Abstract
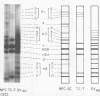
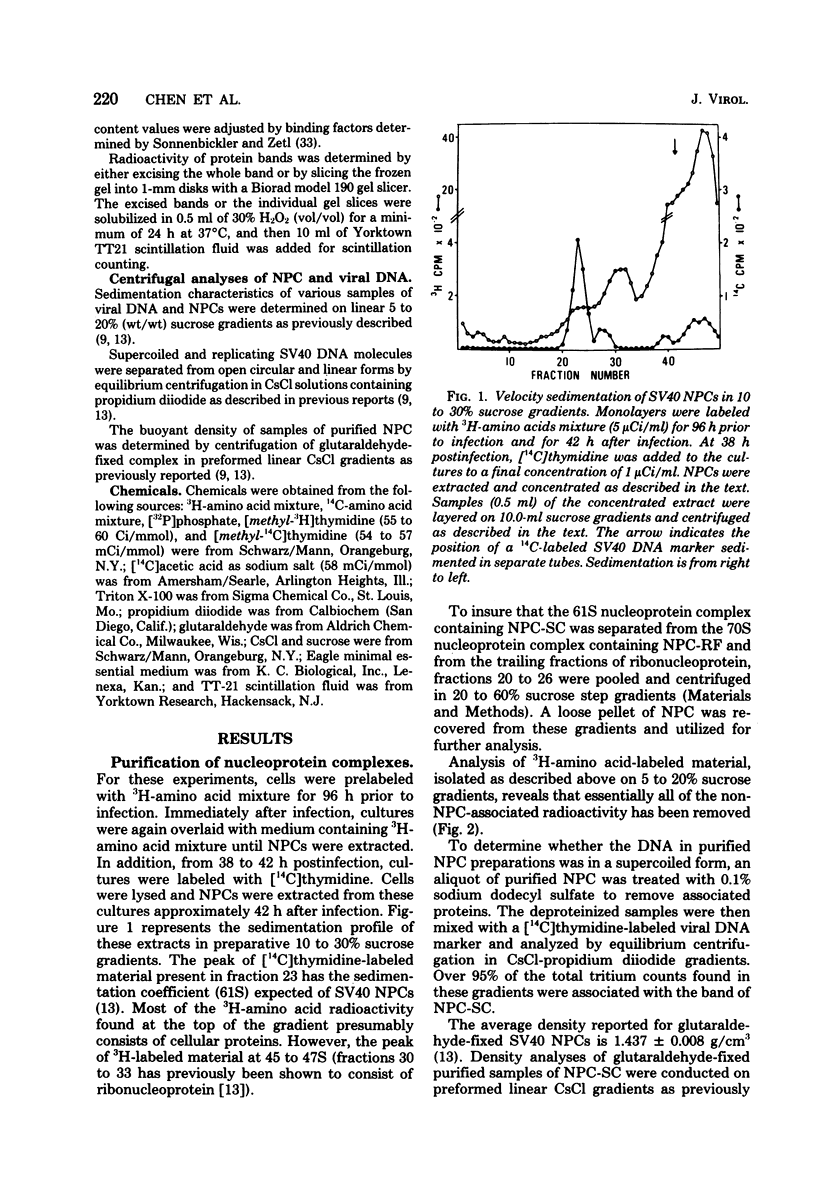
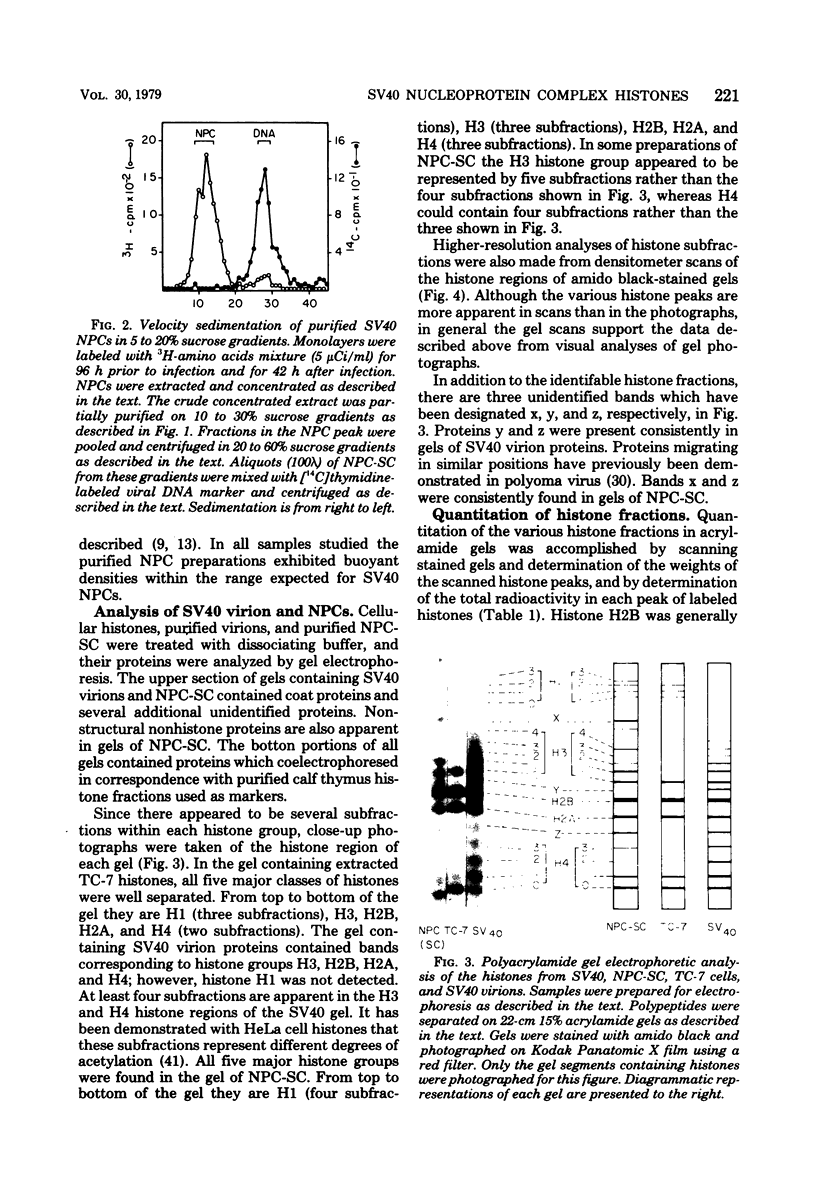
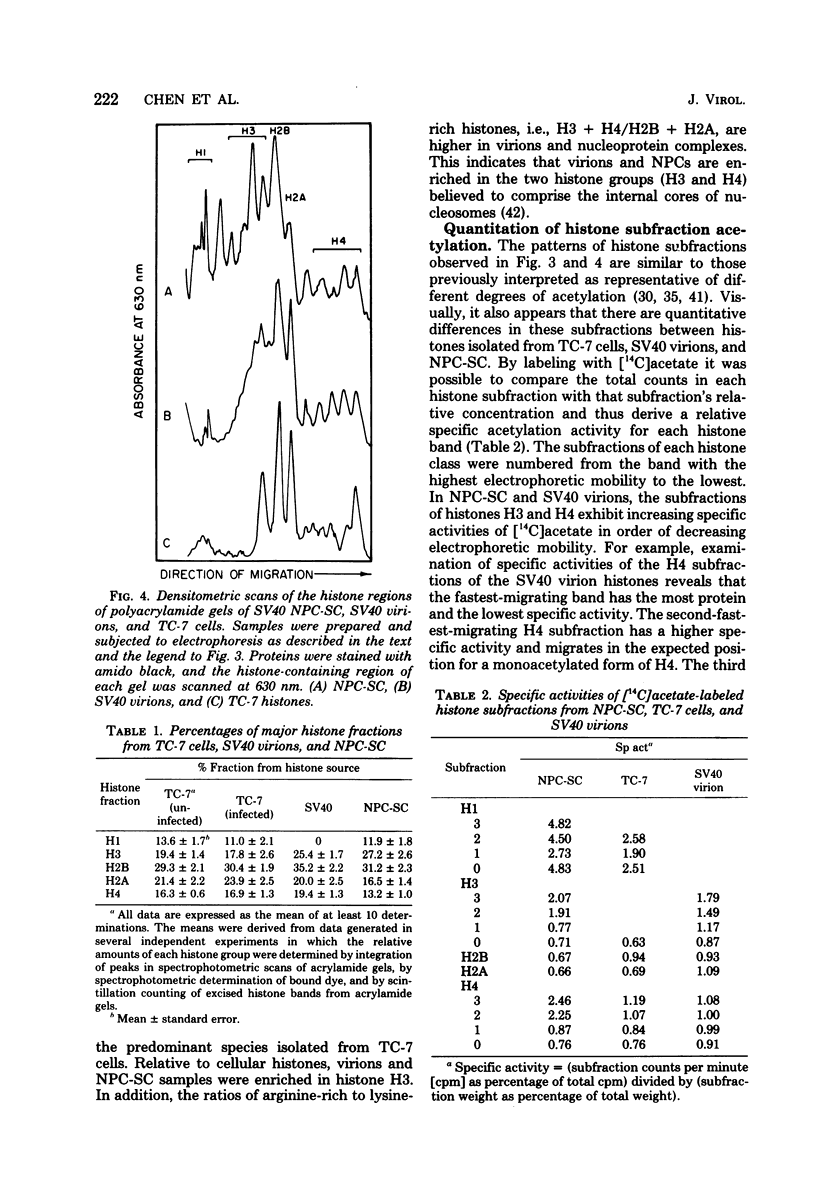
Simian virus (SV40) nucleoprotein complexes containing circular supercoiled viral DNA were extracted from infected cells and purified by differential centrifugation. The protein content of these complexes was compared by electrophoresis on 15% acrylamide gels with the protein content of purified SV40 virions and with histones from virus-infected cells. The electrophoretic patterns of histones from each of the sources revealed several major differences. SV40 virions contained histones H3, H2B, H2A, and H4 but not H1. Nucleoprotein complexes and host cells contained all five major histone groups. Relative to cellular histones, virion and nucleoprotein complex histones were enriched 15 to 40% in histones H3 and H4. In addition to the major classes of histones, several subfractions of histones H1, H3, and H4 were observed in acrylamide gels of proteins from SV40 virions and viral nucleoprotein complexes. Acetate labeling experiments indicated that each subfraction of histones H3 and H4 had a different level of acetylation. The histones from SV40 virions and nucleoprotein complexes were acetylated to significantly higher levels than those of infected host cells. No apparent differences in phosphorylation of the major histone groups were observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkenmeier E. H., Radonovich M. F., Shani M., Salzman N. P. The SV40 DNA template for transcription of late mRNA in viral nucleoprotein complexes. Cell. 1977 Jul;11(3):495–504. doi: 10.1016/0092-8674(77)90067-8. [DOI] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Characterization of a DNA-protein complex and capsomere subunits derived from polyoma virus by treatment with ethyleneglycol-bis-N,N'-tetraacetic acid and dithiothreitol. J Virol. 1978 Jul;27(1):193–204. doi: 10.1128/jvi.27.1.193-204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks T. L., Green M. H. The sv40 transcription complex. I. Effect of viral chromatin proteins on endogenous RNA polymerase activity. Nucleic Acids Res. 1977 Dec;4(12):4261–4277. doi: 10.1093/nar/4.12.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Griffith J. Salt and divalent cations affect the flexible nature of the natural beaded chromatin structure. Nucleic Acids Res. 1977 Jun;4(6):1837–1851. doi: 10.1093/nar/4.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson P. M., Crawford L. V. Polyoma virus basic proteins. J Gen Virol. 1972 Feb;14(2):141–155. doi: 10.1099/0022-1317-14-2-141. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J. The use of fluorocarbon for "unmasking" polyoma virus hemagglutinin. Virology. 1959 Nov;9:488–489. doi: 10.1016/0042-6822(59)90141-2. [DOI] [PubMed] [Google Scholar]
- Goldstein D. A., Hall M. R., Meinke W. Properties of nucleoprotein complexes containing replicating polyoma DNA. J Virol. 1973 Oct;12(4):887–900. doi: 10.1128/jvi.12.4.887-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Brooks T. L. The sv40 transcription complex. II. Non-dissociation of protein from SV40 chromatin during transcription. Nucleic Acids Res. 1977 Dec;4(12):4279–4289. doi: 10.1093/nar/4.12.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Miller H. I., Hendler S. Isolation of a polyoma-nucleoprotein complex from infected mouse-cell cultures. Proc Natl Acad Sci U S A. 1971 May;68(5):1032–1036. doi: 10.1073/pnas.68.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. R., Meinke W., Goldstein D. A. Nucleoprotein complexes containing replicating Simian virus 40 DNA: comparison with polyoma nucleoprotein complexes. J Virol. 1973 Oct;12(4):901–908. doi: 10.1128/jvi.12.4.901-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. R. Transcription and reisolation of the simian virus 40 nucleoprotein complex. Biochem Biophys Res Commun. 1977 Jun 6;76(3):698–704. doi: 10.1016/0006-291x(77)91556-x. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Tan K. B. Nucleoprotein complexes from simian virus 40-infected monkey cells: association of viral DNA with histones and the major viral structural protein. Virology. 1977 May 1;78(1):45–56. doi: 10.1016/0042-6822(77)90077-0. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Estes M. K., Pagano J. S. Structure and function of the polypeptides in simian virus 40. I. Existence of subviral deoxynucleoprotein complexes. J Virol. 1972 Jun;9(6):923–929. doi: 10.1128/jvi.9.6.923-929.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lake R. S., Barban S., Salzman N. P. Resolutions and identification of the core deoxynucleoproteins of the simian virus 40. Biochem Biophys Res Commun. 1973 Sep 18;54(2):640–647. doi: 10.1016/0006-291x(73)91471-x. [DOI] [PubMed] [Google Scholar]
- MacGregor J. P., Chen Y. H., Goldstein D. A., Hall M. R. Properties of the histones associated with simian virus 40 replicative form nucleoprotein complex. Biochem Biophys Res Commun. 1978 Aug 14;83(3):814–821. doi: 10.1016/0006-291x(78)91467-5. [DOI] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Characterization of polyoma DNA-protein complexes. I. Electrophoretic identification of the proteins in a nucleoprotein complex isolated from polyoma-infected cells. J Virol. 1974 Dec;14(6):1326–1336. doi: 10.1128/jvi.14.6.1326-1336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke W., Hall M. R., Goldstein D. A. Proteins in intracellular simian virus 40 nucleoportein complexes: comparison with simian virus 40 core proteins. J Virol. 1975 Mar;15(3):439–448. doi: 10.1128/jvi.15.3.439-448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Bilek D., Chalkley R. An electrophoretic comparison of vertebrate histones. J Biol Chem. 1971 Jul 10;246(13):4206–4215. [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Paul J. General theory of chromosome structure and gene activation in eukaryotes. Nature. 1972 Aug 25;238(5365):444–446. doi: 10.1038/238444a0. [DOI] [PubMed] [Google Scholar]
- Pett D. M., Estes M. K., Pagano J. S. Structural proteins of simian virus 40. I. Histone characteristics of low-molecular-weight polypeptides. J Virol. 1975 Feb;15(2):379–385. doi: 10.1128/jvi.15.2.379-385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., McCarthy B. Location of histones on simian virus 40 DNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2895–2899. doi: 10.1073/pnas.72.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. A., Robbins A. K., Crawford L. V. Phophorylation of polyoma and SV40 virus proteins. J Gen Virol. 1977 Oct;37(1):75–83. doi: 10.1099/0022-1317-37-1-75. [DOI] [PubMed] [Google Scholar]
- Procaccini R. L., Bresnick E. Histone modification in liver after administration of inducers of mixed function oxidase activity. Chem Biol Interact. 1975 Dec;11(6):523–533. doi: 10.1016/0009-2797(75)90028-9. [DOI] [PubMed] [Google Scholar]
- Qureshi A. A., Bourgaux P. Polypeptides of a viral DNA-protein complex form polyoma virus-infected cells. Virology. 1976 Oct 15;74(2):377–385. doi: 10.1016/0042-6822(76)90343-3. [DOI] [PubMed] [Google Scholar]
- Roblin R., Härle E., Dulbecco R. Polyoma virus proteins. 1. Multiple virion components. Virology. 1971 Sep;45(3):555–566. doi: 10.1016/0042-6822(71)90171-1. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Deficiency in histone acetylation in nontransforming host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1092–1096. doi: 10.1073/pnas.73.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebeck T., Weil R. Polyoma viral DNA replicated as a nucleoprotein complex in close association with the host cell chromatin. J Virol. 1974 Mar;13(3):567–576. doi: 10.1128/jvi.13.3.567-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Hancock R., Levine A. J. The properties and origin of the proteins in the SV40 nucleoprotein complex. Virology. 1974 Sep;61(1):11–21. doi: 10.1016/0042-6822(74)90237-2. [DOI] [PubMed] [Google Scholar]
- Sonnenbichler J., Zetl I. The quantitative protein composition of calf thymus chromatin. Hoppe Seylers Z Physiol Chem. 1975 May;356(5):599–603. doi: 10.1515/bchm2.1975.356.1.599. [DOI] [PubMed] [Google Scholar]
- TASHIRO Y., SIEKEVITZ P. ULTRACENTRIFUGAL STUDIES ON THE DISSOCIATION OF HEPATIC RIBOSOMES. J Mol Biol. 1965 Feb;11:149–165. doi: 10.1016/s0022-2836(65)80047-x. [DOI] [PubMed] [Google Scholar]
- Tan K. B. Histones: metabolism in simian virus 40-infected cells and incorporation into virions. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2805–2809. doi: 10.1073/pnas.74.7.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Howe C. C. Studies on viral DNA protein complexes isolated at different times after infection of monkey kidney cells with simian virus 40. Biochim Biophys Acta. 1977 Sep 6;478(1):99–108. doi: 10.1016/0005-2787(77)90248-9. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Structural proteins of simian virus 40: phosphoproteins. J Virol. 1972 Nov;10(5):985–994. doi: 10.1128/jvi.10.5.985-994.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Chumackov P. M., Georgiev G. P. Minichromosome of simian virus 40: presence of histone HI. Nucleic Acids Res. 1976 Aug;3(8):2101–2113. doi: 10.1093/nar/3.8.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Nedospasov S. A., Schmatchenko V. V., Bakayev V. V., Chumackov P. M., Georgiev G. P. Compact form of SV40 viral minichromosome is resistant to nuclease: possible implications for chromatin structure. Nucleic Acids Res. 1977 Oct;4(10):3303–3325. doi: 10.1093/nar/4.10.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangh L., Ruiz-Carrillo A., Allfrey V. G. Separation and analysis of histone subfractions differing in their degree of acetylation: some correlations with genetic activity in development. Arch Biochem Biophys. 1972 May;150(1):44–56. doi: 10.1016/0003-9861(72)90008-2. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Worcel A., Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976 Nov;9(3):409–417. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]
- White M., Eason R. Nucleoprotein complexes in simian virus 40-infected cells. J Virol. 1971 Oct;8(4):363–371. doi: 10.1128/jvi.8.4.363-371.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]