Abstract
Oxidative stress contributes to disease and can alter endothelial cell (EC) function. EC from different vascular beds are heterogeneous in structure and function, thus we assessed the apoptotic responses of EC from lung and heart to oxidative stress. Since protein kinase Cδ (PKCδ) is activated by oxidative stress and is an important modulator of apoptosis, experiments assessed the level of apoptosis in fixed lung and heart sections of PKCδ wild-type (PKCδ+/+) and null (PKCδ−/−) mice housed under normoxia (21% O2) or hyperoxia (~95% O2). We noted a significantly greater number of TUNEL-positive cells in lungs of hyperoxic PKCδ+/+ mice, compared to matched hearts or normoxic organs. We found that 33% of apoptotic cells identified in hyperoxic lungs of PKCδ+/+ mice were EC, compared to 7% EC in hyperoxic hearts. We further noted that EC apoptosis was significantly reduced in lungs of PKCδ−/− hyperoxic mice, compared to lungs of PKCδ+/+ hyperoxic mice. In vitro, both hyperoxia and H2O2 promoted apoptosis in EC isolated from microvasculature of lung (LMVEC), but not from the heart (HMVEC). H2O2 treatment significantly increased p38 activity in LMVEC, but not in HMVEC. Inhibition of p38 attenuated H2O2-induced LMVEC apoptosis. Baseline expression of total PKCδ protein, as well as the caspase-mediated, catalytically active PKCδ cleavage fragment, was higher in LMVEC, compared to HMVEC. PKCδ inhibition significantly attenuated H2O2-induced LMVEC p38 activation.. Conversely, overexpression of wild-type PKCδ or the catalytically-active PKCδ cleavage product greatly increased H2O2-induced HMVEC caspase and p38 activation. We propose that enhanced susceptibility of lung EC to oxidant-induced apoptosis is due to increased PKCδ→p38 signaling, and we describe a PKCδ-centric pathway which dictates the differential response of EC from distinct vascular beds to oxidative stress.
Keywords: Endothelium, apoptosis, heterogeneity, oxidative stress, ROS, PKCδ, p38
Introduction
Increased oxidative stress and reactive oxygen species (ROS) are observed in many acute and chronic disease states, including atherosclerosis, chronic obstructive pulmonary disease (COPD), pulmonary hypertension, acute lung injury (ALI), and acute respiratory distress syndrome (ARDS) (Aoki et al., 2001; Bowers et al., 2004; Chow et al., 2003; Jeremy et al., 2002; Kirkham and Rahman, 2006; Singh and Jialal, 2006). One of the mechanisms by which oxidative stress plays a role in the pathophysiology of these diseases is by modulation of endothelial function (Davignon and Ganz, 2004; Orfanos et al., 2004). Endothelial dysfunction results in increased monolayer permeability, surface activation, abnormal angiogenesis, and apoptosis (Bosch et al., 2010; El Solh et al., 2007; Lum and Roebuck, 2001; Vallet and Wiel, 2001; van den Oever et al., 2010). ROS induce apoptosis through multiple mechanisms; including caspase activation, mitochondrial dysfunction, direct DNA damage, and activation of other pro-apoptotic downstream signaling molecules (Irani, 2000).
Numerous studies have implicated oxidative stress as a cause/ factor in endothelial cell dysfunction and apoptosis; however, there is little data addressing the differential responses of endothelial cells derived from different vascular beds to oxidative stress (Lum and Roebuck, 2001). It is now well recognized that endothelial cells from different vascular beds vary in structure, function, and phenotypic characteristics (Aird, 2007a; Aird, 2007b; Yano et al., 2007). Induction of apoptosis is dependent upon many factors, including genotypic and phenotypic characteristics of individual cells, as well as the environmental milieu of the target tissue; thus it is likely that endothelial cell heterogeneity includes differences in susceptibility to apoptotic stimuli (Aird, 2007a; Aird, 2007b; Yano et al., 2007).
The lung is unique in that it possesses two distinct blood supply networks and three potential sources of tissue oxygenation (Ng et al., 2002). In addition, the pulmonary and systematic circulations behave differently in response to alterations in oxygen levels. While the systemic vasculature dilates in response to hypoxia, hypoxia causes vasoconstriction in the pulmonary circulation. Thus, it is possible that the response of lung endothelial cells to oxidant injury may differ from that of endothelial cells from systemic vascular beds, such as the heart. In this study, we compared the responses of microvascular endothelial cells from the lung and heart to oxidant injury. We investigated the susceptibility of each endothelial cell type to ROS-induced apoptosis and the mechanism(s) by which apoptosis was induced.
We show that lung microvascular endothelial cells are more susceptible to oxidant-induced apoptosis, as compared with heart microvascular endothelial cells. We also present data indicating that the PKCδ/ p38 pathway is important in mediating differences in susceptibility to ROS-induced apoptosis of endothelial cells from heart versus lung.
Materials and Methods
PKCδ+/+ and PKCδ−/− Mice
PKCδ+/− breeding pairs were a kind gift from Dr. Brooke Mossman (U. Vermont, Burlington, VT) (Shukla et al., 2007), originally derived by Dr. Keiichi I. Nakayama (Miyamoto et al., 2002). These mice were subsequently maintained and bred into the C57BL/6 background in the Providence VA Medical Center animal facility. Animals were given food and water ad libitum and monitored daily. The genotype of the mice was confirmed via polymerase chain reaction (PCR) using the following primers (Integrated DNA Technologies): common primer 1, 5’GGAAGAATAAGAAACTGCATCACC3’; amplification of wild type product (240 bp), primer 2, 5’GAAGGAGCCAGAACCGAAAG3’; and amplification of disrupted PKCδ product (150 bp), primer 3, 5’TGGGGTGGGATTAGATAAATG3’, as described by Dr. Keiichi I. Nakayama (Riken BRC, RBRC00457). In parallel, spleen tissue from PKCδ transgenic mice was examined by Western blot analyses using antibodies directed against the –COOH terminus of PKCδ to confirm levels of protein expression.
For experiments, age- and sex-matched adult PKC+/+ or PKC−/− mice (C57/B6 background), were caged under normoxia or hyperoxia (~95% O2) for 72h in a Biospherix A-Chamber, interfaced with a Biospherix ProOx sensor and regulator (Biospherix, Ltd., Lacona, NY). Lungs and hearts were removed following anaesthetization and exsanguination, and fixed with 10% formalin (lungs were inflation fixed).
All animal protocols were approved by the Providence VA Medical Center and Brown University IACUC and comply with the Health Research Extension Act and PHS policy.
Cells and Reagents
Rat lung microvascular (LMVEC) and rat heart microvascular endothelial cells (HMVEC) were purchased from Vec Technologies (Rensselaer, NY). Von Willebrand factor (vWF) and VE-cadherin expression, and uptake of acetylated low density lipoprotein, was confirmed. Cells of the same passage (3–11) were used for comparison. Both endothelial cell types were cultured in complete medium MCDB-131 (Vec Technologies). During experiments, the complete medium was removed and the endothelial cells were cultured in reduced serum medium, which contained 1 part complete MCDB-131 medium and 9 parts basal MCDB-131 medium (i.e., medium lacking any serum or growth factors).
Antibodies and reagents used were obtained from the following vendors: vWF, Dako; VE-cadherin and cytochrome c, Santa Cruz Biotechnologies; caspase-3, total and p~p38(T180/Y182) and total and p~AktS473, Cell Signaling Technology; HSP90, HSP70, and fluorescently-conjugated acetylated LDL, Invitrogen; GRP94, procaspase-9, and procaspase-12, Stressgen/Assay Designs, Inc.; procaspase-8, Stratagene/Agilent Technologies, Inc.; GRP78, Griffonia simplicifolia, Glycine max, Helix pomatia, and rottlerin, Sigma Aldrich; immunohistochemical enzyme substrates, Vector Laboratories; TUNEL Apoptosis Detection kit, Millipore; Antioxidant Assay kit, Cayman Chemicals. The eukaryotic expression vector encoding HA-tagged PKCδ-CAT was obtained from Addgene (Cambridge, MA); GFP expression vector was purchased from Clontech Laboratories (Mountain View, CA); and the eukaryotic vector encoding GFP-conjugated to wild type PKCδ was obtained from Dr. Peter Blumberg (National Cancer Institute) (Wang et al., 1999).
Transfection
Transient transfection of LMVEC and HMVEC was performed using Polyjet reagent (SignaGen), according to the manufacturer’s protocol. Optimal overexpression of GFP, PKCδ-CAT, or wild type PKCδ was confirmed to occur in EC transfected with the eukaryotic expression vectors at 48h post-transfection.
Apoptosis Assessment
Apoptosis was assessed in cultured EC and paraffin-embedded tissue slices via TdT-mediated dUTP nick end labeling (TUNEL) staining, as described (Harrington et al., 2000).
To assess caspase activity, EC were harvested and lysed in 10mM HEPES, pH 7.5, 40mM β-glycerophosphate, 50mM NaCl, 2mM MgCl2, and 5mM EGTA. Following freeze-thaw, debris was removed by centrifugation. Caspase activity was quantified as described (Harrington et al., 2001).
DNA laddering was assessed as described in (Embree-Ku et al., 2002).
Antioxidant Capacity Measurements
Equivalent numbers of LMVEC and HMVEC were collected, re-suspended in assay buffer (5mM potassium phosphate, pH 7.4, 0.9% NaCl, and 0.1% glucose), and sonicated. Following centrifugation, supernatant antioxidant capacity was determined using an Antioxidant Assay kit (Cayman Chemical Company), as per manufacturer’s protocol. This assay measures the oxidation of 2, 2’-Azino-di-[3-ethylbenzthiazoline sulphonate] (ABTS) in a two step reaction. First, metmyoglobin catalytically reacts with hydrogen peroxide forming a ferryl myoglobin radical; which in turn oxidizes ABTS. The radical cation, ABTS®·+, generated is green in colored and measured spectrophotometrically at an absorbance of 405 nm. Antioxidants within experimental samples suppress this reaction by scavenging electrons and inhibiting the formation of the ABTS cationic radical. Trolox [6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid], a water soluble tocopherol analog, is used as a positive control in the assay, serving to inhibit the formation of the radical cation in a dose-dependent manner. The antioxidant capacity of our experimental samples was determined by normalizing to equivalent Trolox units.
Immunohistochemistry
Paraffin-embedded lungs and hearts were sliced into 6µm sections, de-paraffinized, re-hydrated, and processed for antigen retrieval and quenched. Serial sections were immunohistochemically-stained for apoptotic cells using TUNEL. EC were identified using vWF antibodies and nuclei were counterstained with methyl green.
Paraformaldehyde-fixed LMVEC and HMVEC were incubated with the fluorescently-conjugated lectins, Griffonia simplicifolia, Glycine max, and Helix pomatia, as previously described (King et al., 2004; Lu et al., 2009). In parallel, fixed LMVEC and HMVEC were immunofluorescently stained for vWF, VE-cadherin, or cytochrome c, using established protocols (Harrington et al., 2003; Harrington et al., 2005; Klinger et al., 2007). Acetylated LDL uptake was assayed by incubating LMVEC or HMVEC with fluorescently-tagged acetylated LDL for 1h, followed by paraformaldehyde fixation and fluorescence microscopy.
Statistical Analyses
For ≥3 groups, significance was evaluated using ANOVA with Fisher’s least significance difference test. For two groups, significance was assessed using Students’ unpaired t-test or paired t-test. Significance was reached when p<0.05. Data are presented as the mean ± S.E. or ± S.D.
Results
Higher Incidence of Endothelial Cell Apoptosis in Hyperoxic Lungs versus Hearts; PKCδ Deficient Mouse Lungs Display Less Hyperoxia-induced Apoptosis
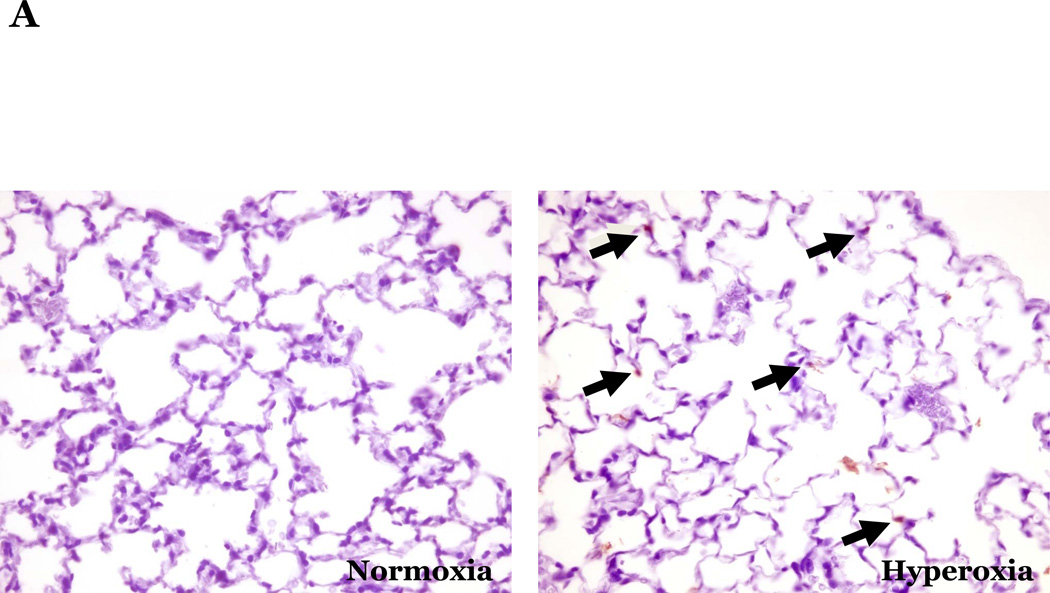
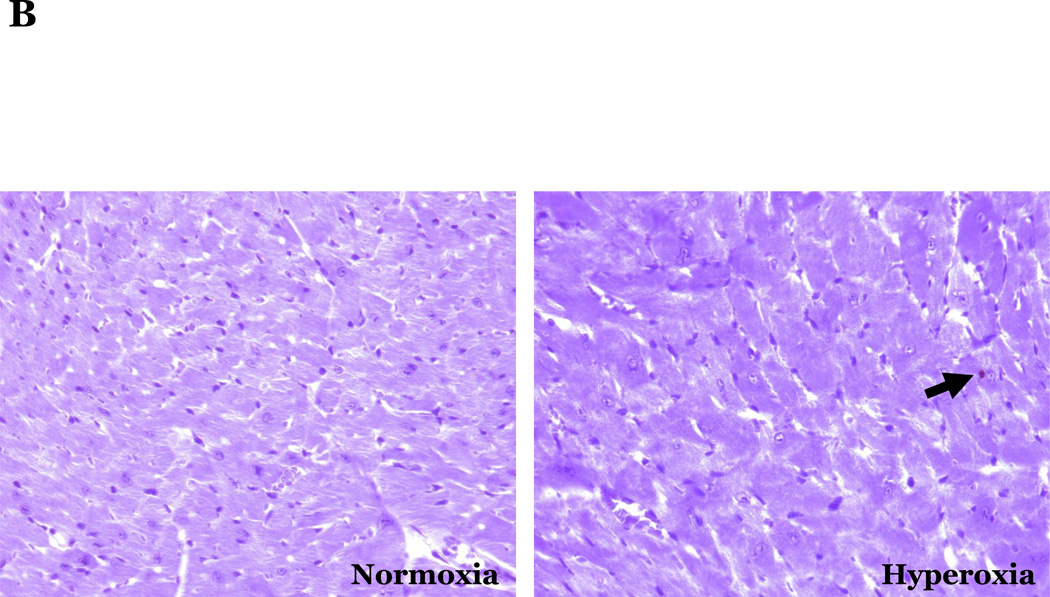
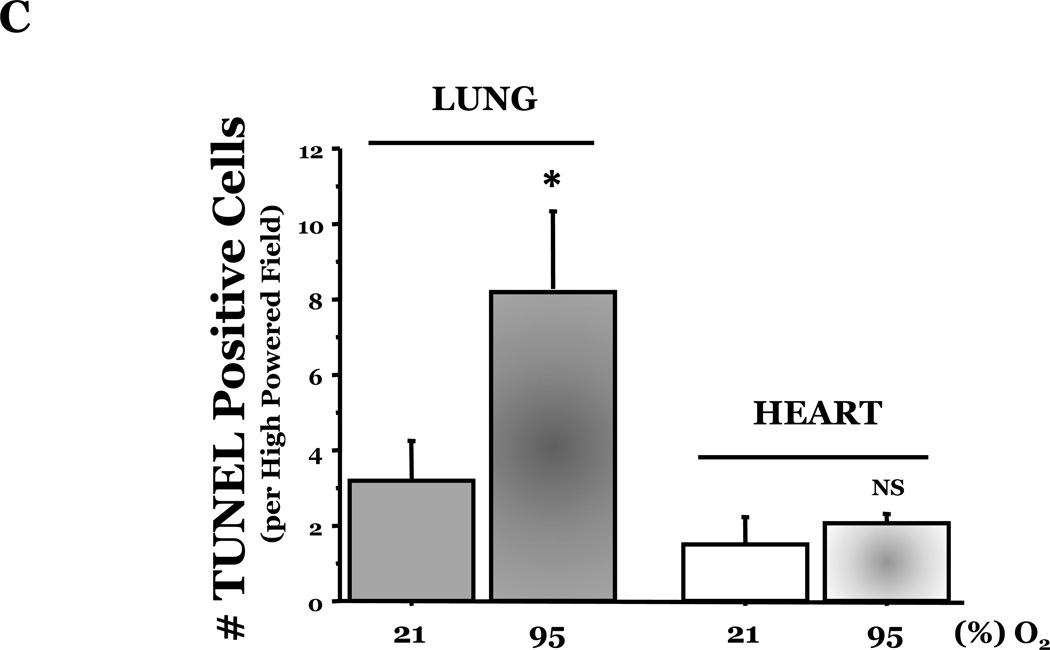
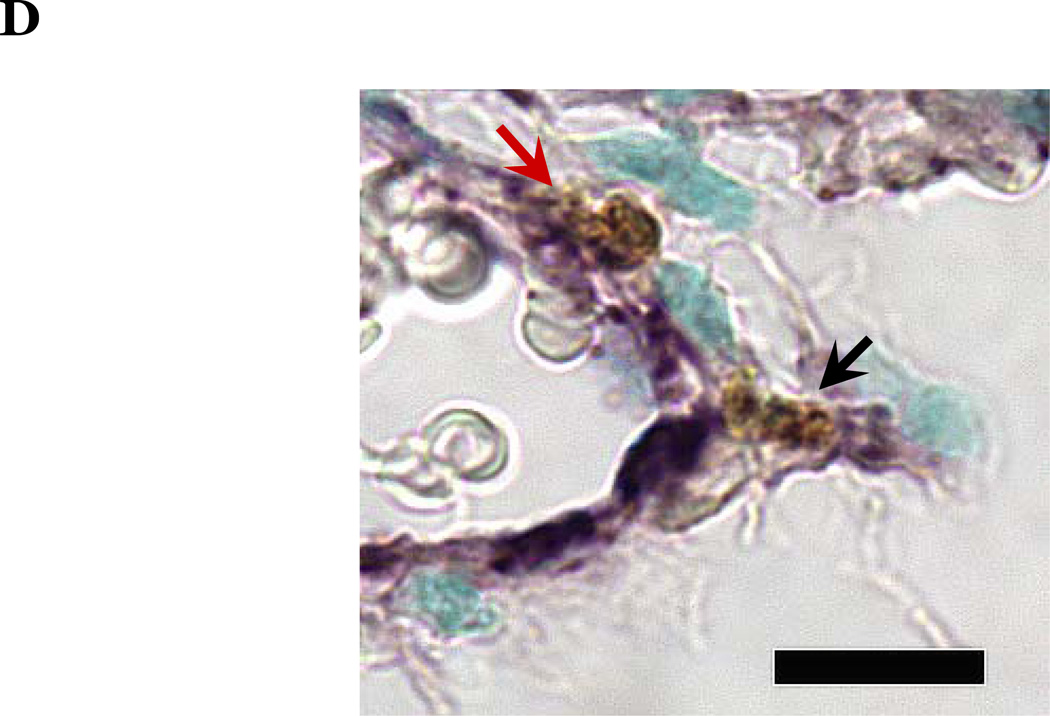
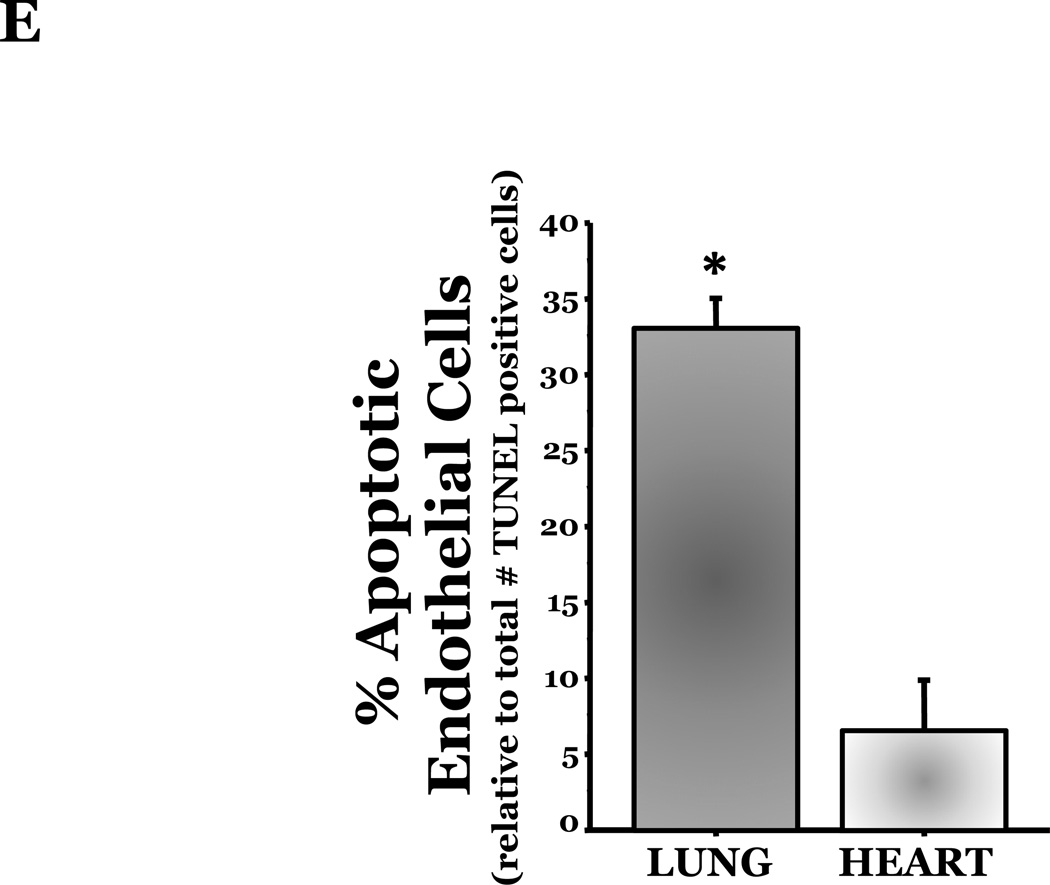
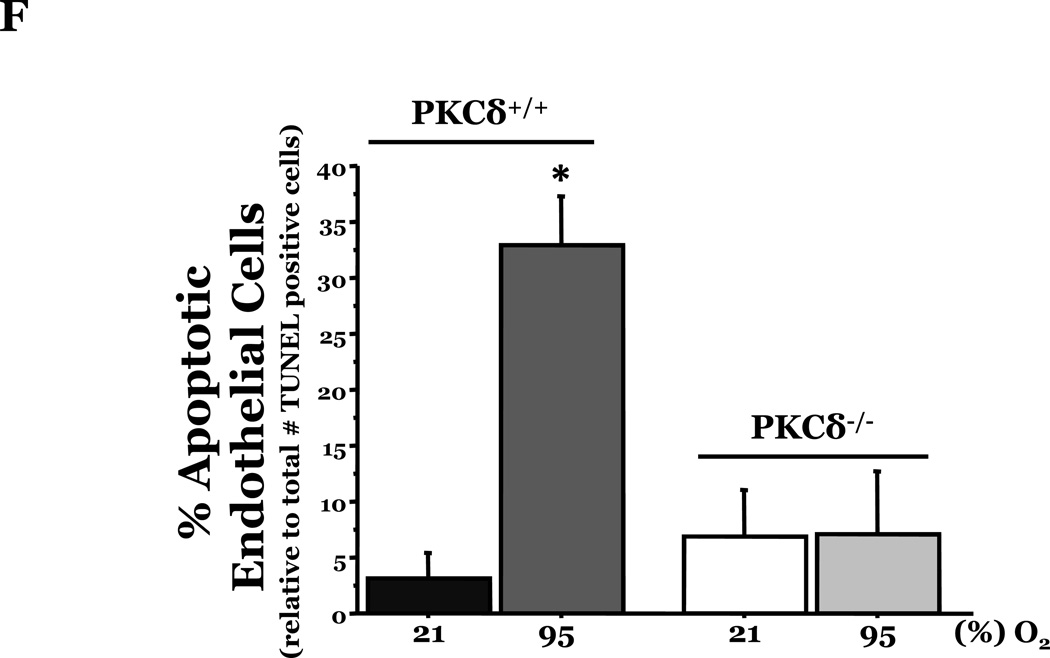
PKCδ, an important modulator of apoptosis, is constitutively activated by caspase-3 in response to oxidative stress. Thus, using PKCδ null (PKCδ−/−) and wild-type (PKCδ+/+) mice, we assayed the number of apoptotic cells in lungs and hearts of the mice following 72h of normoxia or hyperoxia (Figure 1). In the PKCδ+/+ mice, we noted significantly more apoptotic cells in the lungs of mice exposed to high levels of O2, relative to normoxic mice (Figure 1C). In contrast, hyperoxia did not increase apoptosis in the hearts. We next immunohistochemically analyzed the fixed organ sections from PKCδ+/+ mice which were serially stained for DNA fragmentation using a TUNEL assay and for von Wildebrand factor (vWF), to identify if the apoptotic cells were endothelial cells (Figure 1D). Upon enumeration of TUNEL positive endothelial cells in approximately twenty 1000X fields, we noted ~33% of the TUNEL positive cells to be endothelial cells in the lung following hyperoxia exposure, while only ~7% of the TUNEL positive cells were endothelial cells in hearts obtained from the same hyperoxic animal (Figure 1E). The level of endothelial cell apoptosis in the lungs of hyperoxic PKCδ−/− mice was significantly less (15.845 ± 7.031%; n=3; p<0.03) than that noted in hyperoxic PKCδ+/+ mouse lungs (32.942 ± 1.910%; n=6) (Figure 1F), and not statistically different from normoxic PKCδ+/+ or PKCδ−/− mouse lungs. These data demonstrate a differential response of the endothelium from distinct organ vascular beds to undergo apoptosis in settings of acute oxygen injury; an apoptotic response that is dependent upon PKCδ.
Figure 1. Lung endothelial cells are more susceptible to hyperoxia-induced apoptosis than heart endothelial cells; PKCδ deficiency is protective against lung endothelial cell apoptosis.
Age and sex-matched PKCδ+/+ or PKCδ−/− mice were caged in normoxic (room air) or hyperoxic (~95% O2) environments for 72h. The organs from PKCδ+/+ mice were harvested and immunohistochemically processed either for fragmented DNA, using TUNEL, (panels A and B) or for the identification of apoptotic endothelial cells, using antibodies directed against vWF and counterstained using TUNEL and methyl green for nuclei (panel D). Panels A and B, representative sections of TUNEL stained normoxia and hyperoxia exposed lungs (panel A) and hearts (panel B) are shown at 400X magnification in bright field. Arrows indicate cells with TUNEL staining. Panel C, four random fields from each section viewed at 400X magnification were quantitated for the number of apoptotic cells per field. n = 4; * p < 0.05 vs. normoxic lungs. NS, not significant. Panel D, representative section of a hyperoxic mouse lung section co-stained for vWF (VIP positive; purple staining) and fragmented DNA, using TUNEL (diaminobenzidine (DAB) positive; brown staining) viewed at 1000X magnification in bright field; scale bar = 20µm. Panel E, random fields (16–27 fields) containing apoptotic cells from each section viewed at 1000X magnification were quantitated for the number of TUNEL positive endothelial cells relative to the total number of TUNEL positive cells in the cumulative fields of the PKCδ+/+ organ sections. n = 5. * p < 0.0001. Panel F, the number of apoptotic endothelial cells were quantitated in lung sections from PKCδ+/+ and PKCδ−/− mice exposed to normoxia or hyperoxia co-stained for vWF and fragmented DNA, using TUNEL. n = 3–5. *p < 0.0001.
Characterization of LMVEC and HMVEC
To determine if endothelial cells responded similarly to oxidant injury in vitro, we used primary cultures of endothelial cells isolated from the periphery of the lung or heart of rats. Both the primary cultures of lung microvascular endothelial cells (LMVEC) and heart microvascular endothelial cells (HMVEC) displayed typical cobblestone morphology (Supplemental Figure 1A). To confirm the endothelial cell phenotype, we first assayed the cells for expression of several classic markers of endothelial cells, including vWF, VE-cadherin, and uptake of acetylated LDL using immunofluorescence microscopy. As expected, both endothelial cell types stained positive for vWF and VE-cadherin and took up acetylated LDL (Supplemental Figures 1B–1D). Previous work has characterized lung macrovascular and microvascular endothelial cells using a series of lectins (King et al., 2004; Lu et al., 2009). Using the same series of lectins, we noted equivalent levels of staining in both endothelial cell types with Griffonia simplicifolia and Glycine max (Table 1). Conversely, Helix pomatia staining was low in both the LMVEC and HMVEC. Further experiments characterizing the endothelial cell markers and lectin staining patterns in early passaged (P4) and late passaged LMVEC (P11) and HMVEC (P10), demonstrated maintenance of these characteristics over passages (Table 1).
Table 1.
Characterization of Microvascular Endothelial Cells
| LMVEC | HMVEC | |||
|---|---|---|---|---|
| P4 | P11 | P4 | P10 | |
| vWF | +++ | +++ | +++ | +++ |
| VE-cadherin | +++ | Not done | +++ | Not done |
| Ac-LDL | +++ | +++ | +++ | +++ |
| Griffonia simplicifolia | +++ | +++ | +++ | +++ |
| Glycine max | +++ | +++ | +++ | +++ |
| Helix pomatia | + | + | + | + |
N=3 for each staining
Level of immunofluorescence signal: +, low; ++, moderate; +++, high.
Response of LMVEC and HMVEC to Oxidative Stress
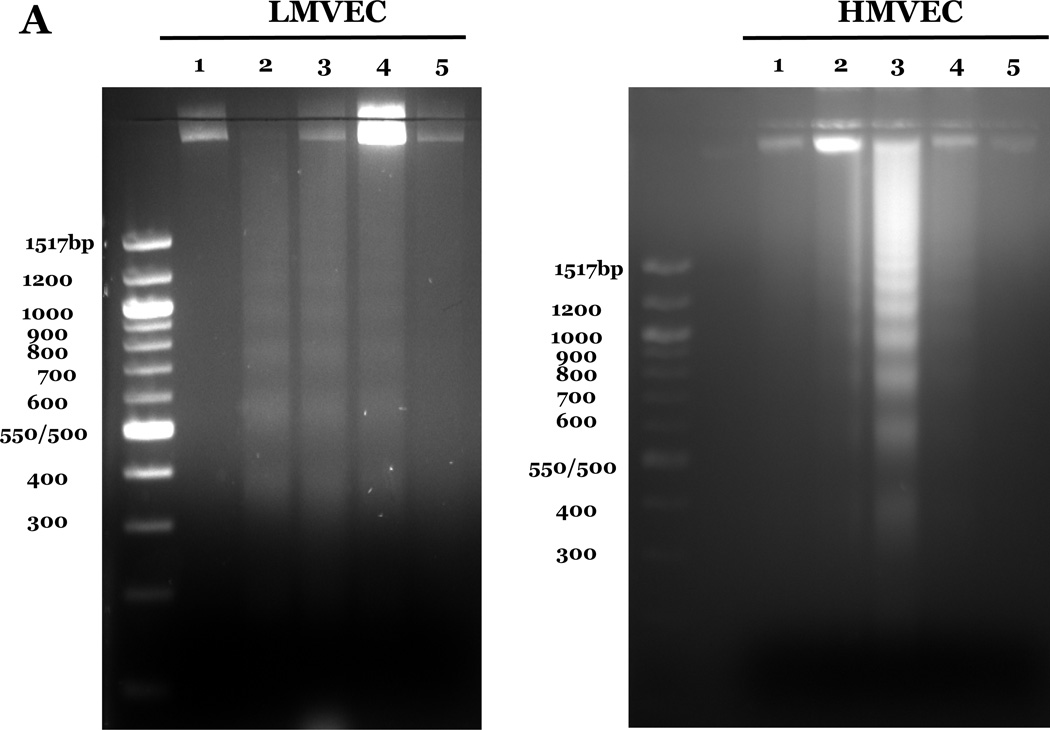
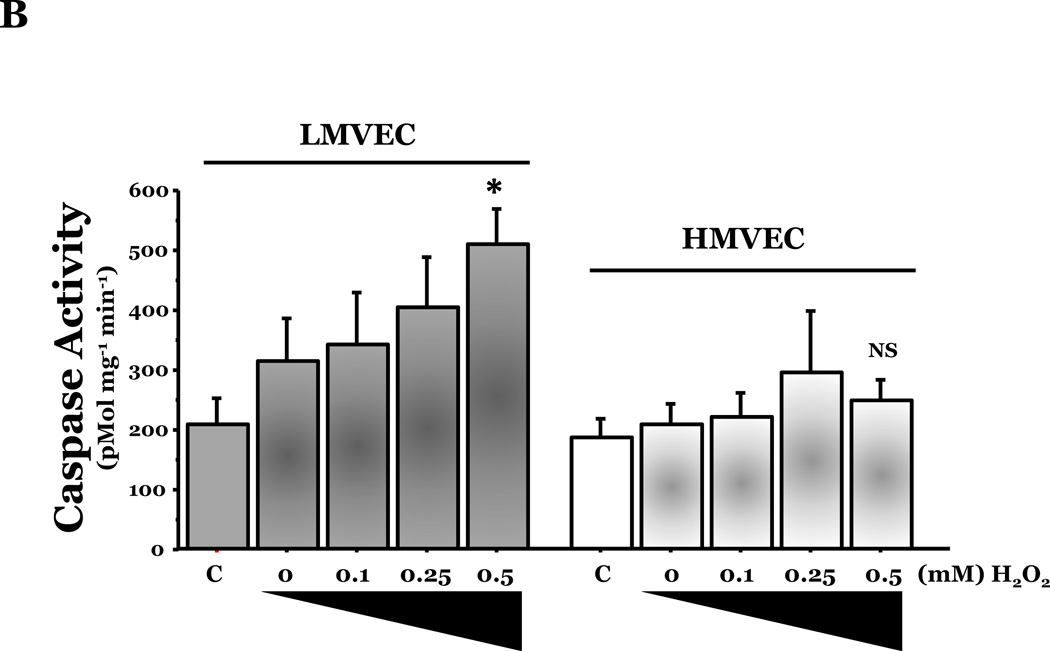
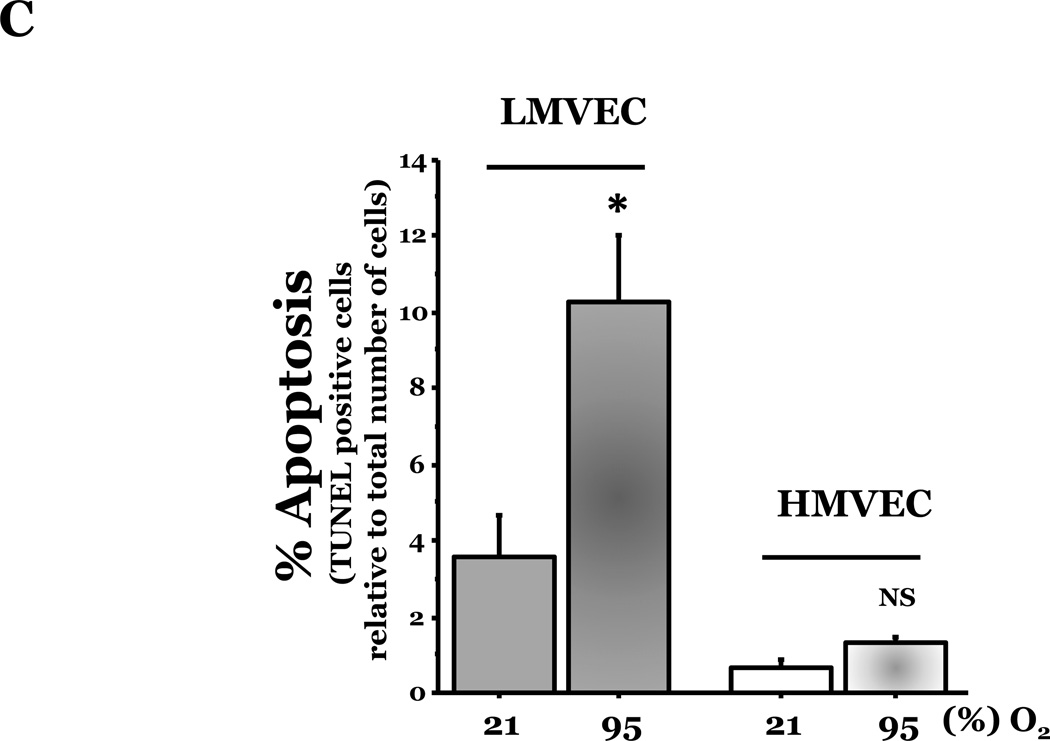
We investigated the response of LMVEC and HMVEC to apoptosis induced by oxidative stresses. Endothelial cells were incubated in the presence or absence of the indicated amount of H2O2 or under normoxic or hyperoxic conditions. Analysis of genomic DNA demonstrated DNA laddering in LMVEC exposed to both types of oxidative stresses (Figure 2A). Further experiments showed a significant increase in caspase activity in LMVEC upon exposure to 0.5mM H2O2 (Figure 2B) and a greater number of TUNEL positive LMVEC upon exposure to hyperoxia (Figure 2C). Interestingly, HMVEC cultured under the same conditions did not undergo a significant level of apoptosis in response to either oxidative stress (Figures 2A – 2C).
Figure 2. Oxidative stress promotes greater degree of apoptosis in LMVEC.
Panel A, microvascular endothelial cells cultured in serum deficient medium were exposed to normoxia, 21% O2, (lane 1) or hyperoxia, 95% O2, (lane 2) for 24h, UV light for 3 minutes and cultured an additional 18h (lane 3), or 0.5mM H2O2 for 24h (lane 4) or vehicle (H2O) (lane 5). Genomic DNA was isolated and electrophoretically resolved on 2% agarose gel. Representative gels are shown. n = 3. Panel B, microvascular endothelial cells were cultured in complete medium (C) or reduced serum medium and exposed to indicated concentration of H2O2 for 6h. Equivalent amounts of lysates were assayed for caspase activity, using release of the fluorescent conjugate (AMC) from the peptide substrate, DEVD. Data is presented as the mean ± S.E. n= 3 – 4; *p < 0.05 vs. complete medium (C) or vehicle treated (0) cells. NS, not significant. Panel C, microvascular endothelial cells were cultured in reduced serum medium under settings of normoxia or hyperoxia for 24h and apoptosis was detected via TUNEL staining. Data is presented as the mean ± S.E. of percent apoptotic cells relative to total cells present. Normoxic endothelial cells, n = 9; hyperoxic endothelial cells, n = 11; *p < 0.0005. NS = not significant.
Antioxidant Capacity of the LMVEC and HMVEC
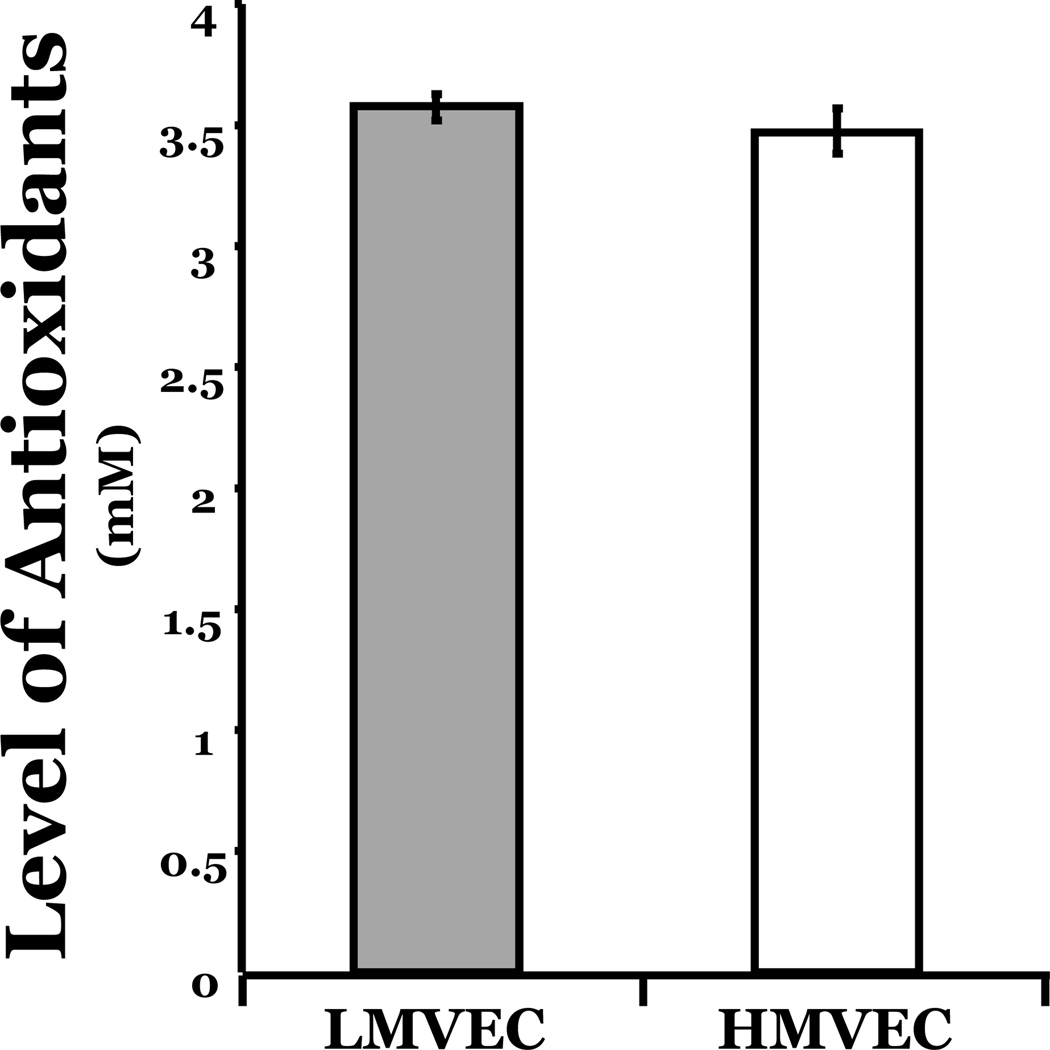
To explore the possibility that the differential apoptotic response of the LMVEC and HMVEC following exposure to oxidative stress was due to differing levels of antioxidant activities within the endothelial cells, we quantitated the overall antioxidant levels. As shown in Figure 3, the antioxidant levels in LMVEC were not statistically different from those noted in HMVEC; thus, it appears that the differential response of the two types of endothelial cells to oxidative stresses is not due to altered antioxidant capacity.
Figure 3. Equivalent levels of antioxidant capacity in both types of microvascular endothelial cells.
Equivalent amounts of cells grown in complete medium were collected by scraping and the antioxidant levels were quantitated within equivalent amounts of lysate of LMVEC and HMVEC using a spectrophotometric antioxidant assay. n = 6.
Effects of Oxidative Stresses on Apoptotic and Unfolded Protein Response (UPR) Pathways in LMVEC and HMVEC
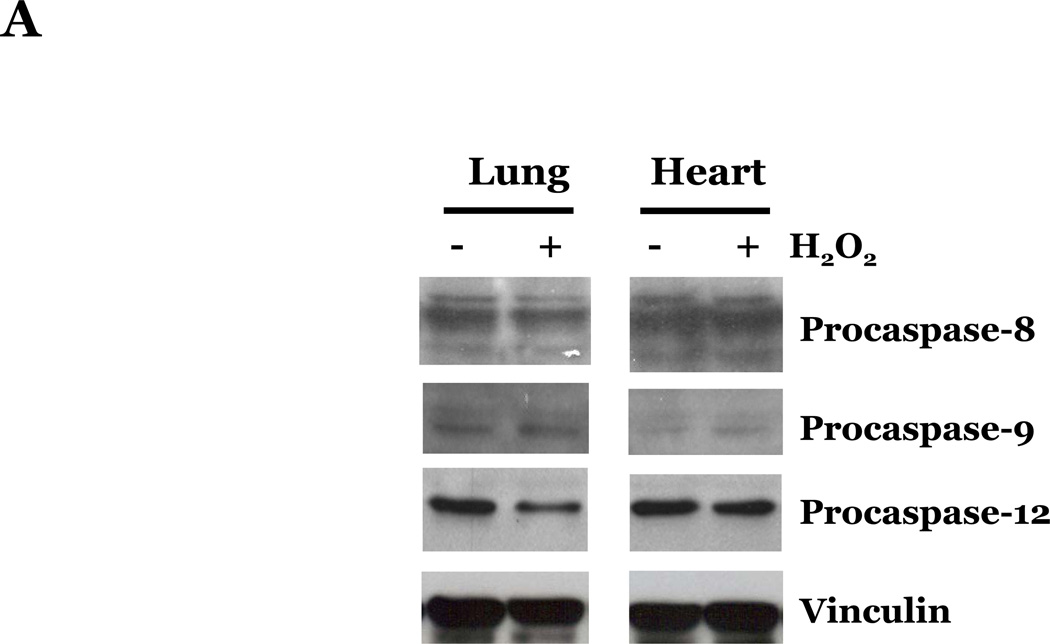
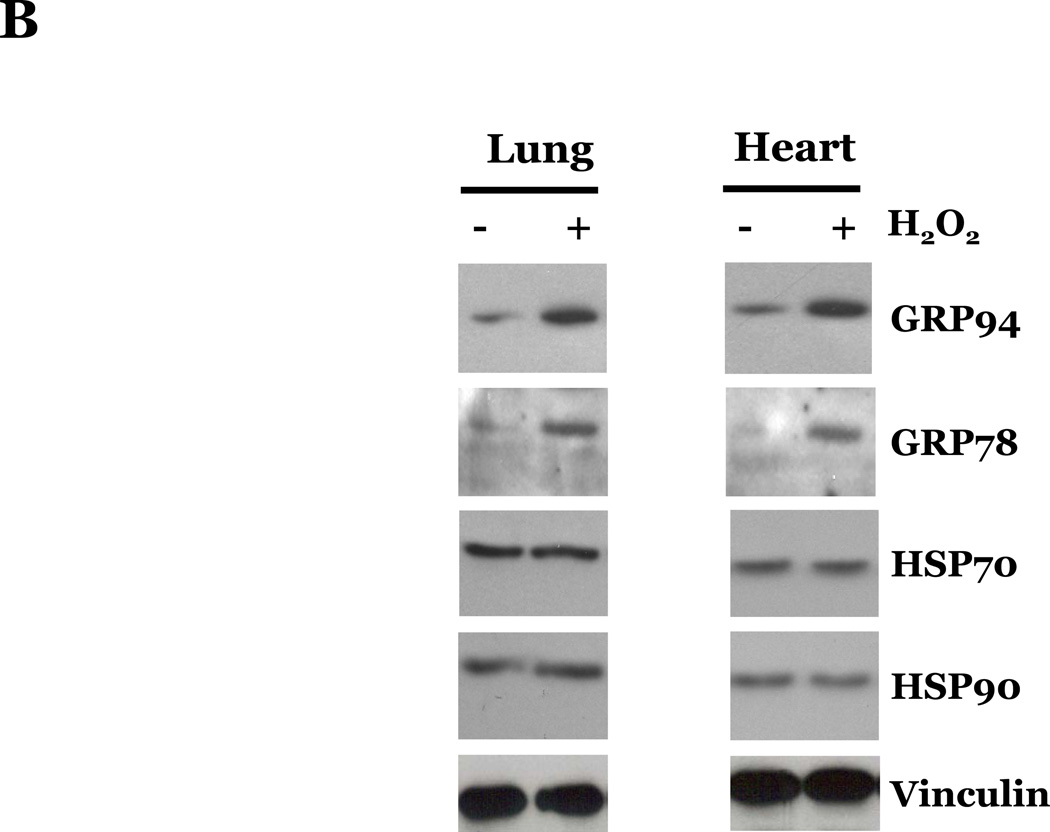
Reactive oxygen species (ROS) have been shown to promote apoptosis via the intrinsic pathway through alterations in mitochondrial function and by activation of the UPR pathway (Buytaert et al., 2007). Thus, we next examined if ROS promoted endothelial cell apoptosis through the classical pathways (i.e., extrinsic or intrinsic pathways) or by promoting endoplasmic reticulum stress and apoptosis through a UPR-mediated pathway, by assaying the activation of caspases known to be involved in these pathways. Caspase-8, caspase-9, and caspase-12 have been described as initiator caspases, involved in the extrinsic-, intrinsic-, and UPR-mediated apoptotic pathways, respectively. Thus, we assayed the effects of ROS exposure on the protein levels of the inactive, pro-peptide forms of these caspases. We did not observe a significant difference in the levels of procaspase-8, -9, or -12 in LMVEC as compared with HMVEC upon exposure to H2O2 (Figure 4A). We noted an increase in the expression of the UPR-associated chaperone proteins GRP94 and GRP78, but not of the cytosolic chaperone proteins HSP90 or HSP70, in both LMVEC and HMVEC upon H2O2 exposure (Figure 4B), suggesting a similar level of UPR activation in both endothelial cell types. Similar results assaying either the pro-caspases or UPR-associated chaperone proteins were obtained in both endothelial cells cultured under normoxic and hyperoxic conditions (data not shown). In addition, we did not detect any significant difference in LMVEC and HMVEC lysates from the various experimental conditions in the level of cleaved, fluorescent VEID-AFC, LEHD-AFC, or ATAD-AFC in in vitro activity assays for caspase-6, -9, or -12, respectively (data not shown). Furthermore, pretreatment of LMVEC or HMVEC with caspase inhibitors z-IETD-fmk (caspase-8), z-LEHD-fmk (caspase-9), or z-ATAD-fmk (caspase-12) did not significantly affect the degree of apoptosis in any experimental condition (data not shown).
Figure 4. Oxidative stress promotes similar effects on caspase activation, UPR-associated chaperone proteins, and cytochrome c release in lung and heart microvascular endothelial cells.
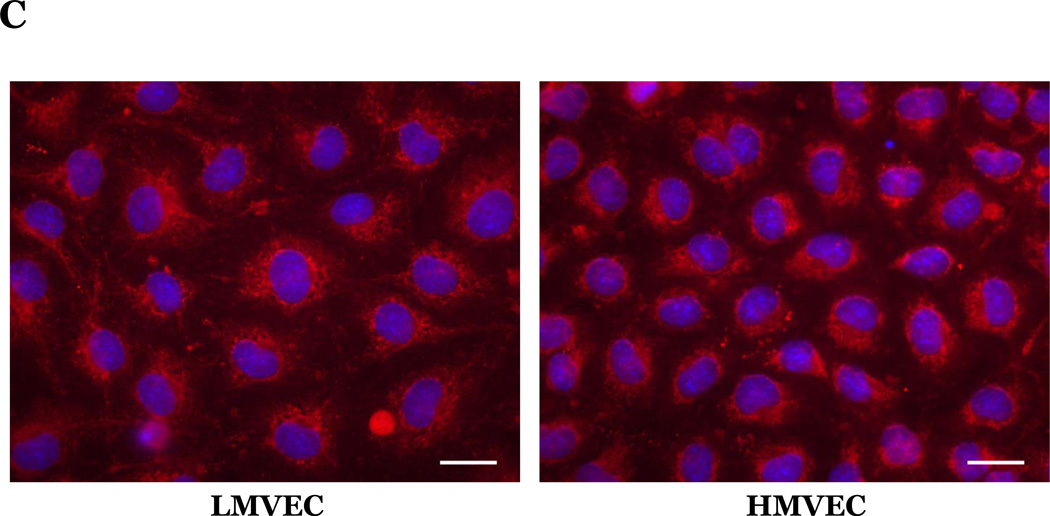
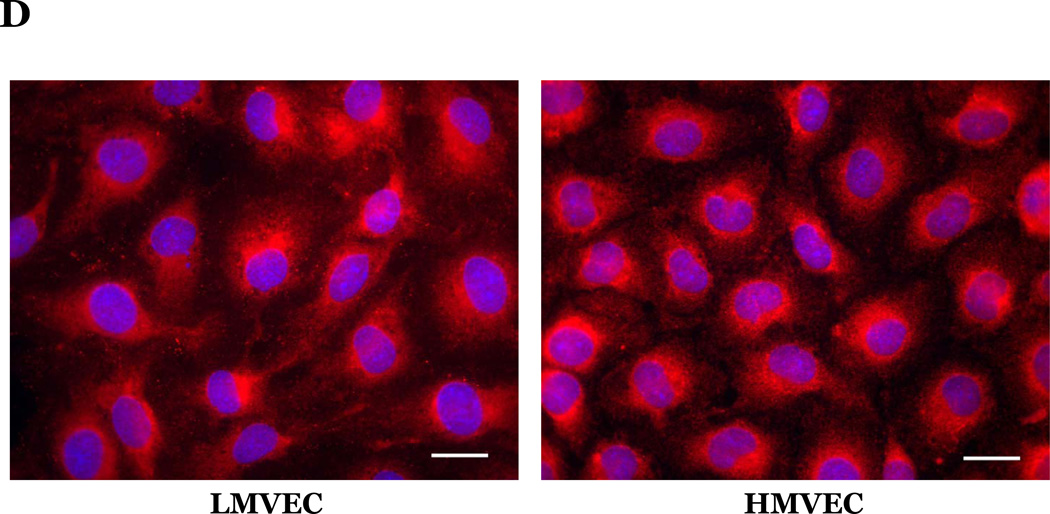
Microvascular endothelial cells cultured in reduced serum medium were exposed to vehicle or H2O2 overnight. Equivalent amounts of lysates were resolved by SDS-PAGE and immunoblotted for indicated proteins. Representative gels are presented. n = 3. (panels A and B). Microvascular endothelial cells were cultured in reduced serum medium in settings of normoxia (panel C) or hyperoxia (panel D) for 24h. Cells were fixed and immunofluorescently stained for cytochrome c and viewed at 1000X magnification. Nuclei are counterstained with DAPI. Scale bar = 20µm. Representative images are presented. n = 3.
Upon activation of the intrinsic pathway, cytochrome c is released from the mitochondria into the cytosol. Thus, to further examine the response of the endothelial cells to oxidative stress we next assessed the effect of hyperoxia or H2O2 on cytochrome c subcellular localization. We noted a defined staining pattern of cytochrome c around the nucleus in both endothelial cell types cultured under normoxic conditions (Figure 4C). We further noted that upon exposure to hyperoxia (Figure 4D) or H2O2 (data not shown), cytochrome c was more diffusely localized throughout the cytosol in both the LMVEC and HMVEC.
Differential Activation of p38 by ROS in Lung and Heart Endothelial Cells
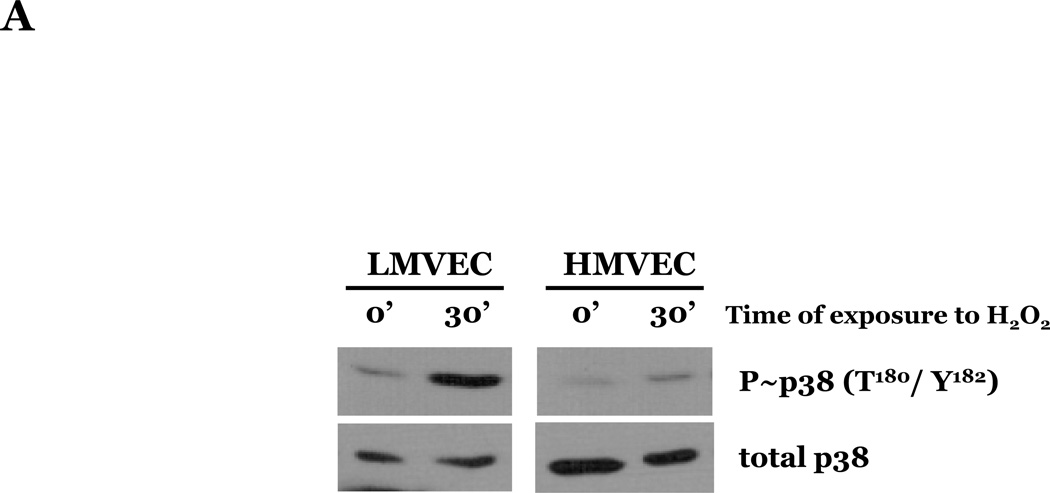
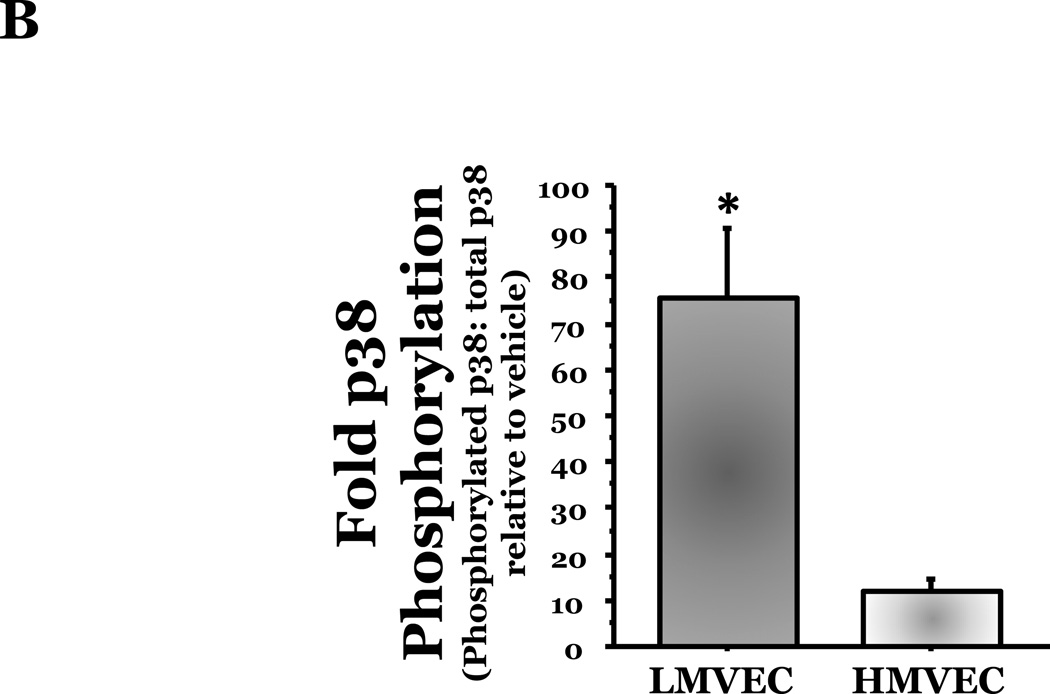
To assess the effect of oxidative stress on p38 activation, we next exposed LMVEC or HMVEC to H2O2 for varying times in serum-reduced medium and lysates were assayed for the phosphorylated, active form of p38 (T180/Y182). We noted a delayed activation of p38 in both cells types with increased levels of phosphorylated p38 occurring at 30 minutes following exposure to H2O2 and levels diminishing by 60 minutes of exposure. We noted an approximate 75-fold elevation in the level of p38 phosphorylation in the LMVEC at 30 minutes exposure to H2O2 (Figures 5A and 5B), relative to vehicle exposed LMVEC. Conversely, the level of p38 phosphorylation in H2O2-exposed HMVEC was not statistically increased relative to the level noted in vehicle exposed HMVEC (p = 0.349).
Figure 5. Differential activation of p38 by oxidative stress in microvascular endothelial cells.
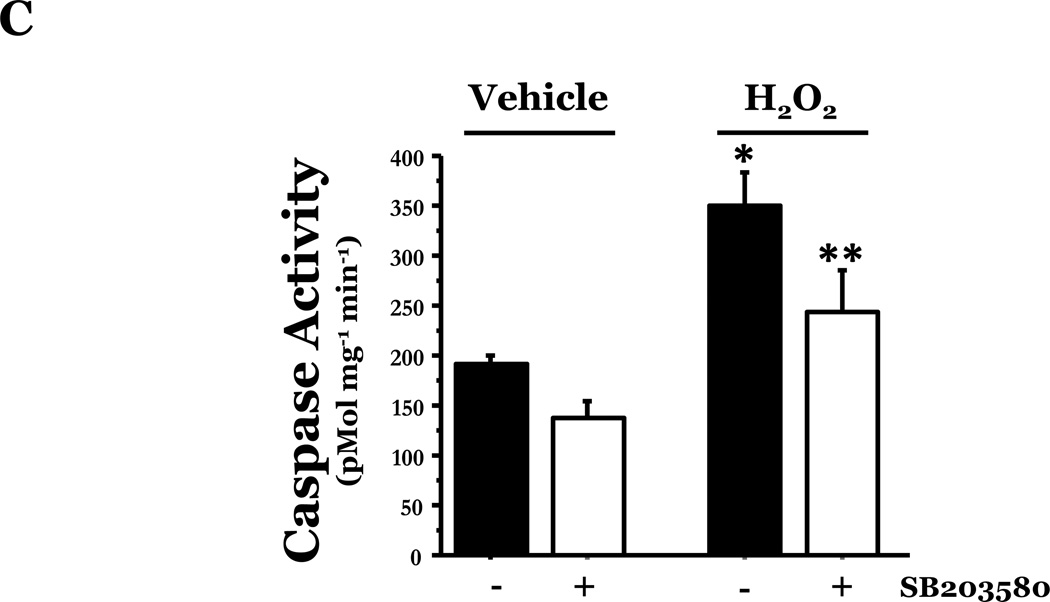
Panel A, equivalent amounts of LMVEC or HMVEC, cultured in reduced serum medium, were exposed to 0.5mM H2O2 for 30 minutes. Equivalent amounts of lysates were resolved by SDS-PAGE and immunoblotted for phosphorylated p38 (T180/Y182). The membranes were stripped and reprobed for total p38. Representative immunoblots are shown, n = 3. Panel B, data is presented as the mean ± S.E. of the level of phosphorylated p38 relative to total p38 and normalized to the level of active p38 in vehicle treated endothelial cells. n = 3; *p < 0.001 vs. HMVEC. Panel C, LMVEC were pretreated with 100nM SB203580 for 30 minutes and then exposed to vehicle or 0.5mM H2O2 for 6h. Equivalent amounts of lysates were assayed for caspase activity, the release of the fluorescent conjugate (AMC) from the peptide substrate, DEVD. Data is presented as mean±SE. n = 6. *p < 0.0001 vs. vehicle ± SB203580. **p < 0.05 vs. 0.5mM H2O2 without SB203580.
We next examined the differential role of p38 in mediating ROS-induced apoptosis in LMVEC, relative to HMVEC. We noted that chemical inhibition of p38 significantly attenuated H2O2-induced caspase activity in LMVEC (Figure 5C). Thus, the data suggest that the enhanced level of activated p38 in response to oxidant stress in LMVEC, but not HMVEC, plays an important role in mediating ROS-induced apoptosis.
Increased PKCδ Expression May Predispose LMVEC to Increased p38 Activation and ROS-Induced Apoptosis
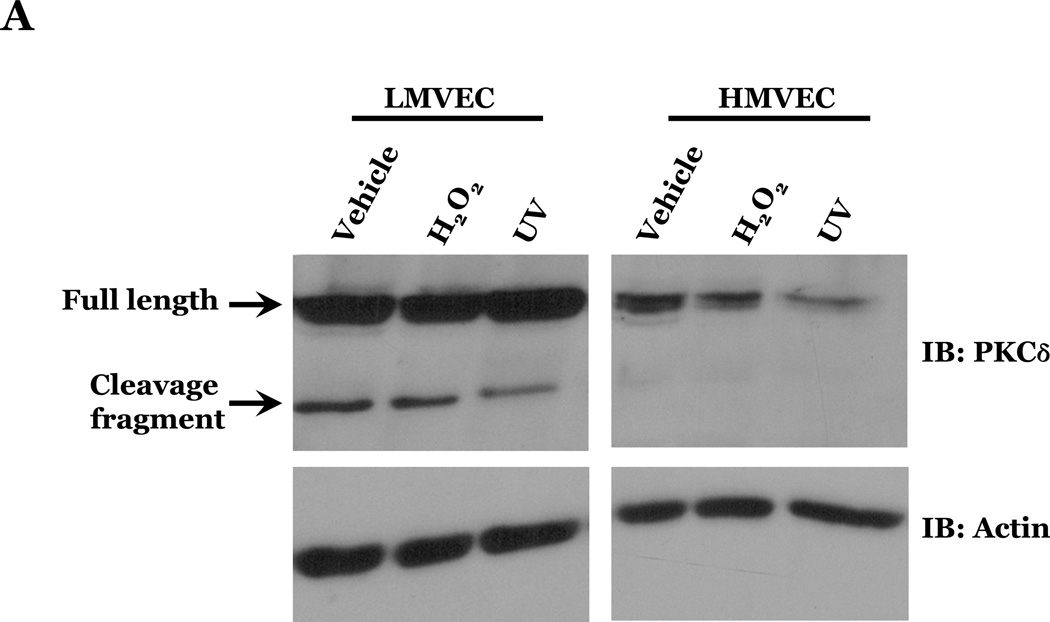
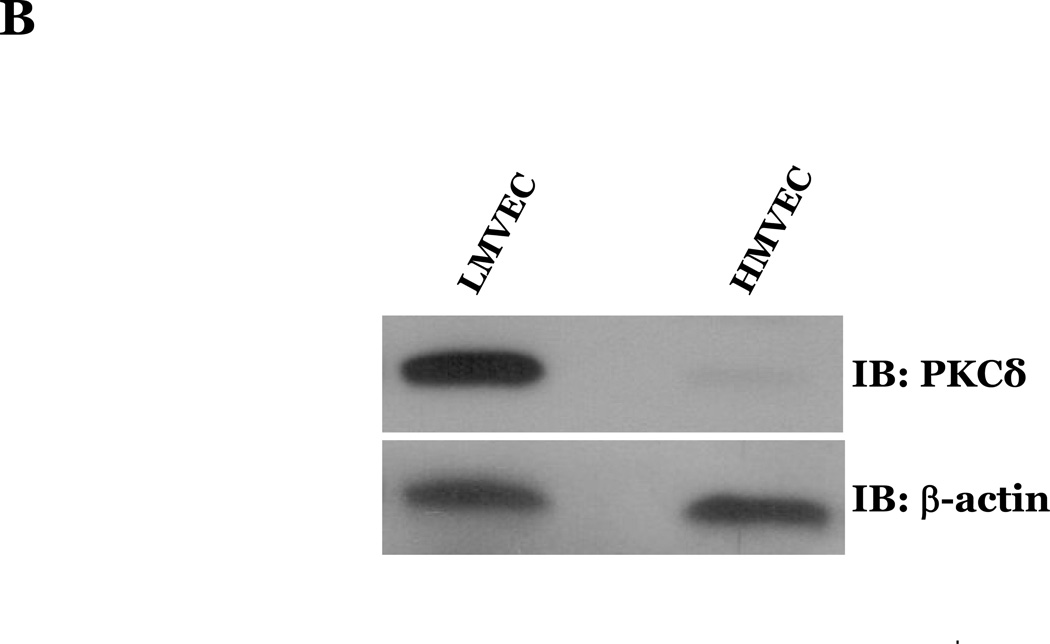
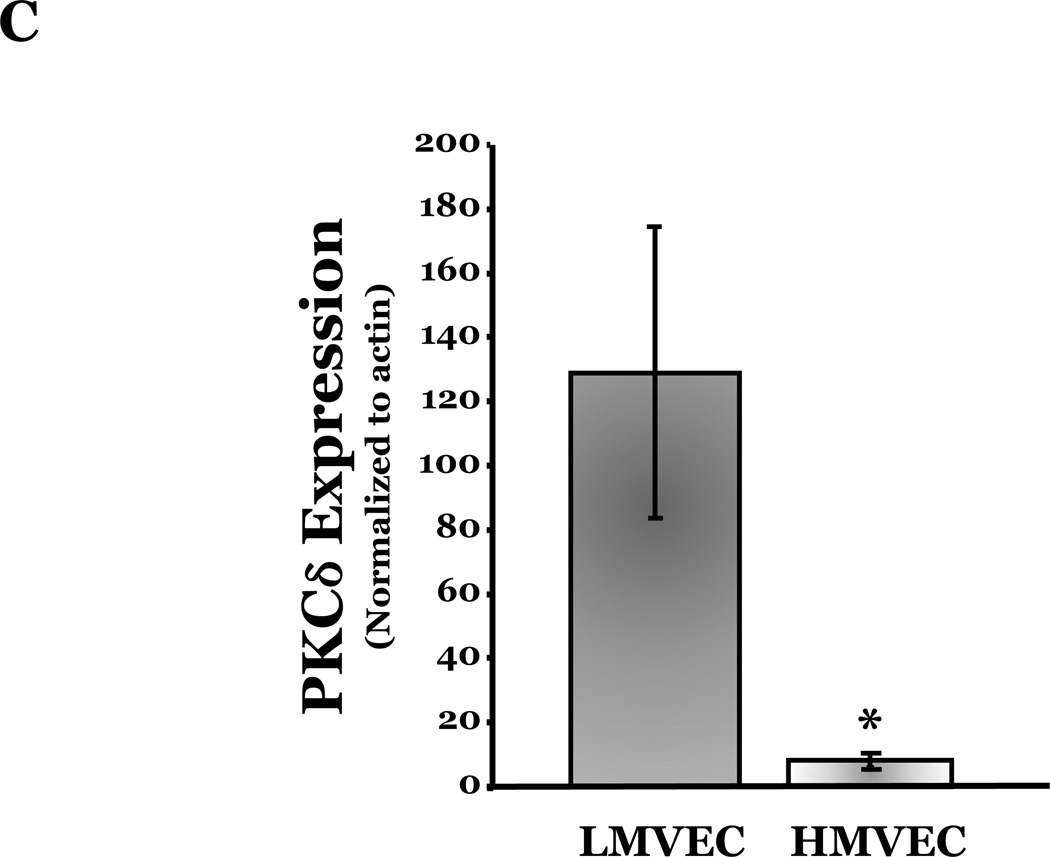
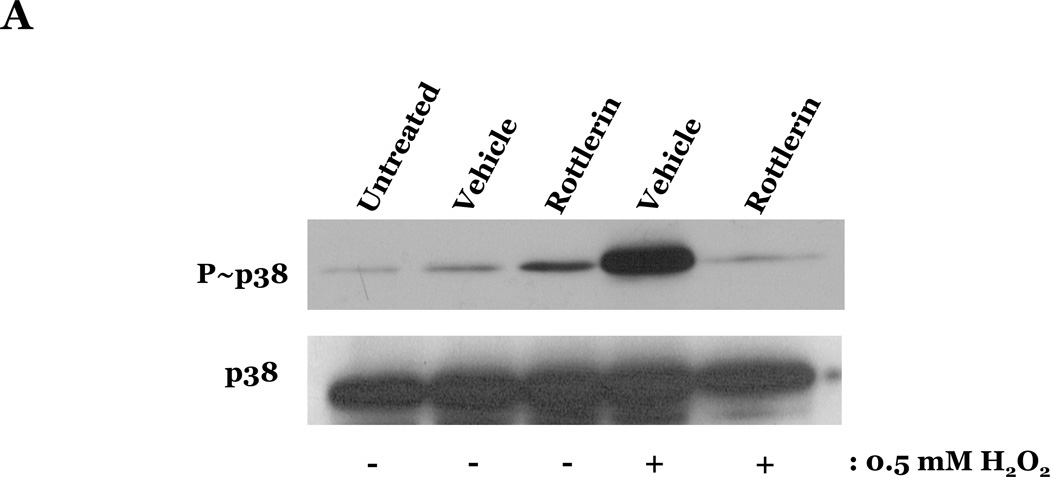
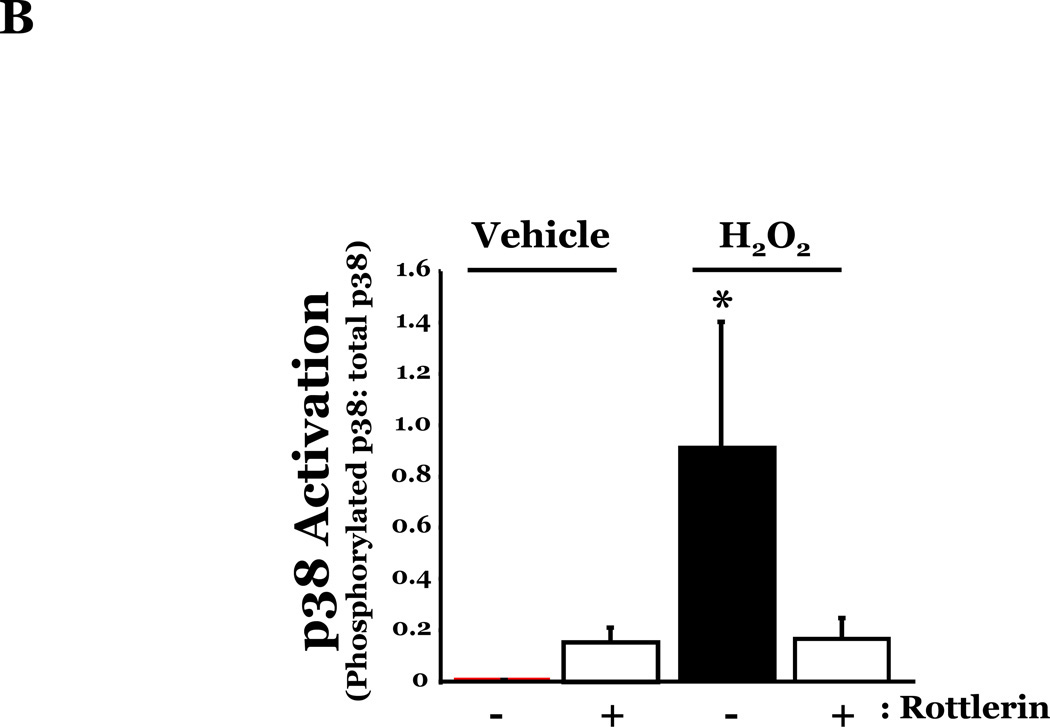
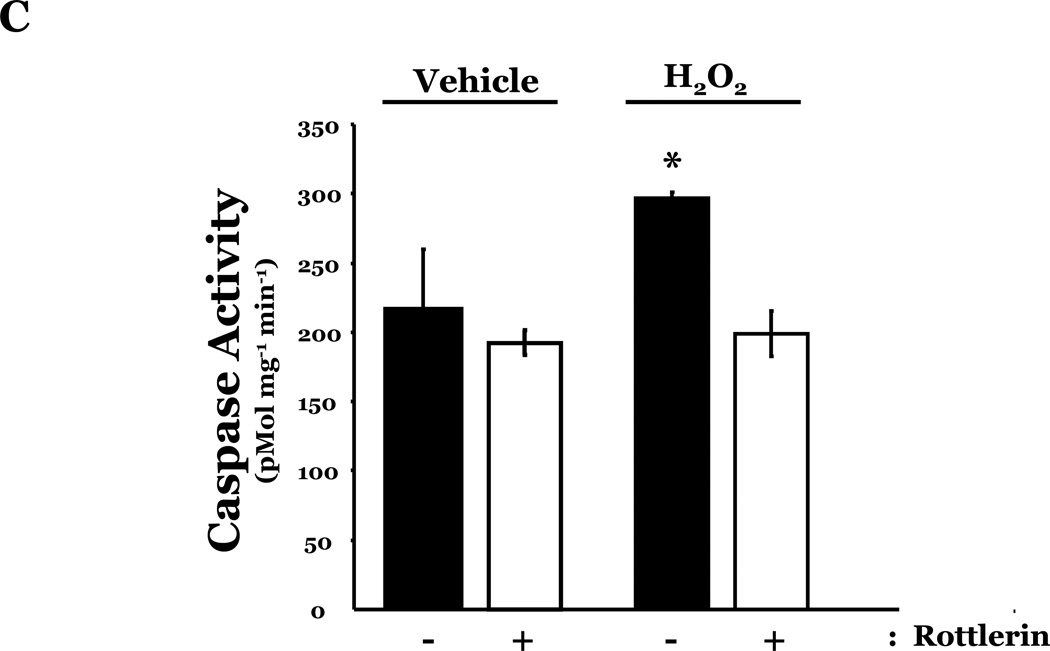
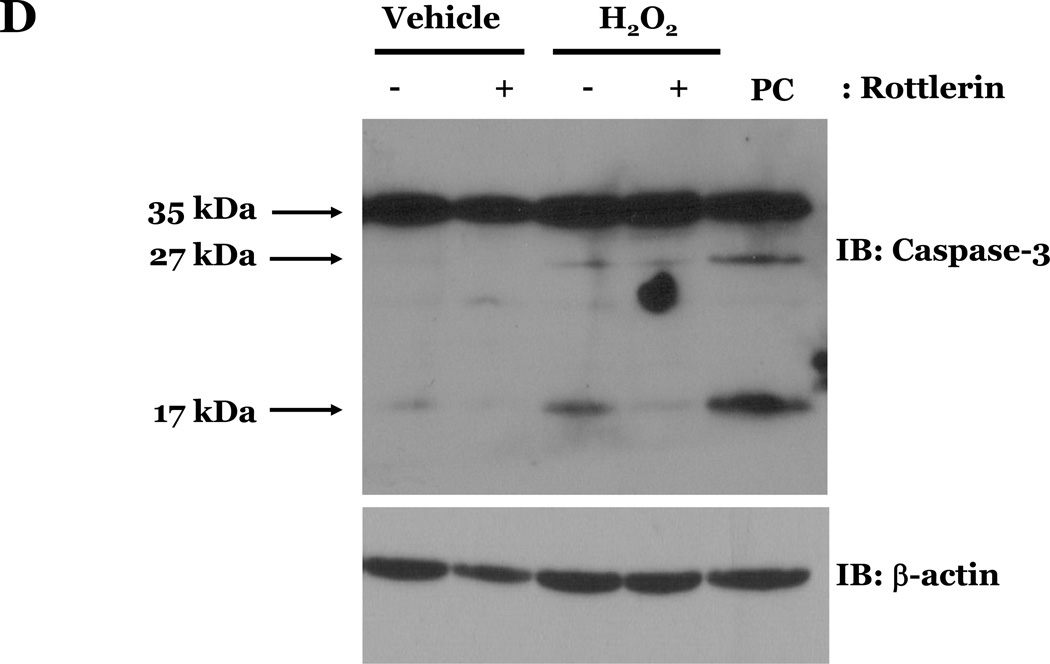
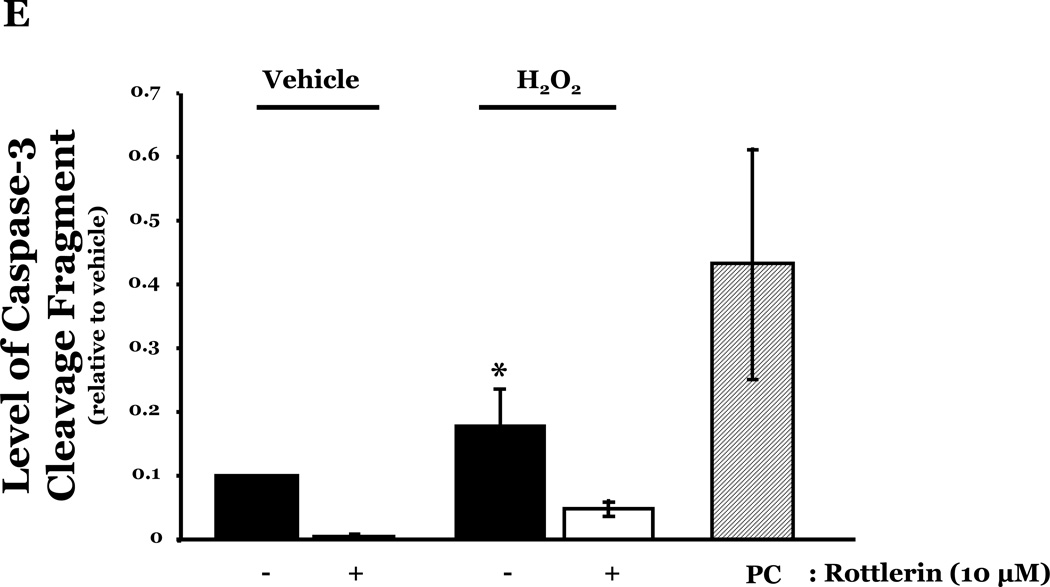
PKCδ has been reported to function as a pro-apoptotic mediator by signaling through p38 (Ryer et al., 2005), thus, we next explored how changes in PKCδ activation correlated with oxidant-induced activation of p38 in LMVEC, relative to HMVEC. We noted an increased presence of the pro-apoptotic PKCδ proteolytic cleavage fragment in LMVEC, as compared to HMVEC, under both normoxic and hyperoxic conditions (Figure 6A). Based on these findings, we then examined total PKCδ protein expression in LMVEC versus HMVEC, using equal numbers of each cell type, at the same passage. As shown in Figures 6B and 6C, LMVEC have a greater level of PKCδ protein expression, relative to HMVEC. We next examined how inhibition of PKCδ might affect p38 activation in LMVEC. We noted that pre-incubation of LMVEC with the PKCδ chemical inhibitor, rottlerin, significantly attenuated p38 activation (Figures 7A and 7B) and apoptosis (Figures 7C–7E) in response to H2O2. The data thus suggest a potential dual role for PKCδ in ROS-induced LMVEC apoptosis, both as an upstream regulator of p38 activation and as an inducer of DNA-damage through its caspase-3-dependent cleavage fragment.
Figure 6. Increased PKCδ expression and cleavage fragment production in LMVEC as compared to HMVEC.
Panel A, equivalent numbers of LMVEC or HMVEC, grown in reduced serum medium, were exposed to 0.5mM H2O2 for 30 minutes (or UV light for 3 minutes). Equivalent amounts of lysates were resolved by SDS-PAGE and immunoblotted using an antibody directed against the carboxyl terminus of PKCδ. Blots were stripped and reprobed for β-actin to confirm equal protein loading. A representative immunoblot is shown, n = 4. Panel B, lysates collected from equal numbers of LMVEC and HMVEC (1.5 × 105 cells) were resolved by SDS-PAGE and immunoblotted for PKCδ. Blots were stripped and reprobed for β-actin to confirm equal protein loading. A representative immunoblot is shown, n=3. Panel C, data is presented as the mean ± S.E. of the level of PKCδ protein relative to β-actin protein. n = 3; *p < 0.05.
Figure 7. Inhibition of PKCδ attenuates oxidant-induced p38 activation in LMVEC.
Panel A, Equal numbers of LMVEC were incubated with 10 µM rottlerin or vehicle for 30 minutes, followed by exposure to 0.5 mM H2O2 for 30 minutes. Equivalent quantities of lysates were resolved by SDS-PAGE and immunoblotted for phosphorylated p38 (T180/Y182). The membranes were stripped and reprobed for total p38. Representative immunoblots are shown, n = 3. Panel B, data is presented as the mean ± S.E. of the level of phosphorylated p38 relative to total p38. *p < 0.0001 vs. vehicle. Panels C and D, Equal numbers of LMVEC were incubated with 10 µM rottlerin (or vehicle) for 30 minutes, followed by exposure to 0.5 mM H2O2 for 6 hours. Panel C, equivalent amounts of lysates were assayed for caspase activity, based on the release of the fluorescent conjugate (AMC) from the peptide substrate, DEVD. Data is presented as the mean±S.E. n = 4; *p < 0.05 vs. or vehicle treated cells. Panel D, equivalent amounts of lysates were immunoblotted with an antibody for cleaved caspase-3. PC = UV-irradiated positive control (Panel D). Panel E, data is presented as the mean±S.E. of the level of cleaved caspase-3 (17 kDa fragment) relative to β-actin, normalized to the level noted in LMVEC pretreated with vehicle and then incubated with vehicle for 30 minutes. n = 3; *p < 0.05 vs. all other conditions.
Overexpression of wild type or catalytically activated PKCδ increases HMVEC susceptibility to ROS-induced p38 activation and apoptosis
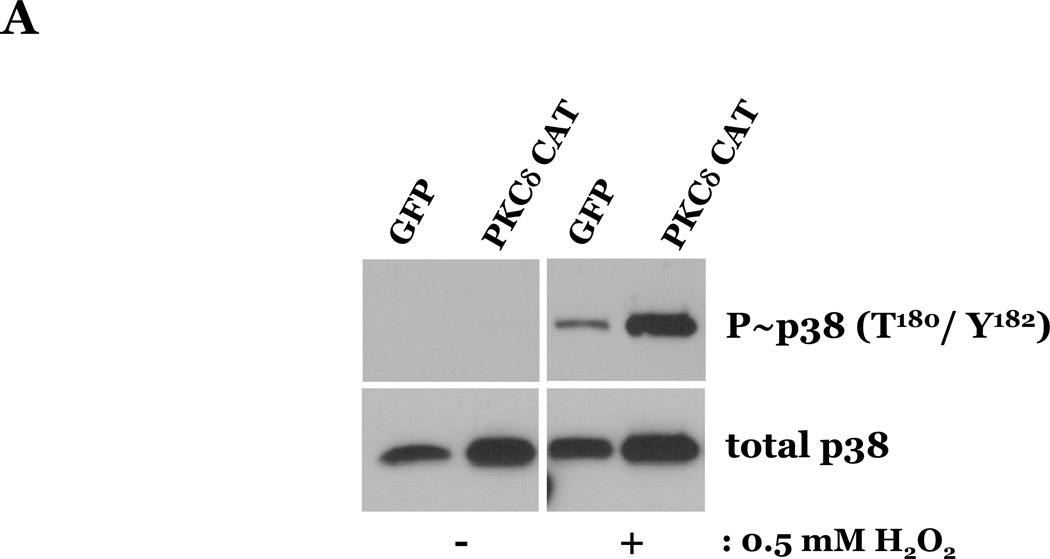
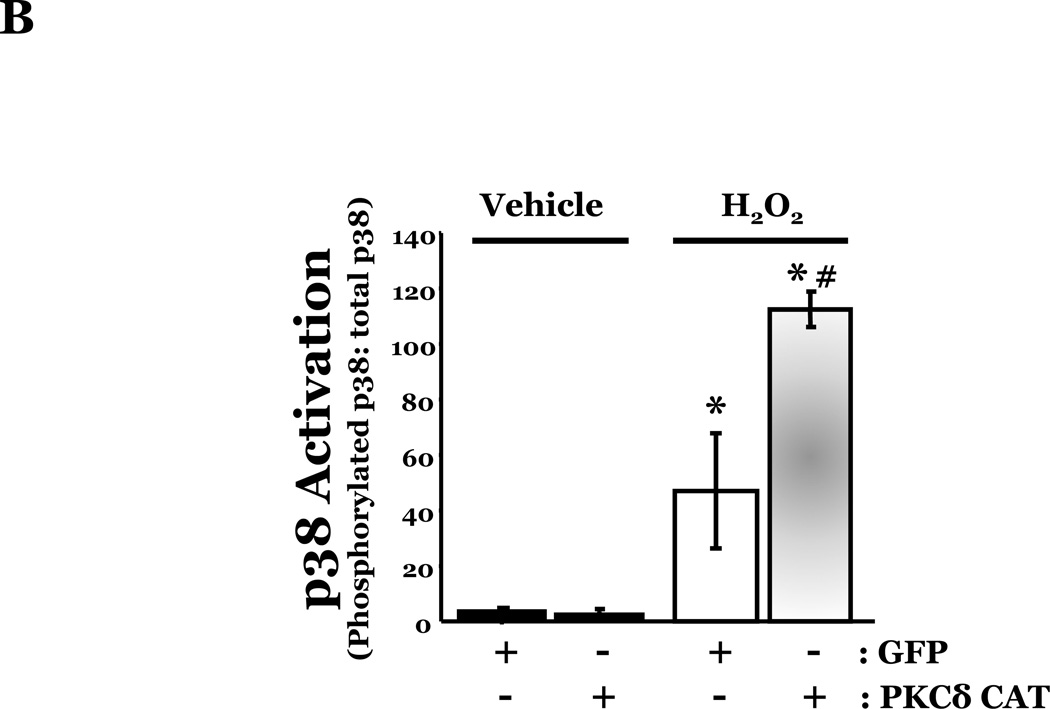
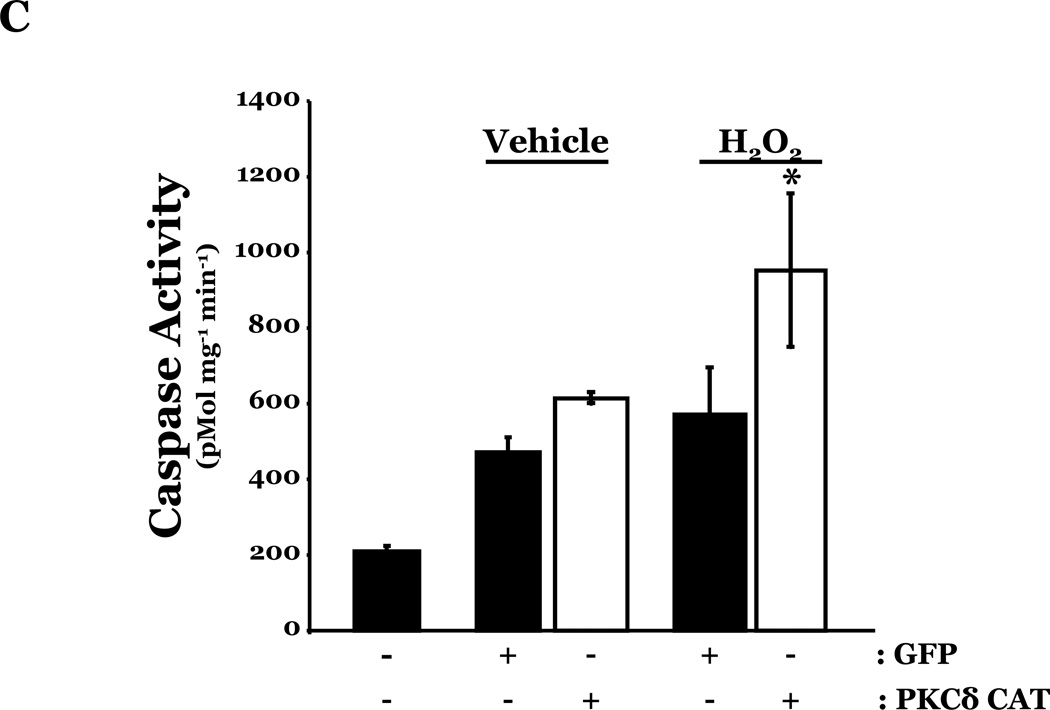
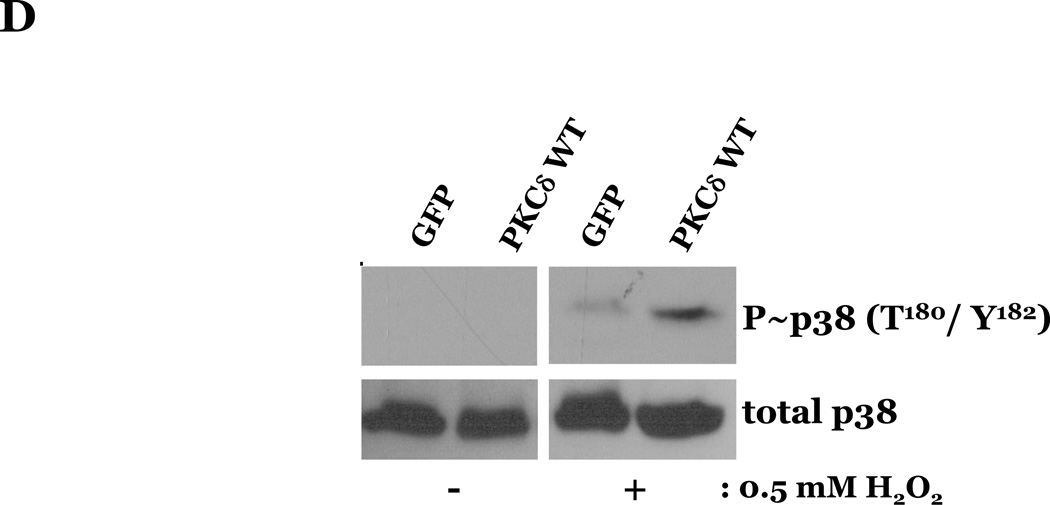
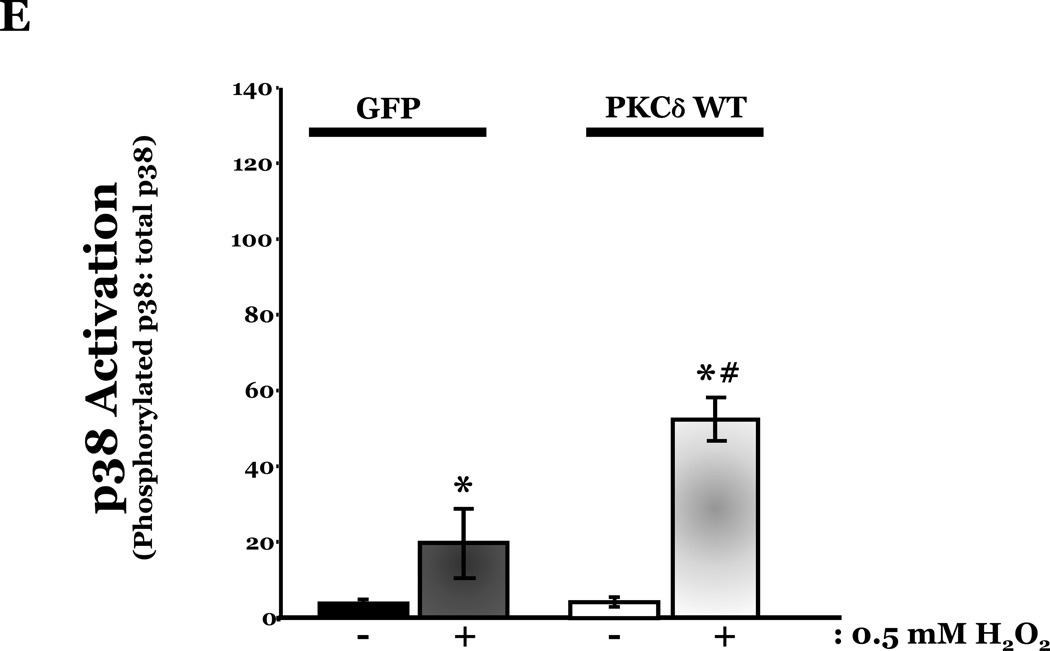
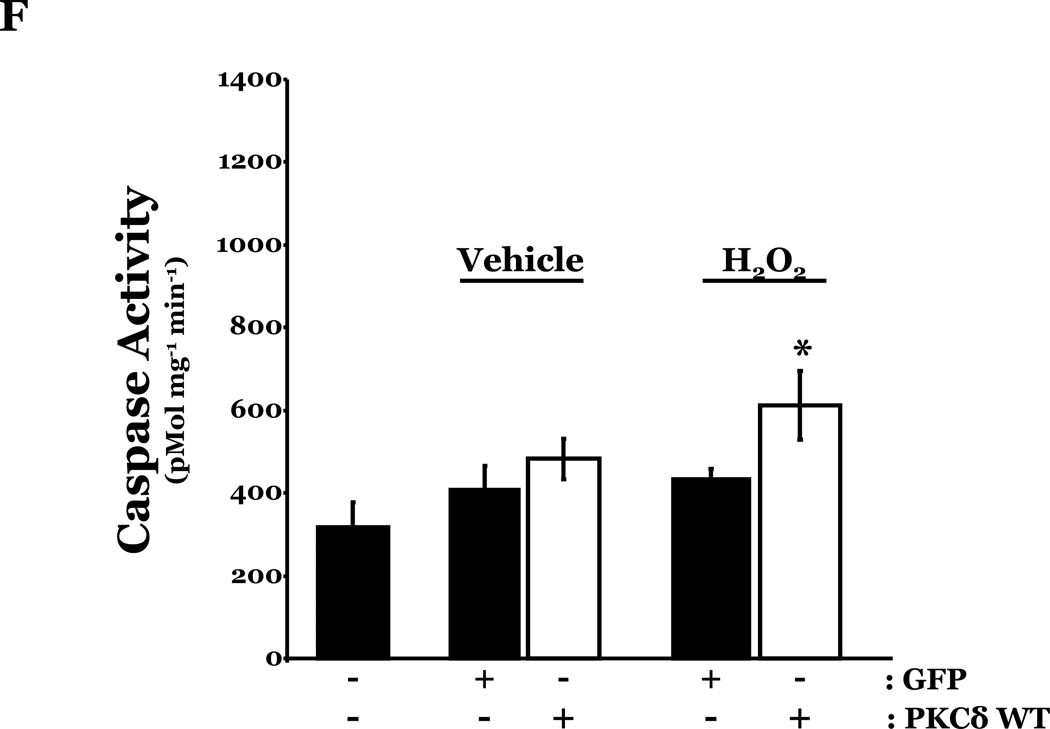
To determine if HMVEC could become susceptible to ROS-induced apoptosis, we next enhanced PKCδ protein expression in HMVEC by transfecting with cDNA encoding PKCδ catalytically active fragment (PKCδ-CAT) or wild type (PKCδ wt) and exposed the cells to H2O2. HMVEC overexpressing either PKCδ-CAT or PKCδ wt displayed significantly increased p38 activation in response to H2O2, as compared with GFP-overexpressing HMVEC (Figures 8A–B, 8D–E). While HMVEC transfected with GFP cDNA demonstrated an increased level of caspase activity upon H2O2 exposure, relative to untransfected HMVEC exposed to H2O2 (Figures 2B, 8C, 8F), HMVEC overexpressing PKCδ-CAT or PKCδ wt displayed a significant elevation in caspase activity, as compared to GFP-overexpressing HMVEC, following H2O2 treatment (Figures 8C, 8F). This data further supports the hypothesis that the differential apoptotic response of LMVEC and HMVEC to oxidant stress is due to the difference in the level of active PKCδ.
Figure 8. Increased susceptibility to ROS-induced p38 activation and apoptosis in HMVEC overexpressing PKCδ.
HMVEC were transfected with cDNA encoding the catalytic cleavage product of PKCδ (PKCδ-CAT) (Panels A–C), with cDNA encoding wild type PKCδ (PKCδ wt) (Panels D–F), or GFP cDNA, as control, for 48h and then transfected HMVEC were expose to H2O2 for 30 minutes. Panels A and D, Equivalent amounts of lysates were resolved by SDS-PAGE and immunoblotted for phosphorylated p38 (T180/Y182). The membranes were stripped and reprobed for total p38. Representative immunoblots are shown, n = 3. Panels B and E, data is presented as the mean±S.E. of the level of phosphorylated p38 relative to total p38. *p < 0.005 vs. PKCδ cDNA transfected endothelial cells exposed to vehicle; #p < 0.02 vs. GFP exposed to H2O2. Panels C and F, HMVEC transfected with GFP or PKCδ-CAT or PKCδ wt were cultured in complete medium (C) or reduced serum medium and exposed to H2O2 for 6h. Equivalent amounts of lysates were assayed for caspase activity, the release of the fluorescent conjugate (AMC) from the peptide substrate, DEVD. Data is presented as the mean±S.E. n= 3; * p < 0.0001 vs. all other conditions.
Discussion
In the present study, we demonstrated heterogeneity in the apoptotic responses of endothelial cells from the lung and heart to oxidant-induced injury. We showed that in mice exposed to hyperoxia, pulmonary endothelial cells underwent significantly more apoptosis, compared to heart endothelial cells. This was confirmed with cultured lung microvascular endothelial cells (LMVEC), which displayed increased caspase-3 activation and DNA laddering in response to both hyperoxia and H2O2, compared to cultured heart microvascular endothelial cells (HMVEC). The higher incidence of ROS-induced apoptosis correlated with signficantly increased activation of p38 MAP kinase (p38) and increased production of the caspase-induced PKCδ cleavage product in LMVEC, as compared with HMVEC. Further analysis revealed that endothelial cells derived from the lung express more PKCδ protein than endothelial cells from the heart. To assess the significance of PKCδ protein level on EC susceptibility to ROS-induced apoptosis, we altered its activation in both LMVEC and HMVEC. Both rottlerin-induced PKCδ inhibition in cultured pulmonary endothelial cells and genetic deletion of PKCδ−/− in mouse lungs significantly attenuated oxidant-induced p38 activation and programmed cell death, respectively. Conversely, overexpression of a constitutively activated PKCδ cleavage product or PKCδ wt in cultured heart endothelial cells increased both p38 activation and caspase activation in response to H2O2. Thus, our findings support the concept of endothelial cell heterogeneity based on tissue of origin, and suggest that the level of PKCδ protein and production of its cleavage product may be a critical factor dictating the response of endothelial cells to oxidant-induced injury.
Depending upon the type of vessel (artery, vein, or capillary) and the particular organ in which it resides, each endothelial cell is exposed to a unique extracellular milieu and is subjected to varying environmental stresses; as a result, endothelial cells differ in both phenotypic and genotypic characteristics. Chi and colleagues demonstrated site-specific differences in the transcriptomes of endothelial cells isolated from different portions of the vasculature (Chi et al., 2003). This study revealed striking differences in the genetic program between microvascular and macrovascular endothelial cells, as well as between arterial and venous endothelial cells. Endothelial heterogeneity has also been shown within vascular beds of the same organ. Dini and Carla demonstrated heterogeneity of the hepatic sinusoidal endothelium with regard to recognition of apoptotic cells (Dini and Carla, 1998). In this study, they noted two distinct subsets of endothelial cells correlating with either recognition of or removal of apoptotic peripheral lymphocytes. Within the pulmonary vasculature, Lu et al. demonstrated a difference in the TGFβ-1-induced apoptotic response between endothelial cells derived from the pulmonary conduit vessels and those derived from the lung microvasculature (Lu et al., 2009)
In the current study, we examined and compared the response of microvascular endothelial cells derived from the lung and heart to ROS exposure. While endothelial cells of the kidney, spleen, heart, and lung are all subjected to fluctuations in oxidant exposure, lung endothelial cells are exposed to the highest oxygen concentrations in the body. As noted by Aird, pulmonary endothelial cells also undergo the most drastic changes in oxidant exposure throughout their lifetime, receiving less than 10% of the maternal cardiac output during embryogenesis, as compared with 100% of the fully oxygenated cardiac output after birth (Aird, 2007b). It was therefore intriguing that we noted increased apoptosis of LMVEC as compared with HMVEC in response to reactive oxygen species (ROS).
Both heart and lung endothelial cell populations displayed similar ROS-induced changes in both the classical and UPR-mediated apoptotic pathways, and there did not appear to be any significant differences in the antioxidant capacity of either cell type. However, p38 and PKCδ were differentially regulated in the two cell types. The stress-activated p38 kinase has a well established role in mediating cellular apoptosis (Deschenes et al., 2001); activation of this kinase has been shown to mediate cytoskeletal changes associated with membrane blebbing, as well as both caspase-dependent and independent nuclear condensation and fragmentation. The two main upstream activators of p38 are the MAP kinase kinase proteins, MKK3 and MKK6 (Sorkin et al., 2009; Zhu et al., 2001). Activation of these proteins can be stimulated by a number of signaling molecules, including PKCδ (Uddin et al., 2002). Activation of PKCδ has been shown to be a crucial component of oxidant-induced apoptosis in a variety of cell types (Efimova et al., 2004; Kato et al., 2009). In human keratinocytes, activation of PKCδ by a variety of stressors, including H2O2, causes the sequential activation of RAS, MEKK1, and MEK6 (Dashti et al., 2001; Efimova et al., 2004; Ono and Han, 2000). Subsequent activation and nuclear localization of p38 is required for H2O2-induced keratinocyte apoptosis. Overexpression of dominant negative PKCδ prevents p38 activation and translocation, suggesting that PKCδ kinase activity is necessary for mediating ROS-induced apoptosis (Efimova et al., 2004).
There are several known mechanisms of PKCδ activation: diacylglycerol-induced activation subsequent to serine/threonine phosphorylation at motif sites; tyrosine phosphorylation; homotypic binding; and generation of a small molecular weight cleavage fragment by proteolytic cleavage (Kikkawa et al., 2002). Tyrosine phosphorylation and caspase-induced cleavage of PKCδ appear to be the primary mechanisms involved in mediating oxidant-induced apoptosis (Brodie and Blumberg, 2003). Specifically, the PKCδ cleavage product mediates DNA cleavage and nuclear fragmentation, and serves to regulate transcription of p53, Rad9, topoisomerase IIα, lamin-β, p73β, and DNA-dependent protein kinase (Brodie and Blumberg, 2003). In the current study, we noted greater total PKCδ protein expression in LMVEC than in HMVEC. We also observed an increased presence of the PKCδ catalytic cleavage fragment in normoxic LMVEC, as compared with normoxic HMVEC, and an increase in cleavage fragment production in response to ROS in LMVEC, but not HMVEC.
The current study is the first to report that PKCδ is differentially expressed in endothelial cells derived from different vascular beds, and that this difference is associated with altered susceptibility to oxidant-induced apoptosis, both in vitro and in vivo. There are several possible mechanisms by which PKCδ protein expression may be differentially regulated in lung and heart endothelial cells. One possibility is that transcriptional activation of PKCδ is enhanced in lung microvascular endothelial cells, compared to heart endothelial cells. Very little is known regarding the transcriptional regulation of PKCδ; promoter studies have revealed several putative binding sites for various transcription factors, including p63, p73, and NFκB (Greene et al., 2010; Ponassi et al., 2006). It is also possible that the turnover of PKCδ protein is somehow delayed in lung microvascular endothelial cells, as compared to cardiac microvascular endothelial cells. Finally, a second isoform of PKCδ (PKCδII) was noted in mice, which possessed similar kinase activity but was insensitive to caspase-induced cleavage (Sakurai et al., 2001). Thus, it is possible that the heterogenic response could also be due to higher PKCδII expression in HMVEC. Future studies are needed to investigate the underlying mechanism of differential expression and activity of PKCδ in lung- and heart-derived endothelial cells, especially under oxidative stress.
In addition to p38, PKCδ has also been implicated in the phosphorylation and subsequent activation of the serine/ threonine kinase, Akt (Sud et al., 2008). Akt has been shown to mediate a myriad of cell functions, including production of endothelial nitric oxide via eNOS, regulation of glycogen synthesis, cell cycle regulation, and promotion of cell survival (El-Deiry, 2001; Roberts et al., 2004; Sud et al., 2008; Thakkar et al., 2001). In this study, we noted a temporal difference in oxidant-induced Akt activation, with HMVEC displaying a delay in activation relative to LMVEC (Supplemental Figure 2). Pre-treatment of LMVEC with any concentration of the Akt inhibitor, wortmanin, however, was unable to attenuate oxidant-induced caspase activation (data not shown), suggesting that, although altered in response to oxidative stress, Akt activation was not a critical component of the differential apoptotic response noted in LMVEC and HMVEC.
Further study is necessary to elucidate the mechanism(s) responsible for mediating differential expression of PKCδ in endothelial cells of the heart and lung, and the possible homeostatic basis for this difference. Identification of both upstream and downstream targets of PKCδ should help to further define the oxidant-induced apoptotic pathway and provide potential targets for attenuation of lung injury in response to ROS. In addition, the elucidation of mechanisms which dictate the different response of microvascular endothelial cells, isolated from different organs, to the same stress may provide an approach for tissue and/ or vascular bed specific targeting for therapeutic modulation in pathophysiological settings.
Supplementary Material
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the Providence VA Medical Center and supported with VA Merit Review and R01 HL67795 grants to E.O. Harrington. K. Grinnell was supported by T32 HL094300. G. Choudhary was supported by a Career Development Award from Department of VA. S. Rounds was supported by RO1 HL64936.
Footnotes
Some of these results were presented at the 2007, 2009, and 2011 American Thoracic Society international meetings and were published in abstract form in American Journal of Respiratory and Critical Care Medicine. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circulation Research. 2007a;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circulation Research. 2007b;100(2):174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- Aoki M, et al. Endothelial apoptosis induced by oxidative stress through activation of NFκB: antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension. 2001;38(1):48–55. doi: 10.1161/01.hyp.38.1.48. [DOI] [PubMed] [Google Scholar]
- Bosch J, Abraldes JG, Fernandez M, Garcia-Pagan JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. Journal of Hepatology. 2010;53(3):558–567. doi: 10.1016/j.jhep.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Bowers R, et al. Oxidative stress in severe pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2004;169(6):764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase Cδ. Apoptosis. 2003;8(1):19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochimica et Biophysica Acta. 2007;1776(1):86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Chi JT, et al. Endothelial cell diversity revealed by global expression profiling. Proceedings of the National Academies of Science USA. 2003;100(19):10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CW, Herrera Abreu MT, Suzuki T, Downey GP. Oxidative stress and acute lung injury. American Journal of Respiratory Cell and Molecular biology. 2003;29(4):427–431. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- Dashti SR, Efimova T, Eckert RL. MEK6 regulates human involucrin gene expression via a p38alpha - and p38delta -dependent mechanism. Journal of Biological Chemistry. 2001;276(29):27214–27220. doi: 10.1074/jbc.M100465200. [DOI] [PubMed] [Google Scholar]
- Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23) Suppl 1:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- Deschenes C, Vezina A, Beaulieu JF, Rivard N. Role of p27(Kip1) in human intestinal cell differentiation. Gastroenterology. 2001;120(2):423–438. doi: 10.1053/gast.2001.21199. [DOI] [PubMed] [Google Scholar]
- Dini L, Carla EC. Hepatic sinusoidal endothelium heterogeneity with respect to the recognition of apoptotic cells. Experimental Cell Research. 1998;240(2):388–393. doi: 10.1006/excr.1998.4015. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. Protein kinase Cδ regulates keratinocyte death and survival by regulating activity and subcellular localization of a p38delta-extracellular signal-regulated kinase 1/2 complex. Molecular and Cellular Biology. 2004;24(18):8167–8183. doi: 10.1128/MCB.24.18.8167-8183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Solh AA, Akinnusi ME, Baddoura FH, Mankowski CR. Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. American Journal of Respiratory and Critical Care Medicine. 2007;175(11):1186–1191. doi: 10.1164/rccm.200611-1598OC. [DOI] [PubMed] [Google Scholar]
- El-Deiry WS. Akt takes centre stage in cell-cycle deregulation. Nature Cell Biology. 2001;3(3):E71–E73. doi: 10.1038/35060148. [DOI] [PubMed] [Google Scholar]
- Embree-Ku M, Venturini D, Boekelheide K. Fas is involved in the p53-dependent apoptotic response to ionizing radiation in mouse testis. Biology of Reproduction. 2002;66(5):1456–1461. doi: 10.1095/biolreprod66.5.1456. [DOI] [PubMed] [Google Scholar]
- Greene MW, Ruhoff MS, Burrington CM, Garofalo RS, Orena SJ. TNFα activation of PKCδ, mediated by NFκB and ER stress, cross-talks with the insulin signaling cascade. Cell Signaling. 2010;22(2):274–284. doi: 10.1016/j.cellsig.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Harrington E, Smeglin A, Newton J, Ballard G, Rounds S. Protein tyrosine phosphatase-dependent proteolysis of focal adhesion complexes in endothelial cell apoptosis. American Journal of Physiology. 2001;280:L342–L353. doi: 10.1152/ajplung.2001.280.2.L342. [DOI] [PubMed] [Google Scholar]
- Harrington EO, et al. Role of protein kinase C isoforms in rat epididymal microvascular endothelial barrier function. American Journal of Respiratory Cell and Molecular biology. 2003;28(5):626–636. doi: 10.1165/rcmb.2002-0085OC. [DOI] [PubMed] [Google Scholar]
- Harrington EO, et al. PKCδ regulates endothelial basal barrier function through modulation of RhoA GTPase activity. Experimental Cell Research. 2005;308(2):407–421. doi: 10.1016/j.yexcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Harrington EO, Smeglin A, Parks N, Newton J, Rounds S. Adenosine induces endothelial apoptosis by activating protein tyrosine phosphatase: a possible role of p38α. American Journal of Physiology. 2000;279(4):L733–L742. doi: 10.1152/ajplung.2000.279.4.L733. [DOI] [PubMed] [Google Scholar]
- Irani K. Oxidant signaling in vascular cell growth, death, and survival: A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circulation Research. 2000;87(3):179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Yim AP, Wan S, Angelini GD. Oxidative stress, nitric oxide, and vascular disease. Journal of Cardiac Surgery. 2002;17(4):324–327. doi: 10.1111/j.1540-8191.2001.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Kato K, et al. Caspase-mediated protein kinase Cδ cleavage is necessary for apoptosis of vascular smooth muscle cells. American Journal of Physiology. 2009;297(6):H2253–H2261. doi: 10.1152/ajpheart.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U, Matsuzaki H, Yamamoto T. Protein kinase Cδ (PKCδ): activation mechanisms and functions. Journal of Biochemistry. 2002;132(6):831–839. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- King J, et al. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvascular Research. 2004;67(2):139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacology & Therapeutics. 2006;111(2):476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Klinger JR, et al. Rottlerin causes pulmonary edema in vivo: a possible role for PKCδ. Journal of Applied Physiology. 2007;103(6):2084–2094. doi: 10.1152/japplphysiol.00695.2007. [DOI] [PubMed] [Google Scholar]
- Lu Q, Patel B, Harrington EO, Rounds S. Transforming growth factor-β1 causes pulmonary microvascular endothelial cell apoptosis via ALK5. American Journal of Physiology. 2009;296(5):L825–L838. doi: 10.1152/ajplung.90307.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. American Journal of Physiology. 2001;280(4):C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cδ. Nature. 2002;416(6883):865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- Ng CS, Wan S, Yim AP, Arifi AA. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121(4):1269–1277. doi: 10.1378/chest.121.4.1269. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signaling. 2000;12(1):1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Orfanos SE, Mavrommati I, Korovesi I, Roussos C. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Medicine. 2004;30(9):1702–1714. doi: 10.1007/s00134-004-2370-x. [DOI] [PubMed] [Google Scholar]
- Ponassi R, et al. p63 and p73, members of the p53 gene family, transactivate PKCδ. Biochemical Pharmacology. 2006;72(11):1417–1422. doi: 10.1016/j.bcp.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Woods AJ, Dale TC, Van Der Sluijs P, Norman JC. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of αvβ3 and α5β1 integrins. Molecular and Cellular Biology. 2004;24(4):1505–1515. doi: 10.1128/MCB.24.4.1505-1515.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryer EJ, et al. Protein kinase Cδ induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. Journal of Biological Chemistry. 2005;280(42):35310–35217. doi: 10.1074/jbc.M507187200. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Onishi Y, Tanimoto Y, Kizaki H. Novel protein kinase Cδ isoform insensitive to caspase-3. Biological & Pharmaceutical Bulletin. 2001;24(9):973–977. doi: 10.1248/bpb.24.973. [DOI] [PubMed] [Google Scholar]
- Shukla A, et al. Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase Cδ knockout mice. American Journal of Pathology. 2007;170(1):140–151. doi: 10.2353/ajpath.2007.060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U, Jialal I. Oxidative stress and atherosclerosis. Pathophysiology. 2006;13(3):129–142. doi: 10.1016/j.pathophys.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, et al. MKK3, an upstream activator of p38, contributes to formalin phase 2 and late allodynia in mice. Neuroscience. 2009;162(2):462–471. doi: 10.1016/j.neuroscience.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud N, Wedgwood S, Black SM. Protein kinase Cδ regulates endothelial nitric oxide synthase expression via Akt activation and nitric oxide generation. American Journal of Physiology. 2008;294(3):L582–L591. doi: 10.1152/ajplung.00353.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thakkar H, et al. Pro-survival function of Akt/protein kinase B in prostate cancer cells. Relationship with TRAIL resistance. Journal of Biological Chemistry. 2001;276(42):38361–38369. doi: 10.1074/jbc.M103321200. [DOI] [PubMed] [Google Scholar]
- Uddin S, et al. Protein kinase C-δ (PKC-δ) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. Journal of Biological Chemistry. 2002;277(17):14408–14416. doi: 10.1074/jbc.M109671200. [DOI] [PubMed] [Google Scholar]
- Vallet B, Wiel E. Endothelial cell dysfunction and coagulation. Critical Care Medicine. 2001;29(7 Suppl):S36–S41. doi: 10.1097/00003246-200107001-00015. [DOI] [PubMed] [Google Scholar]
- van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QJ, et al. Differential Localization of Protein Kinase Cδ by Phorbol Esters and Related Compounds Using a Fusion Protein with Green Fluorescent Protein. Journal of Biological Chemistry. 1999;274(52):37233–37239. doi: 10.1074/jbc.274.52.37233. [DOI] [PubMed] [Google Scholar]
- Yano K, et al. Phenotypic heterogeneity is an evolutionarily conserved feature of the endothelium. Blood. 2007;109(2):613–615. doi: 10.1182/blood-2006-05-026401. [DOI] [PubMed] [Google Scholar]
- Zhu X, et al. Activation of MKK6, an upstream activator of p38, in Alzheimer's disease. Journal of Neurochemistry. 2001;79(2):311–318. doi: 10.1046/j.1471-4159.2001.00597.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.