Abstract
It is widely recognized that ATP, along with other nucleotides, subserves important intercellular signalling processes. Among various nucleotide release mechanisms, the relatively recently identified pannexin 1 (Panx1) channel is gaining prominence by virtue of its ability to support nucleotide permeation and release in a variety of different tissues. Here, we review recent advances in our understanding of the factors that control Panx1 channel activity. By using electrophysiological and biochemical approaches, diverse mechanisms that dynamically regulate Panx1 channel function have been identified in various settings; these include, among others, activation by caspase-mediated channel cleavage in apoptotic immune cells, by G protein-coupled receptors in vascular smooth muscle, by low oxygen tension in erythrocytes and neurons, by high extracellular K+ in various cell types and by stretch/strain in airway epithelia. Delineating the distinct mechanisms of Panx1 modulation that prevail in different physiological contexts provides the possibility that these channels, and ATP release, could ultimately be targeted in a context-dependent manner.
 |
Douglas A. Bayliss (left) and Joanna K. Sandilos (right) work in the Department of Pharmacology at the University of Virginia in Charlottesville, VA. They use a combination of biochemical, pharmacological and electrophysiological approaches to study mechanisms of ion channel modulation. Doug received his PhD at the University of North Carolina, under the supervision of David E. Millhorn and did postdoctoral work with Albert J. Berger at the University of Washington; he is currently the Pharmacology department chair at the University of Virginia. Joanna is a recent graduate from the Department of Pharmacology and earned her PhD in the Bayliss laboratory.
Introduction
ATP, first isolated from muscle tissue in the late 1920s, is most recognized for its fundamental role as an energy substrate in all living cells. Although some very early studies hinted at functions outside the cell, the idea that ATP could act as an extracellular signalling molecule was met with considerable resistance. In 1972, the term ‘purinergic’ was coined by Burnstock on the heels of his initial work implicating a role for ATP in neurotransmission (Burnstock, 1972; Burnstock et al. 1972). His later work led to the distinction between the two classes of purinergic receptors, P1 and P2, for adenosine and ATP, respectively (Ralevic & Burnstock, 1998; Burnstock, 2007). Today, extracellular nucleotides are well recognized for their roles in paracrine signalling within a wide array of tissues, and the list of distinct purinergic receptor subtypes has grown to 23 (Ralevic & Burnstock, 1998; Abbracchio et al. 2006; Gever et al. 2006).
The regulated release of nucleotides was first noted in neurons, where ATP was found to act as a neurotransmitter in the CNS and periphery (Su et al. 1971; Burnstock, 1976, 1999). It is now known that ATP exerts a direct effect on a number of neuronal cell types through P2X and P2Y purinergic receptor agonism (Bean & Friel, 1990; Koles et al. 2011). ATP-mediated signalling has been associated with epilepsy-associated seizures, and with pain transduction within the spinal cord and centrally (Brake & Julius, 1996; Tsuda et al. 2003, 2005, 2009; Chiang et al. 2007; Wei et al. 2008). ATP can also play diverse roles within the cardiovascular system with pathophysiological implications regarding hypertension. For example, ATP can initiate constriction or dilatation of peripheral vasculature depending on the target cell (e.g. endothelial or smooth muscle) and the type of purinergic receptor they express (e.g. P2X or P2Y subtype) (Burnstock, 1985, 2007; Ellsworth, 2004; Sprague et al. 2011; Stokes et al. 2011; Gunduz et al. 2012). Purinergic signalling also plays key roles within the immune system mediating immune response amplification and host–pathogen interactions (Champagne et al. 1995; Davis et al. 2004). ATP, released by damaged cells or immune cells, can modulate immune responses such as interleukin processing and release (Harada et al. 2011; Junger, 2011), chemotaxis (Zigmond, 1977; Chen et al. 2006; Lecut et al. 2009; Junger, 2011) and T-cell activation (Schenk et al. 2008; Woehrle et al. 2010b; Junger, 2011). Nucleotides released from apoptotic cells act as ‘find-me’ signals to attract monocytes or macrophages to the area of cell death (Elliott et al. 2009; Chekeni et al. 2010; Elliott & Ravichandran, 2010).
ATP is generally thought to exit the cell by exocytosis, via plasma membrane channels or transporters, or through the complete breakdown of plasma membrane integrity. Exocytotic release was one of the earliest known mechanisms for regulated ATP release, described in chromaffin cells, pancreatic acini and epithelial cells (Li et al. 2010; Riteau et al. 2010). More recently, a number of candidate ATP-releasing channels have come to the forefront, specifically the connexins and pannexins (Silverman et al. 2009; Chen et al. 2010; Lazarowski et al. 2011). Connexins typically make gap junctions but they can also form so-called plasma membrane hemichannels and have been associated with ATP release in a number of cell types (Beyer & Steinberg, 1991; Cotrina et al. 1998; Romanello et al. 2001; Arcuino et al. 2002; Contreras et al. 2002; Goldberg et al. 2002; Lazarowski et al. 2011). Pannexins are topologically similar to the connexins based on hydrophobicity analysis; however, they function as a membrane channel rather than forming gap junctions (Sosinsky et al. 2011). Pannexin 1 (Panx1), specifically, has recently emerged as a candidate ATP release channel within a variety of different physiological contexts (Bao et al. 2004; Locovei et al. 2006a; Iglesias et al. 2009; Ransford et al. 2009; Silverman et al. 2009; Kawamura et al. 2010; Kim & Kang, 2011).
Pannexin 1 channel properties
Panx1, originally cloned as MRS1 in 1998 (Dahl & Keane, 2012), is the most ubiquitously expressed of the pannexins, while pannexins 2 and 3 are predominantly expressed in brain (Panx2) and skin and bone (Panx3). Perhaps due to its widespread expression and easily recorded plasma membrane channel activity in Panx1-expressing cells, Panx1 has garnered the most attention to date (Bruzzone et al. 2003). Pannexins contain four transmembrane domains, an intracellular loop, intracellular N and C termini as well as regularly spaced, highly conserved cysteine residues within the two extracellular loops (Panchin et al. 2000; Locovei et al. 2006a). By analogy with connexons, and supported by biochemical analyses (Ambrosi et al. 2010), it is believed that six subunits hexamerize to form a single Panx1 channel. Although best known for supporting efflux of nucleotides, Panx1 is a non-selective channel that allows permeation by small molecules up to 1 kDa in size including both positively and negatively charged dyes (Bao et al. 2004; Locovei et al. 2006a; Boassa et al. 2007; Ma et al. 2009; Qiu & Dahl, 2009; Ambrosi et al. 2010); it has also been implicated in release of glutamate, arachidonic acid and its metabolites (Bao et al. 2004; Pelegrin & Surprenant, 2006; Jiang et al. 2007; Chekeni et al. 2010).
Pannexin 1 was first demonstrated to act as an ATP-permeant channel in 2004 (Bao et al. 2004). Subsequent work solidified a role for Panx1 as a major ATP release channel in a variety of cell types including neurons (Kawamura et al. 2010), astrocytes (Iglesias et al. 2009; Silverman et al. 2009; Kim & Kang, 2011), taste bud cells (Dando & Roper, 2009; Huang et al. 2009), T-cells (Schenk et al. 2008; Chekeni et al. 2010; Woehrle et al. 2010a), erythrocytes (Locovei et al. 2006a; Sridharan et al. 2010), airway epithelial cells (Ransford et al. 2009; Seminario-Vidal et al. 2011), endothelial cells (Goedecke et al. 2011), skeletal and smooth muscle cells (Buvinic et al. 2009; Billaud et al. 2011), and pituitary cells (Li et al. 2011a,b).
Panx1 currents can be distinguished pharmacologically from connexin hemichannels by their differential sensitivity to a number of gap junction blockers (Bruzzone et al. 2005). For example, Panx1 is strongly inhibited by carbenoxolone (CBX) (IC50 = 5 μm) and probenecid (IC50 = 150 μm), but only weakly by flufenamic acid (FFA) (IC50 = 0.3 mm). Connexins, however, are much more strongly inhibited by FFA with potency approximately equal to their CBX sensitivity (IC50 = 3–100 μm) and are insensitive to the Panx1 channel blocker probenecid (Silverman et al. 2008; D’hondt et al. 2009). Panx1 currents are weakly outwardly rectifying and are activated at increasingly depolarized potentials (Bruzzone et al. 2003; Ma et al. 2009).
There is some discrepancy in the literature regarding single channel properties of Panx1. Single channel recordings in excised patches from Panx1-expressing oocytes identified a main single channel conductance of ∼500 pS, with no less than four additional subconductance states (Bao et al. 2004). In a recent publication, however, Ma et al. (2009) recorded a 68 pS CBX-sensitive anion-selective channel from Panx1-transfected mammalian cells. The nature of that smaller conductance channel remains uncertain. Although differences in single channel properties could reflect the use of different expression systems (e.g. mammalian cells vs. Xenopus oocytes) and a truly smaller mammalian cell-specific main conductance, the reported anion selectivity of the recorded 68 pS channel is difficult to reconcile with previous work by multiple groups showing permeation by both positively and negatively charged dyes through Panx1 (Locovei et al. 2006a; Pelegrin & Surprenant, 2006; Boassa et al. 2007; Ma et al. 2009; Qiu & Dahl, 2009).
An additional surrogate measure of Panx1 channel function is the cellular uptake of fluorescent DNA binding dyes such as the monomeric cyanine dyes (YO-PRO-1, TO-PRO-3, etc.), allowing for a higher throughput means of assessing Panx1-dependent plasma membrane permeability in larger populations of cells (Fig. 1). The combination of molecular biology techniques (mutagenesis, siRNA and over-expression) with pharmacological characterization, dye uptake and/or electrophysiology, provides the most compelling way to confidently identify Panx1 channel activity in native cells.
Figure 1. C terminal cleavage-mediated Panx1 channel activation using a TEV protease system.
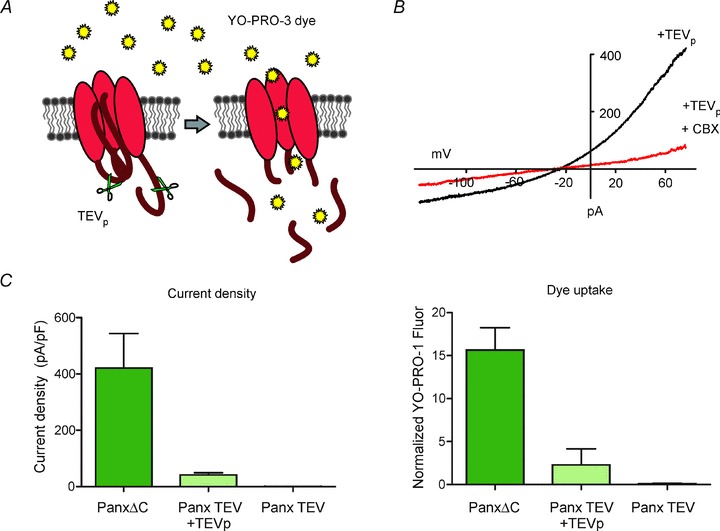
A, schematic of a Panx1 (TEV) construct with TEV protease site substituted for the C terminal caspase site. Co-expression of TEV protease results in C terminal cleavage and subsequent Panx1 channel activation leading to YO-PRO-3 dye uptake into the cell. B, when co-transfected with TEVp in HEK293T cells, Panx1(TEV) generated robust whole cell CBX-sensitive currents with I–V properties characteristic of Panx1. C, comparison of Panx1 channel activity assessed by membrane current recording and by dye uptake. HEK293T cells expressing C terminally deleted, constitutively activated (PanxΔC), cleavage activated (Panx1(TEV) + TEVp), and basally inactive Panx1(TEV) were recorded in whole cell voltage clamp mode (left panel), or treated with YO-PRO-3 dye, fixed and fluorescence measured on a plate reader (right panel). YO-PRO-3 dye uptake assay was performed in a 96-well plate format and can reflect differences in Panx1 channel activity as measured by whole cell voltage clamp recordings.
Given that pannexins allow permeation of ATP and additional large molecules, these channels must be very tightly regulated to avoid dissipation of important electrochemical gradients or loss of critical cellular constituents that would result in the rapid demise of the cell. Regulation of Panx1 function has been observed at the level of the plasma membrane channel activity as well as by dynamics of channel trafficking to the membrane (see Fig. 2).
Figure 2. Mechanisms of pannexin1 channel modulation.
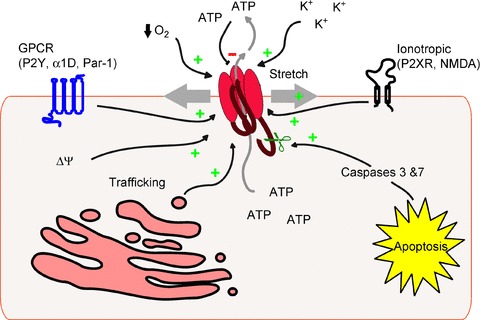
Schematic outlining direct mechanisms of Panx1 channel modulation including activation via plasma membrane stretch/strain, membrane depolarization, extracellular potassium, low oxygen tension, G protein-coupled receptor, caspase cleavage and ionotropic receptors including P2X. Panx1 can be inhibited directly by high levels of extracellular ATP, and Panx1 channel activity at the level of the plasma membrane can be regulated by trafficking dynamics.
Pannexin 1 channel regulation by trafficking
A defining characteristic of Panx1 is its glycosylation at residue N254 that is required for full plasma membrane localization. Three glycosylation species of Panx1 have been identified: non-glycosylated core–Gly0, high mannose–Gly1 and the complex glycosylated–Gly2 species (Boassa et al. 2007). Site-directed mutagenesis of the N-linked glycosylation residue N254 results in intracellular localization of the channel (Boassa et al. 2007). Recent work implicated a role for the Panx1 C terminus in cell surface trafficking as well. A truncated mutant Panx1Δ307 was primarily glycosylated to the high mannose form and did not mature to the Gly2 species, resulting in its retention in the endoplasmic reticulum (Gehi et al. 2011). Pharmacological disruption of actin microfilaments with cytochalasin B results in a loss of cell surface Panx1 and further co-immunoprecipitation studies revealed that the C terminus of Panx1 can bind F-actin. Panx1 was also shown to be highly mobile via COPII (coat protein II)-dependent endoplasmic reticulum-to-Golgi trafficking and dynamin II-dependent internalization pathways (Bhalla-Gehi et al. 2010). Taken together, it appears that Panx1 trafficking is a highly dynamic process that may take part in regulating the level of channel activity on the cell surface at any particular moment. Panx1 trafficking towards membrane protrusions may be indicative of a role in cell motility, a hypothesis supported by the observation that the absence of Panx1 from corneal epithelial leading edge in P2X7−/− mice was associated with compromised corneal wound healing (Mayo et al. 2008).
Pannexin 1 channel regulation by mechanosensitivity
Environmental factors that can activate Panx1 include tonicity and stretch/strain (Bao et al. 2004). These mechanisms are particularly relevant to erythrocytes, which are known to release ATP in response to both low oxygen tension and shear stress (Fig. 2). Since erythrocytes do not express the machinery necessary for vesicular release, Panx1 was a prime candidate and its role in hypoxia- or shear stress-induced ATP release by erythrocytes has been verified pharmacologically and by genetic knockout (Locovei et al. 2006a; Sridharan et al. 2010; Qiu et al. 2011b). Panx1 was recently identified as the mechanosensitive conduit by which ATP is released from airway epithelia. Mechanical forces during breathing, coughing and hypotonic secretions can exert strain on epithelial cell membranes resulting in Panx1 activation. It has also been suggested that mechanosensitivity of Panx1 may underlie T-cell activation during hypertonic saline treatment (Woehrle et al. 2010b). Panx1 mechanosensitivity may be a channel-intrinsic property since stretch activation of Panx1 was observed in cell-free oocyte membrane patches (Bao et al. 2004). A different mechanism may prevail in the case of airway epithelial cells, where osmotic strain-dependent RhoA activation and subsequent Rho kinase-dependent myosin light chain phosphorylation were identified as upstream factors in Panx1 mechanosensitivity (Seminario-Vidal et al. 2011).
Proteolytic cleavage-mediated pannexin 1 channel regulation
It was recently discovered that nucleotides released from apoptotic cells are responsible for recruiting macrophages to areas of cell death (Elliott et al. 2009). Subsequently, a critical role was identified for Panx1 in mediating ATP release during apoptosis, involving a novel caspase-mediated mechanism for the apoptosis-dependent activation of the channel (Chekeni et al. 2010). This molecular identification of Panx1 as the ATP release channel during cell death was verified by another group that used Panx1 knockout mice to show that apoptotic Panx1−/− thymocytes were deficient in dye uptake, ATP release and recruitment of peritoneal macrophages (Qu et al. 2011). Although a caspase cleavage site on the Panx1 C terminus was required for apoptotic channel activation, the role of the C terminal tail in channel gating was not well understood.
In subsequent work, we were able to functionally separate Panx1 C terminal cleavage-activation from apoptosis by using a TEV (tobacco etch virus protease) protease cleavage model (Fig. 1). We also provided evidence for channel activation at the level of the plasma membrane, suggesting that it can be a membrane-delimited and channel-intrinsic process (Sandilos et al. 2012). By serial deletion, we identified a C terminal region just distal to the caspase site that is required for inhibition of Panx1; point mutations within this small region resulted in partial activation of the full length channel. Consistent with the C terminal tail functioning as an independent autoinhibitory region, we found that truncated channels could be inhibited in trans by the isolated Panx1 C terminus either in cells or when applied directly as a purified peptide. A recent structure–function analysis of Panx1 provided evidence that the distal region of the C terminus, along with select residues in the first transmembrane domain, contribute to the channel pore (Wang & Dahl, 2010). Using a cysteine cross-linking approach, we showed that relief of inhibition following cleavage requires dissociation of the C terminus from the channel pore. Collectively, these data suggest a mechanism of Panx1 channel regulation whereby the intact, pore-associated C terminus inhibits the full length channel and a remarkably well-placed caspase cleavage site allows effective removal of key inhibitory C terminal determinants to activate Panx1 (Sandilos et al. 2012).
Receptor-mediated pannexin 1 channel regulation
Ionotropic receptors
A P2X7-dependent ‘large permeation’ pathway had already been observed and was thought to either be the result of P2X receptor pore dilatation and/or coupling with a large permeation channel. Panx1 was first associated with the P2X7 receptors when it was shown by pharmacology and siRNA-mediated knockdown to be required for ATP-stimulated large pore formation and IL-1 beta release from macrophages (Fig. 1) (Pelegrin & Surprenant, 2006). Since then, other P2X family members (P2X1–5) have also been shown to take part in Panx1 activation (Woehrle et al. 2010a,b; Li et al. 2011b). Moreover, this functional P2X–Panx1 coupling has been documented in a wide variety of immune cell processes, especially prevalent in T-cell activation, neutrophil regulation and macrophage chemotaxis (Chen et al. 2006, 2010; Schenk et al. 2008; Elliott et al. 2009; Woehrle et al. 2010a,b; Junger, 2011); it has also been identified in other physiological contexts including purinergic signalling within the pituitary gland (Li et al. 2011a,b), aqueous humour outflow from ciliary epithelial cells (Li et al. 2010), and ATP release from astrocytes, neurons and epithelial cells during ischaemic stress (Domercq et al. 2010; Riteau et al. 2010; Iwabuchi & Kawahara, 2011). The exact molecular mechanism of Panx1 channel activation downstream of P2X receptor activation is not well understood. Although it may involve a direct interaction between P2X receptors and Panx1 channels, one study suggests that it could be a Src kinase-dependent process since P2X7-mediated Panx1 activation is sensitive to the Src kinase inhibitor PP2 and to exogenous application of a peptide comprising a Src homology 3 domain of P2X7 (Iglesias et al. 2008). In addition, recent genetic analysis identified a P451L polymorphism of P2X7 that appears to impair large pore formation, suggesting that the C terminal region of P2X7 encompassing P451 may be required for efficient coupling to Panx1 (Adriouch et al. 2002; Sorge et al. 2012).
While Panx1 can be activated by extracellular ATP through P2 receptor agonism, high levels of extracellular ATP can also act in a negative feedback loop to inhibit Panx1 channels directly (Fig. 1) (Qiu & Dahl, 2009; Qiu et al. 2011a). Further mutational analysis of the extracellular loops identified residues W74, S237, S240, I247 and L266 to be crucial for the inhibitory effect of several ATP analogues and led to the hypothesis that these residues may support ATP binding or represent the ATP binding site itself (Qiu et al. 2011a). This mechanism of ‘a permeant regulating its permeation pore’, both negatively and positively, provides for exquisitely precise modulation of channel activity in response to varied ATP concentrations.
One of the electrophysiological characteristics of Panx1 is its activation by membrane depolarization (Bruzzone et al. 2003). It has been suggested that this voltage-dependent mechanism may play a role in Panx1 activity during seizure and ischaemia (Thompson et al. 2008; MacVicar & Thompson, 2010; Iwabuchi & Kawahara, 2011). The molecular mechanisms through which Panx1 is activated during seizure and ischaemia are not well understood; however, a non-purinergic receptor-mediated activation of Panx1 was implicated in the context of seizure where NMDA receptor–Panx1 channel coupling was observed under conditions of excitotoxicity (Thompson et al. 2008; MacVicar & Thompson, 2010). Most recently, it was suggested that Src kinase phosphorylation of Panx1 C terminus may mediate anoxia-induced NMDA receptor activation of the channel (Weilinger et al. 2012). Additionally, elevated extracellular potassium associated with neuronal hyperactivity may also activate Panx1, independent of membrane depolarization (Silverman et al. 2009; Santiago et al. 2011).
Metabotropic receptors
While coupling between Panx1 and the ionotropic P2X receptors is the most widely studied activation mechanism, there is evidence for Panx1 channel activation through the metabotropic P2Y purinergic receptors (Locovei et al. 2006b). In this case, it was suggested that P2Y1- and P2Y2-mediated activation of Panx1 involves a rise in intracellular calcium through phospholipase C signalling (Locovei et al. 2006b), but this has yet to be tested directly or reproduced by others.
A Gαq-linked G protein-coupled receptor (GPCR)-mediated activation of Panx1 has also been demonstrated in the context of phenylephrine-mediated and ATP-dependent vasoconstriction. Vascular constriction was inhibited by pharmacological blockade and siRNA knockdown of Panx1 channels, by apyrase treatment to hydrolyse extracellular ATP, and by blocking ATP-activated P2Y receptors (Billaud et al. 2011). Co-immunoprecipitation of Panx1 with α1D receptors from native tissue led to the hypothesis of a direct functional coupling between the receptor and channel, a possibility that remains to be demonstrated (Billaud et al. 2011). Most recently, another group identified Panx1-dependent thrombin-induced ATP release in human umbilical vein endothelial cells (Goedecke et al. 2011). They identified the channel pharmacologically, and showed a marked reduction in thrombin-stimulated ATP release after siRNA-mediated knockdown of Panx1. Like the α1DR, the thrombin receptor PAR-1 is a Gαq/11-linked GPCR. The mechanisms that underlie GPCR-mediated Panx1 activation remain to be identified and it is possible that a common downstream signalling pathway is engaged by these different receptors.
Discussion
Over the past decade many roles for Panx1 in normal physiology and disease have emerged. Although the pannexin field is still in its infancy, it is developing rapidly and contributions of Panx1 channels are being recognized in a growing number of physiological and pathophysiological contexts. It has been reported that Panx1 functions as a tumour suppressor in the context of glioma tumorigenesis and metastasis (Lai et al. 2007; Bao et al. 2012), while in the context of melanoma Panx1 expression correlates with increased tumour cell aggressiveness (Penuela et al. 2012). This raises the possibility that Panx1 may act as a tumour suppressor in one context and as an oncogene in another, as was recently demonstrated for E-cadherin (Lewis-Tuffin et al. 2010). In addition, increased gap junctional coupling observed in the glioma cells may point to an up-regulation of connexins, which are also known to act as tumour suppressors (Naus & Laird, 2010). Future efforts should be directed towards understanding the context-specific roles of Panx1 in tumour cell aggressiveness and metastasis. The potential for targeting Panx1 channel activity in other human diseases is now being recognized in diverse areas covering many fields, including inflammatory diseases, and even HIV, as discussed in an excellent recent review (Dahl & Keane, 2012).
Several recently generated lines of Panx1−/− mice have already led to insights into how Panx1 contributes to the development of seizures and its role in the immune system (Anselmi et al. 2008; Qu et al. 2011; Santiago et al. 2011). These global knockout animals, as well as tissue-specific Panx1 knockouts, will undoubtedly continue to be valuable tools in understanding the diverse physiological roles for Panx1. That said, it is worth noting that two different mouse models yielded contradictory results regarding the roles of pannexins in astrocytic ATP release. Astrocytes from Panx1−/−:Panx2−/− double knockout mice appear to have unchanged outward current, ATP release and dye uptake following P2X7 stimulation. On the other hand, astrocytes from a different line of Panx1−/− mice show a marked reduction in these same surrogate measures of Panx1 channel function (Bargiotas et al. 2011; Suadicani et al. 2012). The reasons for these differences are not immediately obvious, but such discrepant results reinforce the need to be mindful of the well-known caveats associated with the use of knockout animals (e.g. compensation, genetic background, etc.).
In addition, a better understanding of Panx1 activation mechanisms will also be required to elucidate how Panx1 functions in each physiological context. There are probably a number of tissue-specific mechanisms of Panx1 channel modulation. For example, the cleavage-based mechanism of Panx1 activation observed in apoptosis seems appropriate for a terminal process; however, more subtle mechanisms may account for other forms of channel modulation associated with different physiological conditions. A dye uptake assay that we have developed in a 96-well plate format represents a higher-throughput means for measuring channel function and may be a useful tool for studying additional Panx1 activation mechanisms (Fig. 1). This assay could also lead to the identification of more specific pharmacological blockers and agonists for pannexins, the lack of which remains a limitation in the field. Several widely used Panx1 channel blockers inhibit connexin hemichannels as well, making it necessary to use a combinatorial approach to pharmacologically distinguish the two in native tissue preparations and animal models.
Additionally, although most publications to date focus on physiological consequences of ATP release, Panx1 is thought to release other cellular contents including glutamate and arachidonic acid (Bao et al. 2004; Pelegrin & Surprenant, 2006; Jiang et al. 2007; Chekeni et al. 2010). The physiological consequences of Panx1-dependent release of these additional factors are not known. Also, in Panx1-expressing cells, the effects of rundown of electrochemical gradients or altered electroresponsive properties following Panx1 activation are not well understood. Continued efforts to develop better tools and to elucidate mechanisms of Panx1 modulation within various physiological contexts will allow us to understand better the processes influenced by Panx1 in both apoptotic and healthy tissues, and to identify new agents for modulating Panx1 channel function in the context of human disease.
Glossary
- CBX
carbenoxolone
- FFA
flufenamic acid
- GPCR
G protein-coupled receptor
- Panx1
pannexin 1
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010;285:24420–24431. doi: 10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao B, Lai CP, Naus CC, Morgan JR. Pannexin1 drives multicellular aggregate compaction via a signaling cascade that remodels the actin cytoskeleton. J Biol Chem. 2012;287:8407–8416. doi: 10.1074/jbc.M111.306522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A. 2011;108:20772–20777. doi: 10.1073/pnas.1018262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP, Friel DD. ATP-activated channels in excitable cells. Ion Channels. 1990;2:169–203. doi: 10.1007/978-1-4615-7305-0_5. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Steinberg TH. Evidence that the gap junction protein connexin-43 is the ATP-induced pore of mouse macrophages. J Biol Chem. 1991;266:7971–7974. [PubMed] [Google Scholar]
- Bhalla-Gehi R, Penuela S, Churko JM, Shao Q, Laird DW. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J Biol Chem. 2010;285:9147–9160. doi: 10.1074/jbc.M109.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE. Pannexin1 regulates α1-adrenergic receptor-mediated vasoconstriction. Circ Res. 2011;109:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Julius D. Signaling by extracellular nucleotides. Annu Rev Cell Dev Biol. 1996;12:519–541. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976;1:239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Nervous control of smooth muscle by transmitters, cotransmitters and modulators. Experientia. 1985;41:869–874. doi: 10.1007/BF01970003. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Current status of purinergic signalling in the nervous system. Prog Brain Res. 1999;120:3–10. doi: 10.1016/s0079-6123(08)63541-4. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Satchell DG, Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br J Pharmacol. 1972;46:234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvinic S, Almarza G, Bustamante M, Casas M, Lopez J, Riquelme M, Saez JC, Huidobro-Toro JP, Jaimovich E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem. 2009;284:34490–34505. doi: 10.1074/jbc.M109.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DE, Smartt CT, Ribeiro JM, James AA. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5′-nucleotidase family. Proc Natl Acad Sci U S A. 1995;92:694–698. doi: 10.1073/pnas.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci. 2007;27:9068–9076. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- Dahl G, Keane RW. Pannexin: From discovery to bedside in 11±4 years? Brain Res. 2012;1487:150–159. doi: 10.1016/j.brainres.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L112–L120. doi: 10.1152/ajplung.00218.2003. [DOI] [PubMed] [Google Scholar]
- Domercq M, Perez-Samartin A, Aparicio D, Alberdi E, Pampliega O, Matute C. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia. 2010;58:730–740. doi: 10.1002/glia.20958. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- Gehi R, Shao Q, Laird DW. Pathways regulating the trafficking and turnover of pannexin1 protein and the role of the C-terminal domain. J Biol Chem. 2011;286:27639–27653. doi: 10.1074/jbc.M111.260711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflugers Arch. 2006;452:513–537. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- Goedecke S, Roderigo C, Rose CR, Rauch BH, Goedecke A, Schrader J. Thrombin-induced ATP release from human umbilical vein endothelial cells. Am J Physiol Cell Physiol. 2011;302:C915–C923. doi: 10.1152/ajpcell.00283.2010. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- Gunduz D, Aslam M, Krieger U, Becker L, Grebe M, Arshad M, Sedding DG, Hartel FV, Abdallah Y, Piper HM, Voss RK, Noll T. Opposing effects of ATP and adenosine on barrier function of rat coronary microvasculature. J Mol Cell Cardiol. 2012;52:962–970. doi: 10.1016/j.yjmcc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Harada K, Hide I, Seki T, Tanaka S, Nakata Y, Sakai N. Extracellular ATP differentially modulates Toll-like receptor 4-mediated cell survival and death of microglia. J Neurochem. 2011;116:1138–1147. doi: 10.1111/j.1471-4159.2011.07170.x. [DOI] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29:13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi S, Kawahara K. Functional significance of the negative-feedback regulation of ATP release via pannexin-1 hemichannels under ischemic stress in astrocytes. Neurochem Int. 2011;58:376–384. doi: 10.1016/j.neuint.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X7 receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol. 2007;151:1033–1040. doi: 10.1038/sj.bjp.0707311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci. 2010;30:3886–3895. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Kang TC. The P2X7 receptor-pannexin-1 complex decreases muscarinic acetylcholine receptor-mediated seizure susceptibility in mice. J Clin Invest. 2011;121:2037–2047. doi: 10.1172/JCI44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koles L, Leichsenring A, Rubini P, Illes P. P2 receptor signaling in neurons and glial cells of the central nervous system. Adv Pharmacol. 2011;61:441–493. doi: 10.1016/B978-0-12-385526-8.00014-X. [DOI] [PubMed] [Google Scholar]
- Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res. 2007;67:1545–1554. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol. 2011;61:221–261. doi: 10.1016/B978-0-12-385526-8.00008-4. [DOI] [PubMed] [Google Scholar]
- Lecut C, Frederix K, Johnson DM, Deroanne C, Thiry M, Faccinetto C, Maree R, Evans RJ, Volders PG, Bours V, Oury C. P2X1 ion channels promote neutrophil chemotaxis through Rho kinase activation. J Immunol. 2009;183:2801–2809. doi: 10.4049/jimmunol.0804007. [DOI] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Rodriguez F, Giannini C, Scheithauer B, Necela BM, Sarkaria JN, Anastasiadis PZ. Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype. PLoS One. 2010;5:e13665. doi: 10.1371/journal.pone.0013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol. 2010;299:C1308–C1317. doi: 10.1152/ajpcell.00333.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bjelobaba I, Yan Z, Kucka M, Tomic M, Stojilkovic SS. Expression and roles of pannexins in ATP release in the pituitary gland. Endocrinology. 2011a;152:2342–2352. doi: 10.1210/en.2010-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tomic M, Stojilkovic SS. Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels. Gen Comp Endocrinol. 2011b;174:202–210. doi: 10.1016/j.ygcen.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006a;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006b;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Mayo C, Ren R, Rich C, Stepp MA, Trinkaus-Randall V. Regulation by P2X7: epithelial migration and stromal organization in the cornea. Invest Ophthalmol Vis Sci. 2008;49:4384–4391. doi: 10.1167/iovs.08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus CC, Laird DW. Implications and challenges of connexin connections to cancer. Nat Rev Cancer. 2010;10:435–441. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S, Gyenis L, Ablack A, Churko JM, Berger AC, Litchfield DW, Lewis JD, Laird DW. Loss of pannexin 1 attenuates melanoma progression by reversion to a melanocytic phenotype. J Biol Chem. 2012;287:29184–29193. doi: 10.1074/jbc.M112.377176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296:C250–C255. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Wang J, Dahl G. Alanine substitution scanning of pannexin1 reveals amino acid residues mediating ATP sensitivity. Purinergic Signal. 2011a;8:81–90. doi: 10.1007/s11302-011-9263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Wang J, Spray DC, Scemes E, Dahl G. Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett. 2011b;585:3430–3435. doi: 10.1016/j.febslet.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol. 2011;186:6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- Romanello M, Pani B, Bicego M, D’Andrea P. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun. 2001;289:1275–1281. doi: 10.1006/bbrc.2001.6124. [DOI] [PubMed] [Google Scholar]
- Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C terminal autoinhibitory region. J Biol Chem. 2012;287:11303–11311. doi: 10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, Meda P, Scemes E. Targeting pannexin1 improves seizure outcome. PLoS One. 2011;6:e25178. doi: 10.1371/journal.pone.0025178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WR, Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med. 2012;18:595–599. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5:193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RS, Bowles EA, Achilleus D, Ellsworth ML. Erythrocytes as controllers of perfusion distribution in the microvasculature of skeletal muscle. Acta Physiol (Oxf) 2011;202:285–292. doi: 10.1111/j.1748-1716.2010.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes L, Scurrah K, Ellis JA, Cromer BA, Skarratt KK, Gu BJ, Harrap SB, Wiley JS. A loss-of-function polymorphism in the human P2X4 receptor is associated with increased pulse pressure. Hypertension. 2011;58:1086–1092. doi: 10.1161/HYPERTENSIONAHA.111.176180. [DOI] [PubMed] [Google Scholar]
- Su C, Bevan JA, Burnstock G. [3H]adenosine triphosphate: release during stimulation of enteric nerves. Science. 1971;173:336–338. doi: 10.1126/science.173.3994.336. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Iglesias R, Wang J, Dahl G, Spray DC, Scemes E. ATP signaling is deficient in cultured Pannexin1-null mouse astrocytes. Glia. 2012;60:1106–1116. doi: 10.1002/glia.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Wang J, Dahl G. SCAM analysis of Panx1 suggests a peculiar pore structure. J Gen Physiol. 2010;136:515–527. doi: 10.1085/jgp.201010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilinger NL, Tang PL, Thompson RJ. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci. 2012;32:12579–12588. doi: 10.1523/JNEUROSCI.1267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010a;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, Chen Y, Junger WG. Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010b;88:1181–1189. doi: 10.1189/jlb.0410211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977;75:606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]


