Abstract
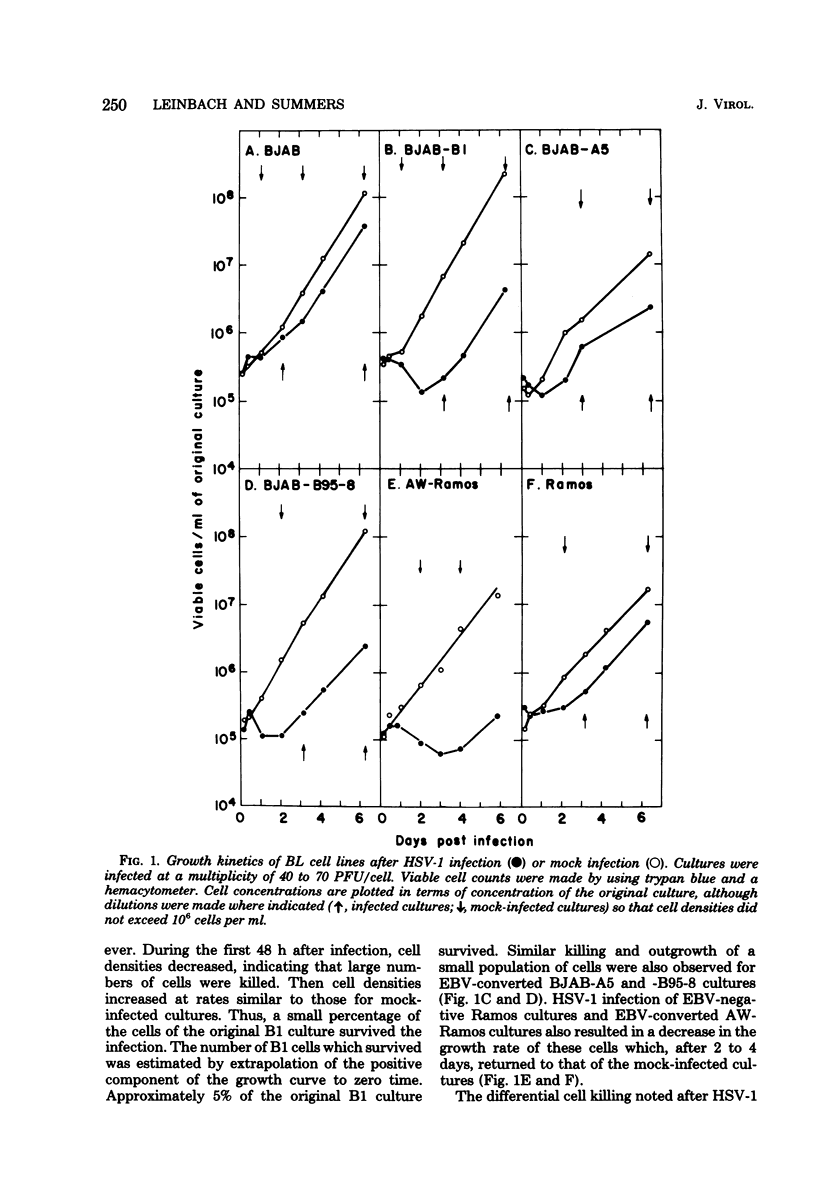
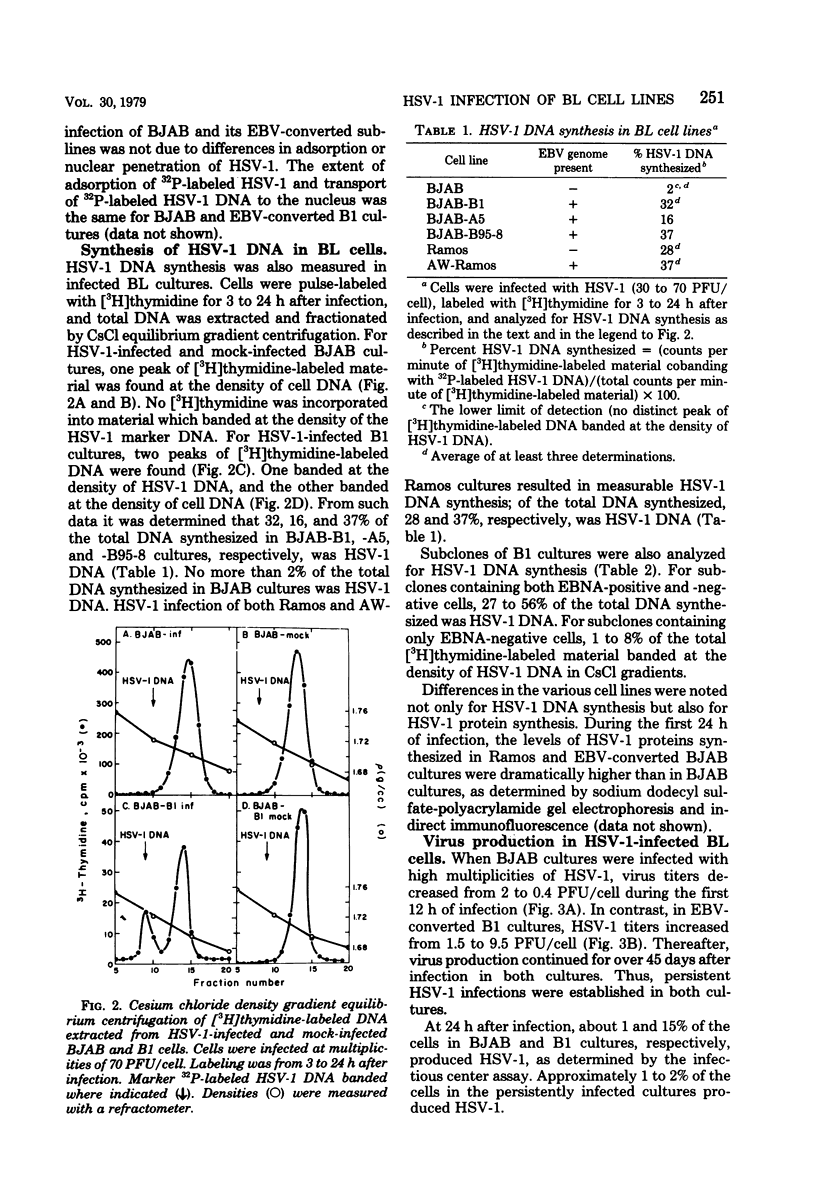
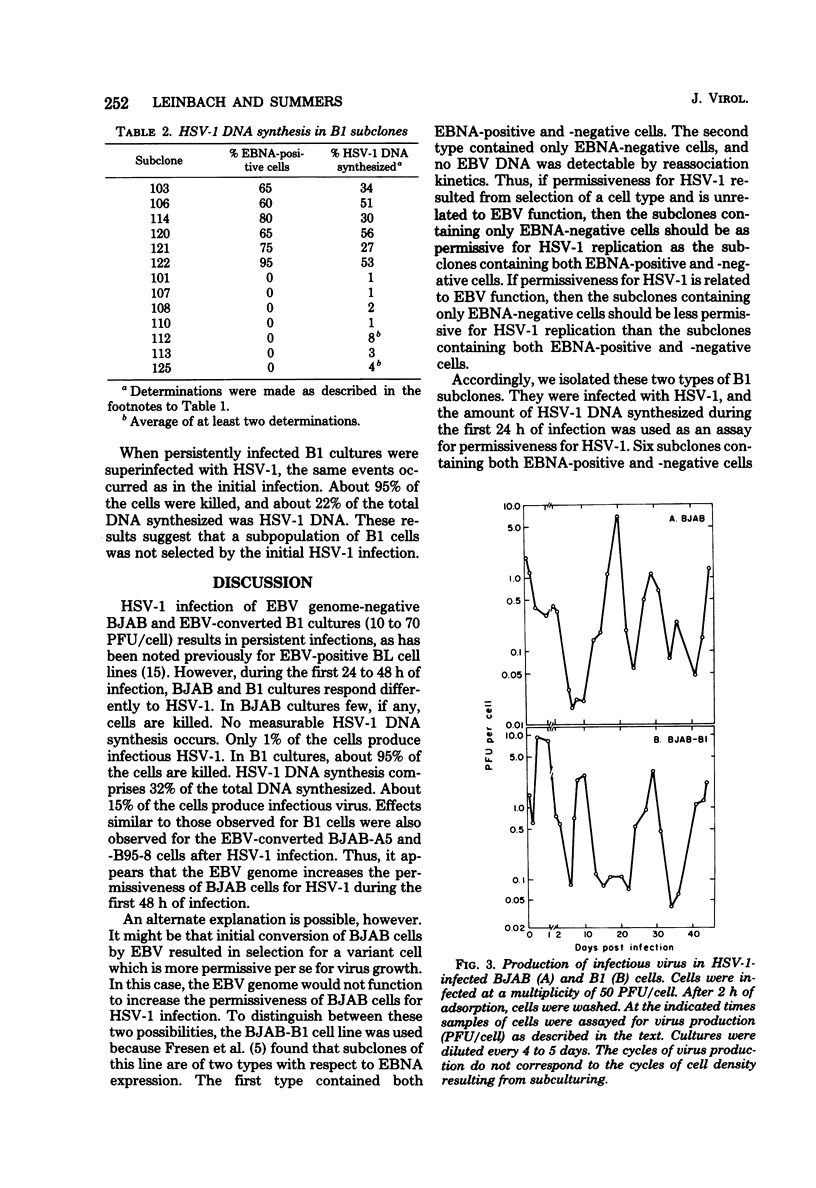
The Epstein-Barr virus (EBV) genome-negative Burkitt's lymphoma-derived cell lines BJAB and Ramos and their in vitro EBV-converted sublines BJAB-B1, BJAB-A5, BJAB-B95-8, and AW-Ramos were infected with high multiplicities of herpes simplex virus type 1 (HSV-1; 10 to 70 PFU/cell). Cultures were monitored for cell growth and HSV-1 DNA synthesis. EBV-converted BJAB cultures were more permissive for HSV-1 infection than BJAB cultures. Significant cell killing and HSV-1 DNA synthesis were observed during the first 48 h of infection in the EBV-converted BJAB cultures but not in the BJAB cultures. The EBV-converted BJAB-B1 cell line contains an appreciable fraction of EBV-negative cells. Therefore, it was cloned. EBV-positive and -negative cells were identified by using EBV-determined nuclear antigen anti-complement immunofluorescence. Two types of subclones were identified: (i) those which contained both EBV-determined nuclear antigen-positive and -negative cells and (ii) those which contained only EBV-determined nuclear antigen-negative cells. When levels of HSV-1 DNA synthesis were measured in these subclones, it was found that the former were more permissive for HSV-1 infection than the latter. Thus, the presence of the EBV genome in BJAB cells correlates with increased permissiveness of these cells for HSV-1 during the first 48 h of infection. Nonetheless, persistent HSV-1 infections were established in both BJAB and EBV-converted BJAB-B1 cultures. No differences in extent of permissiveness for HSV-1 infection were found for Ramos and EBV-converted AW-Ramos cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Lindahl T. Epstein-Barr virus DNA in human lymphoid cell lines: in vitro conversion. Virology. 1976 Aug;73(1):96–105. doi: 10.1016/0042-6822(76)90064-7. [DOI] [PubMed] [Google Scholar]
- Clements G. B., Klein G., Povey S. Production by EBV infection of an EBNA-positive subline from an EBNA-negative human lymphoma cell line without detectable EBV DNA. Int J Cancer. 1975 Jul 15;16(1):125–133. doi: 10.1002/ijc.2910160114. [DOI] [PubMed] [Google Scholar]
- Cremer K. J., Summers W. C., Gesteland R. F. Cell-free synthesis of herpes simplex virus proteins. J Virol. 1977 Jun;22(3):750–757. doi: 10.1128/jvi.22.3.750-757.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Glasser R., Vonka V., Benyesh-Melnick M. Studies on the growth of herpes simplex virus in lymphoblastoid cells. Acta Virol. 1971 Mar;15(2):133–142. [PubMed] [Google Scholar]
- Fresen K. O., Hausen H. Establishment of EBNA-expressing cell lines by infection of Epstein-Barr virus (EBV)-genome-negative human lymphoma cells with different EBV strains. Int J Cancer. 1976 Feb 15;17(2):161–166. doi: 10.1002/ijc.2910170203. [DOI] [PubMed] [Google Scholar]
- Fresen K. O., Merkt B., Bornkamm G. W., Hausen H. Heterogeneity of Epstein-Barr virus originating from P3HR-1 cells. I. Studies on EBNA induction. Int J Cancer. 1977 Mar 15;19(3):317–323. doi: 10.1002/ijc.2910190306. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., zur Hausen H. Effect of herpes simplex virus on cultured Burkitt tumor cells and its failure to influence the Epstein-Barr virus carrier state. Cancer Res. 1969 Feb;29(2):489–494. [PubMed] [Google Scholar]
- Huang E. S., Pagano J. S. Replication of cytomegalovirus DNA in human lymphoblastoid cells. IARC Sci Publ. 1975;(11 Pt 1):475–482. [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Zeuthen J., Terasaki P., Billing R., Honig R., Jondal M., Westman A., Clements G. Inducibility of the Epstein-Barr virus (EBV) cycle and surface marker properties of EBV-negative lymphoma lines and their in vitro EBV-converted sublines. Int J Cancer. 1976 Nov 15;18(5):639–652. doi: 10.1002/ijc.2910180513. [DOI] [PubMed] [Google Scholar]
- McConnell I., Klein G., Lint T. F., Lachmann P. J. Activation of the alternative complement pathway by human B cell lymphoma lines is associated with Epstein-Barr virus transformation of the cells. Eur J Immunol. 1978 Jul;8(7):453–458. doi: 10.1002/eji.1830080702. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Andersson L. C., Gahmberg C. G., Wigzell H. Surface glycoprotein patterns of normal and malignant human lymphoid cells. II. B cells, B blasts and Epstein-Barr virus (EBV)-positive and -negative B lymphoid cell lines. Int J Cancer. 1977 Nov 15;20(5):708–716. doi: 10.1002/ijc.2910200510. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Graham B. J., Harris C. L., Madden M. J., Pearson G. R., Vande Woude G. F. Persistent herpes simplex virus infections established in two Burkitt lymphoma derived cell lines. J Gen Virol. 1976 Jul;32(1):51–62. doi: 10.1099/0022-1317-32-1-51. [DOI] [PubMed] [Google Scholar]
- Steinitz M., Klein G. Comparison between growth characteristics of an Epstein--Barr virus (EBV)-genome-negative lymphoma line and its EBV-converted subline in vitro. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3518–3520. doi: 10.1073/pnas.72.9.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz M., Klein G. Epstein-Barr virus (EBV)-induced change in the saturation sensitivity and serum dependence of established, EBV-negative lymphoma lines in vitro. Virology. 1976 Apr;70(2):570–573. doi: 10.1016/0042-6822(76)90302-0. [DOI] [PubMed] [Google Scholar]
- Yefenof E., Klein G., Ben-Bassat H., Lundin L. Differences in the ConA-induced redistribution and agglutination patterns of EBV genome-free and EBV-carrying human lymphoma lines. Exp Cell Res. 1977 Aug;108(1):185–190. doi: 10.1016/s0014-4827(77)80024-4. [DOI] [PubMed] [Google Scholar]
- Yefenof E., Klein G. Difference in antibody induced redistribution of membrane IgM in EBV geonome free and EBV positive human lymphoid cells. Exp Cell Res. 1976 Apr;99(1):175–178. doi: 10.1016/0014-4827(76)90693-5. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Fresen K. O. Heterogeneity of Epstein-Barr virus. II. Induction of early antigens (EA) by complementation. Virology. 1977 Aug;81(1):138–143. doi: 10.1016/0042-6822(77)90065-4. [DOI] [PubMed] [Google Scholar]


