 Feb;25(2):687–692. doi: 10.1128/jvi.25.2.687-692.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J. A., Nermut M. V. A morphological study of the M-protein of Sendai virus. J Gen Virol. 1977 Jan;34(1):127–136. doi: 10.1099/0022-1317-34-1-127. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy N. L., Auerbach P. S., Hayes E. C. A blood test for multiple sclerosis based on the adherence of lymphocytes to measles-infected cells. N Engl J Med. 1976 Jun 24;294(26):1423–1427. doi: 10.1056/NEJM197606242942604. [DOI] [PubMed] [Google Scholar]
- Marx P. A., Portner A., Kingsbury D. W. Sendai virion transcriptase complex: polyeptide composition and inhibition by virion envelope proteins. J Virol. 1974 Jan;13(1):107–112. doi: 10.1128/jvi.13.1.107-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Lackland H., Choppin P. W. Isolation and characterization of the nonglycosylated membrane protein and a nucleocapsid complex from the paramyxovirus SV5. Virology. 1975 Oct;67(2):365–374. doi: 10.1016/0042-6822(75)90438-9. [DOI] [PubMed] [Google Scholar]
- Moore P. M., Hayes E. C., Miller S. E., Wright L. L., Machamer C. E., Zweerink H. J. Measles virus nucleocapsids: large-scale purification and use in radioimmunoassays. Infect Immun. 1978 Jun;20(3):842–846. doi: 10.1128/iai.20.3.842-846.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Choppin P. W. A comparison of the polypeptides of four measles virus strains. Virology. 1977 May 15;78(2):463–474. doi: 10.1016/0042-6822(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Caliguiri L. A., Choppin P. W. Nucleocapsid protein subunits of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1970 Nov;6(5):677–684. doi: 10.1128/jvi.6.5.677-684.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Lackland H., Choppin P. W. Proteolytic cleavage of subunits of the nucleocapsid of the paramyxovirus simian virus 5. J Virol. 1974 Nov;14(5):1253–1261. doi: 10.1128/jvi.14.5.1253-1261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. doi: 10.1016/0042-6822(74)90419-x. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Ogura H., Klenk H. Studies on the assembly of the envelope of Newcastle disease virus. Virology. 1976 Feb;69(2):523–538. doi: 10.1016/0042-6822(76)90482-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Norrby E., Gollmar Y. Identification of measles virus-specific hemolysis-inihibiting antibodies separate from hemagglutination-inhibiting antibodies. Infect Immun. 1975 Feb;11(2):231–239. doi: 10.1128/iai.11.2.231-239.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Isida N. The smallest protein of Sendi virus: its candidate function of binding nucleocaspsid to envelope. Virology. 1975 Oct;67(2):427–437. [PubMed] [Google Scholar]
- Tyrrell D. L., Norrby E. Structural polypeptides of measles virus. J Gen Virol. 1978 May;39(2):219–229. doi: 10.1099/0022-1317-39-2-219. [DOI] [PubMed] [Google Scholar]
- Waters D. J., Bussell R. H. Isolation and comparative study of the nucleocapsids of measles and canine distemper viruses from infected cells. Virology. 1974 Sep;61(1):74–79. [PubMed] [Google Scholar]
- Waters D. J., Bussell R. H. Polypeptide composition of measles and canine distemper viruses. Virology. 1973 Oct;55(2):554–557. doi: 10.1016/0042-6822(73)90202-x. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Differences between the intracellular polypeptides of measles and subacute sclerosing panencephalitis virus. Nature. 1978 Mar 30;272(5652):458–460. doi: 10.1038/272458a0. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Intracellular synthesis of measles virus-specified polypeptides. J Virol. 1978 Jan;25(1):285–297. doi: 10.1128/jvi.25.1.285-297.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y'Yoshii S., Maeno K., Matsumoto T. Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology. 1976 May;71(1):143–161. doi: 10.1016/0042-6822(76)90101-x. [DOI] [PubMed] [Google Scholar]