Abstract
The α7 nicotinic acetylcholine receptor (nAChR) subtype is abundantly expressed in the central nervous system and in the periphery. Recent evidence suggests that α7 nAChR subtypes, which can be activated by an endogenous cholinergic tone, comprising acetylcholine and the α7 nAChR agonist choline, play an important role in subchronic pain and inflammation. This study’s objective was to test whether α7 nAChR positive allosteric modulators (PAMs) produce antinociception in in vivo mouse models of acute and persistent pain. Testing type I [N-(5-chloro-2-hydroxyphenyl)-N′-[2-chloro-5-(trifluoromethyl)phenyl] (NS1738)] and type II [1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl) (PNU-120596)] α7 nAChR PAMs in acute and persistent pain, we found that, although neither reduced acute thermal pain, only PNU-120596 dose-dependently attenuated paw-licking behavior in the formalin test. The long-acting effect of PNU-120596 in this test was in discordance with its pharmacokinetic profile in mice, which suggests the involvement of postreceptor signaling mechanisms. Our results with selective mitogen-activated protein kinase kinase inhibitor 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene monoethanolate (U0126) argues for an important role of extracellular signal-regulated kinase-1/2 pathways activation in PNU-120596’s antinociceptive effects. The α7 antagonist MLA, administered intrathecally, reversed PNU-120596’s effects, confirming PNU-120596’s action, in part, through central α7 nAChRs. Importantly, tolerance to PNU-120596 was not developed after subchronic treatment of the drug. Surprisingly, PNU-120596’s antinociceptive effects were blocked by NS1738. Our results indicate that type II α7 nAChR PAM PNU-120596, but not type I α7 nAChR PAM NS1738, shows significant antinociception effects in persistent pain models in mice.
Introduction
Nicotinic acetylcholine receptors (nAChRs) are pentameric structures composed of five subunits that form a central ion-conducting pore, allowing permeability of cations, such as sodium, potassium, and calcium (Millar and Gotti, 2009). These receptors are composed of either homomeric α or heteromeric α/β subunit combinations. Twelve neuronal nicotinic subunits have been identified (α2–α10; β2–β4) (Paterson and Nordberg, 2000; Gotti et al., 2006). The homomeric α7 subunit is one subtype that is expressed abundantly in the central nervous system and in the periphery (Girod et al., 1999). nAChR α7 subtypes are characterized by their high calcium permeability and their rapid desensitization during agonist stimulation (Feuerbach et al., 2009) compared with other nAChR subtypes. In recent years, the α7 nAChR agonists were proposed as possible targets for cognitive enhancement, antinociception, and anti-inflammation properties (Damaj et al., 2000; Wang et al., 2005; de Jonge and Ulloa, 2007; Rowley et al., 2010; Thomsen et al., 2010). α7 nAChRs are present in supraspinal and spinal pain-transmission pathways (Gillberg and Aquilonius, 1985; Wada et al., 1989; Seguela et al., 1993; Khan et al., 1994; Cordero-Erausquin and Changeux, 2001; Cordero-Erausquin et al., 2004). These receptors are also present on immune and nonimmune cytokine-producing cells, including macrophages and keratinocytes (Wang and Wang, 2003; Pavlov and Tracey, 2004). Furthermore, α7 nAChR agonists, such as choline, CDP-choline, compound B, JN403 [(S)-(1-azabicyclo[2.2.2]oct-3-yl)-carbamic acid (S)-1-(2-fluoro-phenyl)-ethyl ester], and (2)-spiro[1-azabicyclo[2.2.2]octane-3,59-oxazolidin]-29-one (AR-R17779, exhibited anti-inflammatory effects in various inflammation and pain models in rodents (Damaj et al., 2000; Medhurst et al., 2008; Feuerbach et al., 2009; Gurun et al., 2009; van Maanen et al., 2009; Rowley et al., 2010; Marrero et al., 2011; Munro et al., 2012). Although α7 nAChR agonists showed beneficial effects in inflammatory animal models in some studies, these effects were not seen consistently in other studies (Gao et al., 2010). Furthermore, subchronic treatment with such compounds may provide suboptimal therapeutic efficacy because of sustained activation and/or desensitization of the α7 nAChRs.
Activation of α7 nAChR function can occur via direct agonist activation of the orthosteric site. Enhancement of α7 nAChR function can also occur via positive allosteric modulation, which can strengthen the endogenous cholinergic neurotransmission without directly stimulating the α7 nAChR. Recently, several structurally diverse and selective positive allosteric modulators (PAMs), including 1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl) (PNU-120596) (Hurst et al., 2005), 4-naphthalen-1-yl-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonic acid amide (Grønlien et al., 2007), and N-(5-chloro-2-hydroxyphenyl)-N′-[2-chloro-5-(trifluoromethyl)phenyl] (NS1738) (Timmermann et al., 2007), were reported. These PAMs were shown to increase the potency and/or maximal efficacy of endogenous (acetylcholine [ACh] and choline) or exogenous agonists for the α7 nAChRs. α7 nAChR PAMs have been classified as either type I, such as NS1738, or type II, such as PNU-120596, based on differences in their effect on desensitization (Bertrand and Gopalakrishnan, 2007; Timmermann et al., 2007). The primary difference between these two types lies in their ability to evoke a response at the receptor level. The PAMs classified as type I predominantly affect the apparent peak current, with little effect on desensitization kinetics, whereas type II increase the apparent peak current and evoke a distinct weakly decaying current (Hurst et al., 2005).
Both I and II type PAMs were shown to exhibit cognitive enhancement in vivo in rodents. For example, PNU-120596 reversed amphetamine-induced gating deficits in rats, and NS1738 improved performance in the rat social recognition (Hurst et al., 2005; Timmermann et al., 2007). Although these observations show that α7 nAChR PAMs, belonging to both types, are effective in certain rodent cognitive models, their effects in animal models of pain and inflammation are not well characterized. Recently, Munro et al. (2012) reported that PNU-120596 reversed mechanical hyperalgesia in the carrageenan and complete Freund’s adjuvant tests in the rat.
Therefore, the present study was designed to investigate the effects of type I and II PAMs in acute and tonic pain models in the mouse. PNU-120596 and NS1738 effects were tested after different routes of administration in acute thermal (tail-flick and hot plate tests) and tonic (formalin test) pain models in mice. Site of actions and receptor mechanisms were also determined. Since α7 nAChR PAMs were reported to enhance extracellular signal–regulated kinase (ERK) signaling in PC12 cells (El Kouhen et al., 2009; Hu et al., 2009), we hypothesized that activation of ERK by these allosteric modulators might play an important role in their antinociceptive effects. Therefore, we explored this possibility by examining the effects of 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene monoethanolate (U0126), a specific mitogen-activated protein kinase kinase (MEK)inhibitor (Duncia et al., 1998), on the antinociceptive actions of α7 nAChR PAMs.
Here, we report that systemic administration of PNU-120596 possesses significant activity in tonic, but not acute, pain models, thus providing the first demonstration of in vivo antinociceptive efficacy for α7 nAChR PAMs in the mouse. Importantly, this work also demonstrates a fundamental in vivo difference between type I and II α7 nAChR PAMs in pain models.
Materials and Methods
Animals
Male ICR mice obtained from Harlan Laboratories (Indianapolis, IN) and male C57BL/6 mice from The Jackson Laboratory (Bar Harbor, ME) were used throughout the study. Mice null for the α7 subunits (The Jackson Laboratory) and their wild-type (WT) littermates were bred in an animal care facility at Virginia Commonwealth University. For all experiments, mice were backcrossed for ≥8–10 generations. Mutant and WT mice were obtained by crossing heterozygote mice. This breeding scheme controlled for any irregularities that might occur with crossing solely mutant animals. Mice were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care–approved animal care facility. They were housed in groups of six and had free access to food and water. The rooms were on a 12-hour light/dark cycle (lights on at 7:00 AM). Mice were 8–10 weeks of age and weighed ∼20–25 g at the start of all experiments. All experiments were performed during the light cycle (between 7:00 AM and 7:00 PM), and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyridyl) pyrrolidine (−)-bitartrate salt] was purchased from Sigma-Aldrich (St. Louis, MO). Methyllycaconitine citrate (MLA) and dihydro-β-erythroidine (DHβE) were purchased from RBI (Natick, MA). Naloxone hydrochloride dehydrate, PNU-120596, and PHA-543613 [N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide] was obtained from the Drug Supply Program of the National Institute on Drug Abuse (Rockville, MD). NS1738 was purchased from Tocris Biosciences (Minneapolis, MN). U0126 was purchased from Cell Signaling Technology (Danvers, MA). All drugs, with the exception of PNU-120596, NS1738, and U0126, were dissolved in physiologic saline (0.9% sodium chloride) and injected s.c. at a total volume of 1 ml/100 g body weight, unless noted otherwise. PNU-120596, NS1738, and U0126 were dissolved in a mixture of 1:1:18 [1 volume ethanol/1 volume Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ)/18 volumes distilled water] and administered intraperitoneally. All doses are expressed as the free base of the drug.
Subchronic PNU-120596 Administration Protocol.
Mice were administered PNU-120596 (4 mg/kg i.p.) once a day for 6 days and were challenged with PNU-120596 (4 mg/kg i.p.) on day 7 and tested in analgesic assay. Another group was exposed to vehicle for 6 days and then challenged with PNU-120596 on the seventh day. A vehicle-control group, in which mice were exposed to 7 days of vehicle, was also included.
Intratracheal Injections.
Intratracheal injections were performed free-hand between the L5 and L6 lumbar space in unanesthetized male mice, according to the method of Hylden and Wilcox (1980). The injection was performed using a 30-gauge needle attached to a glass microsyringe. The injection volume in all cases was 5 μl. The accurate placement of the needle was evidenced by a quick “flick” of the mouse’s tail. Thus, the accurate placement of all injections could be assured by watching the tail motion of the mouse.
Antinociceptive Tests
Tail-Flick Test.
The antinociceptive effect of drugs was assessed by the tail-flick method of D’Amour and Smith (1941), as modified by Dewey et al. (1970). A control response (2–4 seconds latency) was determined for each mouse before treatment, and test latency was determined after drug administration. To minimize tissue damage, a maximum latency of 10 seconds was imposed. Antinociceptive response was calculated as the percentage maximum possible effect (%MPE), where %MPE = [(test value − control value)/(cut-off (10 s) − control value)] × 100. Groups of six to eight animals were used for each dose and for each treatment. For the tail-flick test, mice were pretreated for 5 minutes with either vehicle or nicotine (2.5 mg/kg s.c.) or for 15 minutes with PHA-543613 (8 mg/kg s.c.), PNU-120596 (4 and 8 mg/kg i.p.), or NS1738 (10 and 30 mg/kg i.p.).
Hot-Plate Test.
Mice were placed into a 10-cm-wide glass cylinder on a hot plate (Thermojust Apparatus, Columbus, OH) to measure supraspinal antinociception. The hot plate is a rectangular heated surface surrounded by Plexiglas and maintained at 55°C. The device is connected to a manually operated timer that records the amount of time that the mouse spends on the heated surface before showing signs of nociception (e.g., jumping, paw licks). Two control latencies ≥10 minutes apart were determined for each mouse. The normal latency (reaction time) of 8–12 seconds was assessed with a saline injection. To avoid tissue damage, the hot plate automatically disengages after 40 seconds. Antinociceptive response was calculated as %MPE, where %MPE = [(test value − control)/(cut-off time (40 s) − control) × 100]. The reaction time was recorded when the animal jumped or licked its paws. Mice were tested at different times after i.p. injection of NS1738 and PNU-120596. For the hot plate test, mice were pretreated for 5 minutes with either vehicle or nicotine (2.5 mg/kg s.c.) or for 15 minutes with PHA-543613 (8 mg/kg s.c.), PNU-120596 (4 and 8 mg/kg i.p.), or NS1738 (10 and 30 mg/kg i.p.).
Mechanical Sensitivity Test.
Mechanical sensitivity was determined according to the method of Chaplan et al. (1994). Mice were placed in a Plexiglas cage with mesh metal flooring and allowed to acclimate for 30 minutes before testing. A series of calibrated von Frey hairs (Stoelting, Wood Dale, IL) with logarithmically incremental stiffness, ranging from 2.83 to 5.88, expressed dslog10 of [10 × force in (mg)] were applied to the paw with a modified up-down method (Dixon, 1965). In the absence of a paw-withdrawal response to the initially selected hair, a thicker hair corresponding to a stronger stimulus was presented. In the event of paw withdrawal, the next weaker stimulus was chosen. Each hair was presented perpendicularly against the paw, with sufficient force to cause slight bending, and held for 2 to 3 seconds. Stimulation with the same intensity was applied five times to the hind paw at intervals of a few seconds. The mechanical threshold was expressed as force in (g), indicating the force of the Von Frey hair to which the animal reacted (paw withdrawn, licking, or shaking). The mechanical allodynia thresholds were measured before (predrug) and 30 minutes after i.p treatment with PNU-120596 or NS1738.
Formalin Test.
The formalin test was carried out in an open Plexiglas cage, with a mirror placed under the floor to allow an unobstructed view of the paws. Mice were allowed to acclimate for 15 minutes in the test cage before formalin injection. Each animal was injected with 20 μl of 2.5% formalin in the intraplantar region of the right hind paw. Mice were then observed (two at a time) 0–5 minutes (phase 1) and 20–45 minutes (phase 2) postformalin, and the amount of time spent licking the injected paw was recorded. The period between the two phases of nociceptive response is generally considered to be a phase of weak activity. The amount of time spent licking the injected paw was recorded with a digital stopwatch.
PNU-120596, NS1738, or vehicle was injected i.p. at different times before the formalin injection. For the antagonist studies, MLA, DHβE, or naloxone was injected s.c. 5 minutes before the formalin injection. Site studies were carried out by pretreating the mice with PNU-120596 (4 mg/kg i.p.) 15 minutes before i.t. injection of MLA (10 µg/mouse).
Locomotor Activity
Mice were placed into individual Omnitech photocell activity cages (28 × 16.5 cm) 15 minutes after the i.p. administration of either vehicle or PNU-120596 at different doses. Interruptions of the photocell beams (two banks of eight cells each), which assess walking and rearing, were recorded for the next 30 minutes. Data are expressed as the number of photocell interruptions.
Motor Coordination
We used a Rotarod (IITC Life Science Inc., Woodland Hills, CA) to measure motor coordination. The animals are placed on textured drums (1.25 inch diameter) to avoid slipping. When an animal drops onto the individual sensing platforms, test results are recorded. Mice tested at a rate of 4 rpm. Naive mice were trained until they could remain on the Rotarod for 3 minutes. Animals that failed to meet this criterion within three trials were discarded. Fifteen minutes after the injection of vehicle or drugs, mice were placed on the Rotarod for 3 minutes. If a mouse fell from the Rotarod during this time period, it was scored as motor impaired. Percentage impairment was calculated as follows: % impairment = [(180 − test time/180) × 100]. Mice were treated i.p. with either vehicle or PNU-120596 at different doses 15 minutes before the test.
Time Course of PNU-120596 and NS1738 Levels in Brain and Plasma
For the determination of plasma and brain concentrations of the parent compound, naive mice were dosed with the compounds, as indicated, and sacrificed at various time points postdosing. For analytical determination of plasma concentrations, blood was collected into heparinized tubes and then centrifuged, and the separated plasma was frozen at −20°C until analysis. For the determination of brain concentrations, animals were decapitated at the various time points, and the brains were removed immediately and rapidly freed from blood vessels as much as possible. The resulting brain tissues were immediately frozen at −20°C, weighed, and homogenized, and the homogenate was stored at −20°C. For analysis, compounds were extracted from the samples via liquid-liquid extraction and were quantified by liquid chromatography/mass spectroscopy. As a result, concentrations of either NS1738 or PNU-120596 can be measured down to 1 ng/ml in plasma (∼3 nM) and 10 ng/ml in brain (∼30 nM) (Timmermann et al., 2007).
Statistical Analysis
The data obtained were analyzed using the GraphPad software program (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. Statistical analysis was done using the one-way or two-way analysis of variance test, followed by the post hoc, Tukey’s, or Bonferroni’s test. The P values < 0.05 were considered significant.
Results
Lack of Antinociceptive Effect of α7 PAMs in Acute Thermal and Mechanical Pain in Mice.
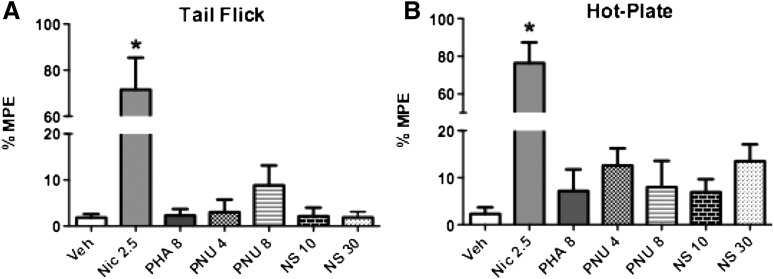
The tail-flick and hot plate tests were used to determine the antinociceptive effects of the α7 nAChR agonist and α7 nAChR PAMs after i.p. administration in acute thermal pain models. A 5-minute pretreatment with nicotine (2.5 mg/kg) induced significant antinociceptive effects in the tail-flick [F(6,35) = 20.91, P < 0.0001] and hot-plate [F(6,35) = 22.68, P < 0.0001] tests compared with vehicle (Fig. 1). In contrast, neither of the α7 PAMs showed significant antinociceptive effects at any dose tested in the tail-flick [F(5,30) = 1.330, P = 0.2783] or hot-plate [F(5,30) = 1.154, P = 0.3546] tests. Figure 1 shows the lack of antinociceptive activity 15 minutes after the administration of PNU-120596 (4 and 8 mg/kg i.p.), NS1738 (10 and 30 mg/kg i.p.), and PHA-543613 (8 mg/kg s.c.) in both tests. A similar lack of effect was observed at later pretreatment times (1 and 3 hours) after injection (data not shown).
Fig. 1.
Antinociceptive effect of various α7 nicotinic compounds in the tail-flick and hot-plate tests after acute administration in mice. (A) Effects of nicotine (2.5 mg/kg s.c.), PHA543613 (8 mg/kg s.c.), PNU-120596 (4 and 8 mg/kg i.p.), and NS1738 (10 and 30 mg/kg i.p.) in the tail-flick test. Mice were treated s.c. with nicotine 5 minutes before testing. The other treatment groups received PHA-543613, PNU-120596, or NS1738 15 minutes before the tail-flick test. (B) Effects of nicotine, PHA-543613, PNU-120596, and NS1738 in the hot plate test. Mice were treated using the same doses and pretreatment times as for the tail-flick test. Each group represents the mean ± S.E. of six to eight mice. *P < 0.05 versus vehicle. Nic, nicotine; NS, NS1738; PNU, PNU-120596; Veh, vehicle.
The effects of PNU-120596 (8 mg/kg i.p.) and NS1738 (30 mg/kg i.p.) on mechanical sensitivity were measured using calibrated von Frey filaments 15 minutes after injection. As shown in Table 1, neither PNU-120596 nor NS1738 produced significant [F(2,3) = 2.172, P = 0.2610] differences in mechanical sensitivity in response to hind paw stimulation using von Frey filaments.
TABLE 1.
Effects of α7 nAChR PAMs of NS1738 (30 mg/kg i.p.) and PNU-120596 (4 mg/kg i.p.) on mechanical sensitivity in naive mice
The two α7 nAChR PAMs were given i.p. to animals; their withdrawal thresholds (g) were measured 30 minutes later. Data are presented as the mean ± S.E. of six mice.
| Contralateral | Ipsilateral | |
|---|---|---|
| Vehicle | 5.33 ± 0.84 | 4.67 ± 0.67 |
| NS1738 | 5.50 ± 0.96 | 5.00 ± 0.57 |
| PNU-120596 | 4.33 ± 0.61 | 4.67 ± 0.42 |
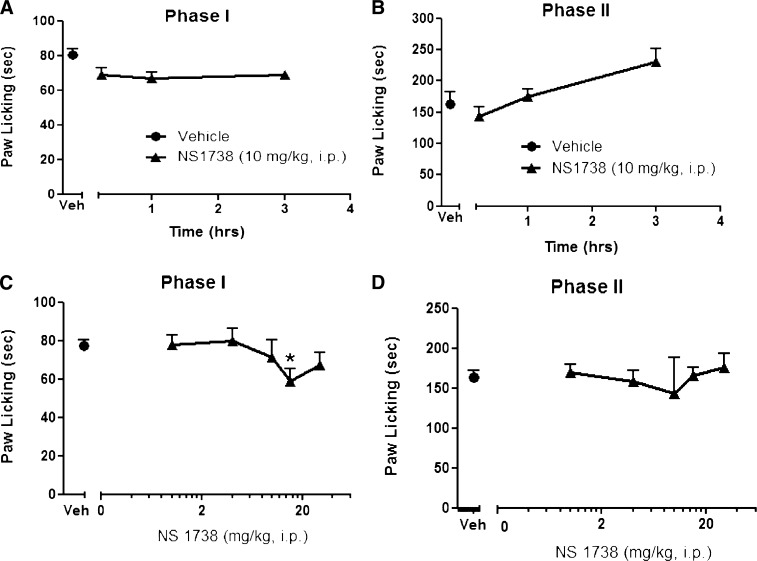
Time Course and Dose-Response Curve of Type I α7 PAM in the Formalin Test.
NS1738, a type I α7 nAChR PAM, was evaluated for its effect in the formalin test, a mouse model of tonic pain. We first tested NS1738 in the formalin test at different times after injection. As shown in Fig. 2, A and B, NS1738 (10 mg/kg i.p.) did not produce a significant decrease [F(3,35) = 2.108, P = 0.1169] in the duration of intraplantar formalin-induced nociceptive behavior during phases 1 and 2 at different times (15 minutes and 1 and 3 hours) after injection. We then tested several NS1738 doses (1, 4, 10, 15, and 30 mg/kg i.p.) 15 minutes after injection in the formalin test. As shown in Fig. 2C, NS1738 produced a small, but significant, decrease [F(5,37) = 7.660, P < 0.0001] in formalin-induced nociceptive behavior at the two highest doses (15 and 30 mg/kg) (∼30% decrease compared with vehicle). However, compared with vehicle, NS1738 failed to elicit a significant decrease [F(3,33) = 1.450, P = 0.2326] in the nociceptive effects during phase 2 at any of the doses tested (Fig. 2D).
Fig. 2.
Effects of α7 type I PAM NS1738 in the mouse formalin test. Time course of the effects of NS1738 during phase 1 (A) and phase 2 (B) in the formalin test after i.p. administration. Mice were treated with either vehicle (Veh) or NS1738 (10 mg/kg, i.p) at different times after injection (15 minutes, 1 hour, and 3 hours) before intraplantar formalin injection in the right paw. The dose-response relationship for NS1738 was established in mice 15 minutes after i.p. injection of various doses of drugs during phase 1 (C) and phase 2 (D) of the formalin test. Each symbol represents the mean ± S.E.M. of total time spent licking for 8–10 mice/group. *P < 0.05 versus vehicle.
Time Course and Dose-Response Curve of Type II α7 nAChR PAM in the Formalin Test.
The type II α7 nAChR PAM PNU-120596 was evaluated for its effect in the formalin test. We first determined the time course of PNU-120596’s effects after injection. In contrast to NS1738, PNU-120596 resulted in a significant decrease in the spontaneous pain-related (nociceptive) behavioral response to formalin. As shown in Fig. 3, A and B, the onset of action for PNU-120596 (4 mg/kg i.p.) was fairly rapid, with maximum antinociception occurring between 15 and 60 minutes. However, the duration of PNU-120596–induced antinociception was long, having a significant effect for up to 16 hours but not at 24 hours. As illustrated in Fig. 3B, PNU-120596’s effect during phase 2 gradually diminished to a 40% decrease at 16 hours (significantly different from vehicle) [F(6,51) = 22.99, P < 0.0001], and it disappeared completely within 24 hours after injection. A similar time course [F(6,51) = 30.78, P < 0.0001] was seen during phase 1, with a rebound at 8 h (Fig. 3A).
Fig. 3.
Effects of α7 type II PAM PNU-120596 in the mouse formalin test. Time course of the effects of PNU-120596 during phase 1 (A) and phase 2 (B) in the formalin test after i.p. administration. Mice were treated with either vehicle (Veh) or PNU-120596 (4 mg/kg i.p) at different times after injection (15 minutes, 1 hour, and 3 hours) before intraplantar formalin injection in the right paw. The dose-response relationship for PNU-120596 was established in mice 15 minutes after i.p. injection of various doses of drugs during phase 1 (C) and phase 2 (D) of the formalin test. Each symbol represents the mean ± S.E.M. of total time spent licking for 8–10 mice/group. *P < 0.05 versus vehicle.
The dose-response relationship was then established for PNU-120596 in mice by measuring antinociception at the time of maximal effect (15 minutes) (Fig. 3, C and D). PNU-120596 produced a dose-responsive decrease in formalin nociceptive behavior, with an ED50 (±Confidence limits) of 12.8 (6.9–23.3) and 2.78 (2.3–3.3) mg/kg in phases 1 and 2, respectively. The dose of 4 mg/kg PNU-120596 was used for the subsequent studies.
Since NS1738 was not effective after peripheral administration (i.p.), we decided to test the drug centrally via the i.t. route. Mice were treated i.t. with 15 μg of NS1738 and tested 5 minutes later in the formalin test. However, NS1738 did not elicit a significant decrease in nociceptive behavior compared with vehicle [phase 1: vehicle = 78 ± 3, NS1738 = 71 ± 6; phase 2: vehicle = 145 ± 12.5, NS1738 = 110 ± 14.6].
Although NS1738 failed to elicit antinociceptive effects after peripheral and central administration, it blocked the actions of PNU-120596 in the formalin test. Indeed, pretreatment of mice with NS1738 (30 mg/kg i.p.) partially blocked the antinociceptive response [F(3,23) = 24.14, P < 0.0001] elicited by an active dose of PNU-120596 (4 mg/kg i.p.) during phase 2 of the formalin test (Table 2).
TABLE 2.
NS1738 blocks PNU-120596’s antinociceptive effect in the formalin test
Mice were treated with NS1738 (30 mg/kg i.p.) 15 minutes before an active dose of PNU-120596 (4 mg/kg i.p.) and were tested 15 minutes after the second injection in the formalin test. The total time spent licking the right hind paw was measured during the late phase. Data are presented as the mean ± S.E.M for each group of six to eight mice.
| Paw Licking (Phase II) | |
|---|---|
| Vehicle | 173.85 ± 8.06 |
| NS1738 | 176.16 ± 7.10 |
| PNU-120596 | 65 ± 5.06 |
| NS + PNU | 130.44 ± 11.46 |
NS, NS1738; PNU, PNU-120596.
Lack of Effect of α7 Type II nAChR PAM on Locomotor Activity and Coordination of Mice.
To determine whether the effects of PNU-120596 in the formalin test are due to disruption of locomotor activity during testing, we evaluated the effect of antinociceptive doses of PNU-120596 on spontaneous activity and motor coordination of mice. As seen in Tables 3 and 4, mice treated i.p. with 4 or 8 mg/kg PNU-120596 did not show significant changes in locomotor activity (locomotor test) [F(2,11) = 0.8252, P = 0.4365] or motor coordination (Rotarod test) [F(2,12) = 0.08455, P = 0.9195] 15 minutes after testing.
TABLE 3.
Effects of PNU-120596 on locomotor activity of mice
The effects of PNU-120596 (4 and 8 mg/kg i.p.) on mouse locomotor activity. Animals were tested 15 minutes after injection with either PNU-120596 or vehicle and their locomotor activity were measured for 30 minutes. Data are presented as the mean ± SE of six to eight mice.
| Number of Photocell Interruptions | |
|---|---|
| Vehicle | 1475.0 ± 395.0 |
| PNU-120596 (4 mg/kg) | 1510.7 ± 268.5 |
| PNU-120596 (8 mg/kg) | 1115.5 ± 177.8 |
TABLE 4.
Effects of PNU-120596 on motor coordination of mice
The effects of PNU-120596 (4 and 8 mg/kg i.p.) on the Rotarod test after administration in mice. Fifteen minutes after injection with either PNU-120596 or vehicle, mice were placed on the Rotarod for 3 minutes. Data are presented as the mean ± S.E. of six to eight mice.
| Percentage Impairment | |
|---|---|
| Vehicle | 10.2 ± 10.2 |
| PNU-120596 (4 mg/kg) | 17.2 ± 17.2 |
| PNU-120596 (8 mg/kg) | 11.0 ± 11.0 |
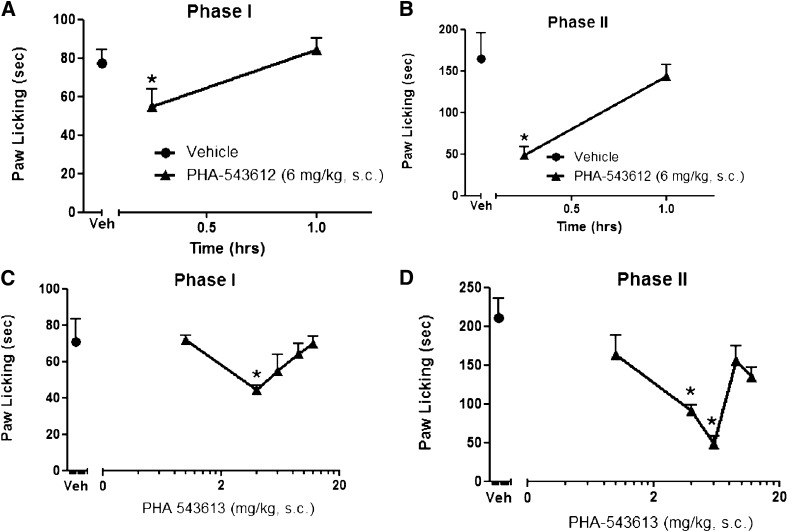
Time Course and Dose-Response Curve of a Selective α7 nAChR Agonist in the Formalin Test.
We compared the effects of the type II α7 nAChR PAM PNU-120596 in the formalin test with a full α7 nAChR agonist PHA-543613. PHA-543613 (6 mg/kg s.c.) significantly reduced the formalin-induced nociceptive behavior during both the early [F(2,15) = 4.366, P = 0.0320] and late [F(2,15) = 25.80, P < 0.0001] phases (Fig. 4, A and B). The onset of action was relatively fast, with the maximum effect occurring between 0 and 15 minutes; the effects disappeared within 60 minutes after the injection (Fig. 4, A and B). When a dose-response relationship was established, a U-shape curve emerged for both phases 1 and 2 (Fig. 4, C and D). PHA-543613 reduced formalin nociceptive behaviors at a narrow range of doses (4 and 6 mg/kg in phase 2), but the antinociceptive effect of the drug was lost at higher doses.
Fig. 4.
Effects of α7 agonist PHA-543613 in the mouse formalin test. Time course of the effects of PHA-543613 during phase 1 (A) and phase 2 (B) in the formalin test after s.c. administration. Mice were treated with either vehicle (Veh) or PHA-543613 (6 mg/kg s.c.) 15 and 60 minutes after injection before intraplantar formalin injection in the right paw. The dose-response relationship for PHA-543613 was then established in mice 15 minutes after s.c. injection of various doses of drugs during phase 1 (C) and phase 2 (D) of the formalin test. Each symbol represents the mean ± S.E.M. of total time spent licking for 8–10 mice/group. *P < 0.05 versus vehicle.
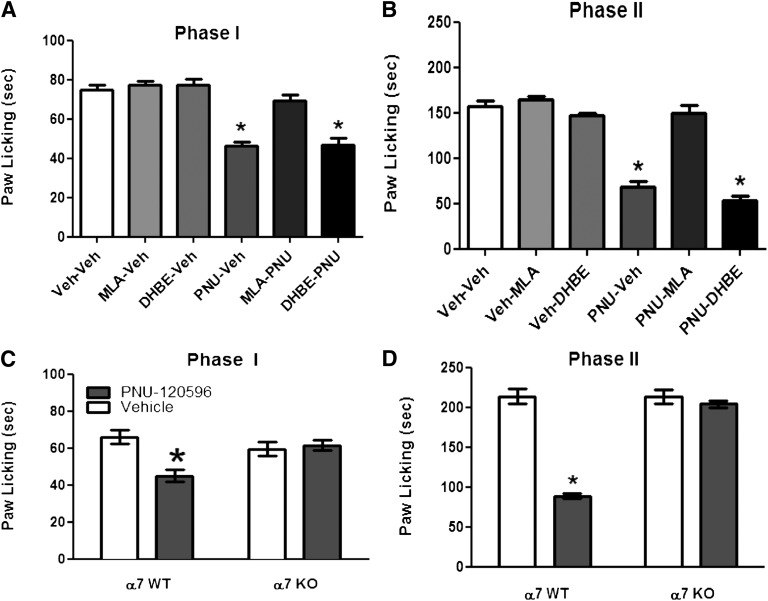
Role of α7 and β2* nAChR Subtypes in PNU-120596–Induced Antinociception in the Formalin Test.
We examined the role of β2* and α7 nAChR subtypes in the mediation of the antinociceptive effect of PNU-120596 (4 mg/kg i.p.). As predicted, the α7 nAChR antagonist MLA (10 mg/kg s.c.) completely blocked PNU-120596’s effects in both phase 1 [F(5,34) = 30.77, P < 0.0001] and phase 2 [F(5,34) = 63.11, P < 0.0001] (Fig. 5, A and B). In contrast, DHβE (2 mg/kg s.c.), a β2-containing selective antagonist, failed to block PNU-120596’s actions in the formalin test (Fig. 5, A and B). We confirmed, using α7 knockout (KO) mice, that blockade of PNU-120596’s effects in the formalin test was mediated through α7 nAChRs. As shown in Fig. 5, C and D, PNU-120596–induced antinociception during both phase 1 (treatment: [F(1,9) = 8.136, P = 0.0106]; gene: [F(1,9) = 2.197, P = 0.1556]; interaction: [F(1,18) = 11.56, P = 0.0032]) and phase 2 (treatment: [F(1,9) = 107.3, P < 0.0001]; gene: [F(1,9) = 79.20, P < 0.0001]; interaction: [F(1,18) = 79.20, P < 0.0001]) was lost in α7 KO mice compared with their WT littermates.
Fig. 5.
Nicotinic receptor subtypes involved in PNU-120596–induced antinociception in the formalin test. Blockade of the antinociceptive effect of PNU-120596 during phase 1 (A) and phase 2 (B) of the formalin test by different nicotinic antagonists. Mice were treated with MLA (10 mg/kg s.c.) or DHβE (2 mg/kg s.c.) 15 minutes before an active dose of 4 mg/kg PNU-120596. Fifteen minutes later, mice were injected with formalin (2.5% intraplantar, 20 μl) and then observed for pain behaviors. Antinociceptive effects of PNU-120596 in the formalin test were tested in α7 WT and KO mice. α7 WT and KO mice received an i.p. dose of 4 mg/kg PNU-120596 and were tested 15 minutes later during phase 1 (C) and phase 2 (D) of the formalin test. Data are mean ± S.E.M. licking time for six to eight mice/group. *P < 0.05 versus vehicle-vehicle group. PNU, PNU-120596; Veh, vehicle.
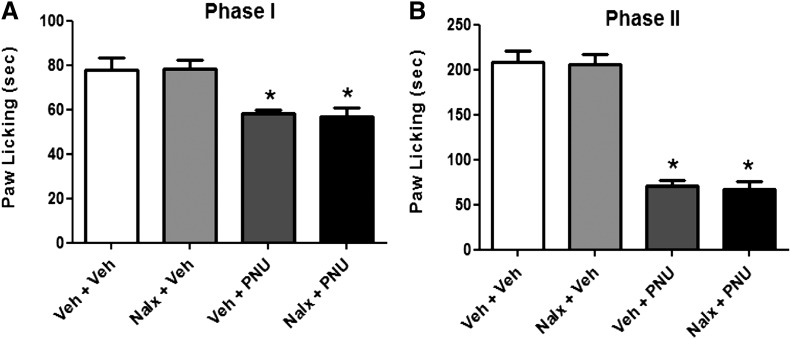
Furthermore, pretreatment with naloxone (1 mg/kg s.c.), an opioid receptor antagonist, did not abolish the antinociceptive effect of PNU-120596, given at the dose of 4 mg/kg (i.p.), during phase 1 [F(3,16) = 9.237, P = 0.0009] or phase 2 [F(3,16) = 69.05, P < 0.0001] (Fig. 6B).
Fig. 6.
Lack of blockade of PNU-120596–induced antinociception in the formalin test by naloxone. Mice were treated with s.c. naloxone 15 minutes prior to PNU-120596 (4 mg/kg i.p.) injection. They were tested 15 minutes after the second injection in the formalin test. The time spent licking the injected paw was recorded during the early (A) and late phase (B) after formalin injection. Each group represents the mean ± S.E. of six to eight mice. *P < 0.05 versus vehicle-vehicle group.
Contribution of Central α7 nAChRs to PNU-120596’s Antinociceptive Response.
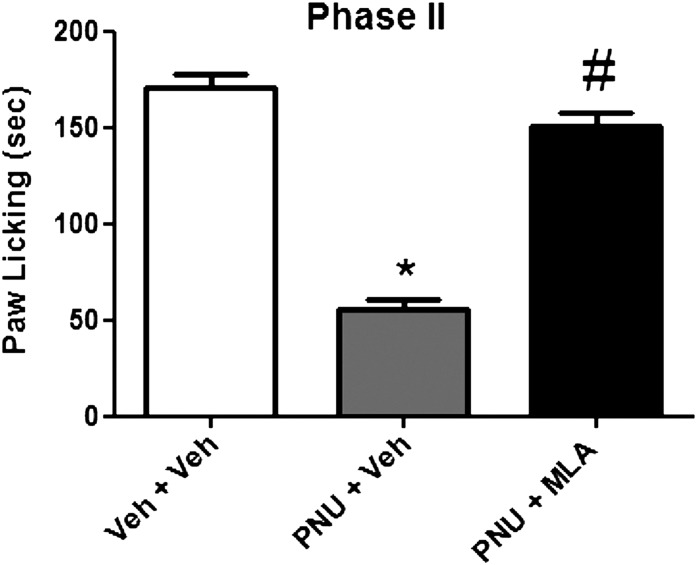
Since α7 nAChRs subtypes are present in both the periphery and the spinal cord, we examined the contribution of these sites to PNU-120596’s antinociceptive response in the formalin test. When the α7 nAChR antagonist MLA was given i.t. (10 µg/mouse) 5 minutes before PNU-120596 (4 mg/kg i.p.) administration, the effect of the drug was completely reversed during phase 2 [F(2,18) = 17.33, P < 0.0001] (Fig. 7).
Fig. 7.
Blockade of PNU-120596's antinociceptive effect after i.t. MLA administration in the phase 2 of the formalin test. Mice were treated with either PNU-120596 (4 mg/kg i.p.) or vehicle (i.p.) 15 minutes prior to MLA (10 μg/5 μl i.t.) injection and were tested 5 minutes later in the formalin test. Each bar represents the mean ± S.E.M. for each group of six to eight mice. *P < 0.05 versus vehicle-vehicle group; #P < 0.05 versus PNU-120596 (4 mg/kg). PNU, PNU-120596; Veh, vehicle.
The Effects of Intrathecal Administration of the MEK Inhibitor U0126 on PNU-120596’s Antinociceptive Effect in the Formalin Test.
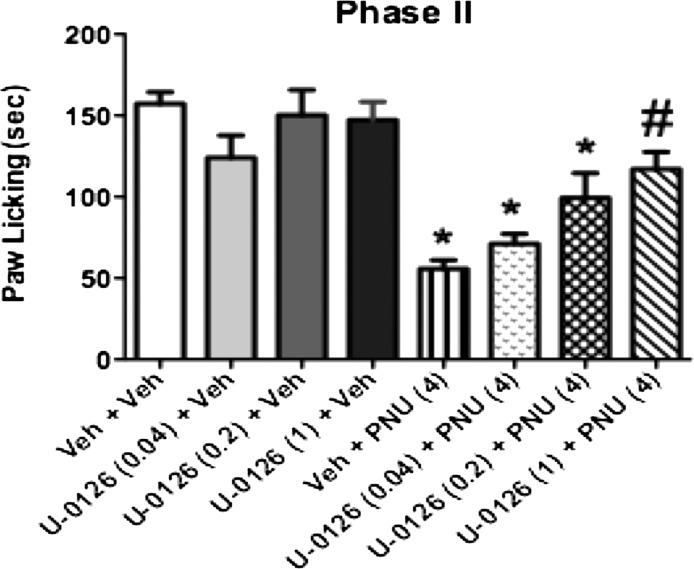
We next investigated whether the antinociceptive effect of PNU-120596 was mediated through ERK activation in mice. Pretreatment of mice with gradually increasing doses (0.04, 0.2, and 1 µg/mouse i.t.) of U0126 alone, a selective MEK inhibitor (Aley et al., 2001; Kominato et al., 2003), did not affect nociceptive responses induced by formalin injection compared with their vehicle controls (Fig. 8). However, U0126 administered 5 minutes before PNU-120596 (4 mg/kg i.p.) dose-dependently blocked [F(7,36) = 13.38, P < 0.0001] PNU-120596–induced antinociception during phase 2 of the formalin test. U0126 (1 µg/mouse) completely reversed PNU-120596’s actions in this test.
Fig. 8.
Effects of MEK inhibitor U0126 on PNU-120596–induced antinociception in the formalin test. Mice were treated with the MEK inhibitor U0126 (0.04, 0.2, and 1 µg/mouse i.t.) or vehicle 5 minutes prior to PNU-120596 (4 mg/kg. i.p.) injection. Mice were tested 15 minutes later during phase 2 of the formalin test. Each bar represents the mean ± S.E.M. for each group of six to eight mice. *P < 0.05 versus vehicle-vehicle group; #P < 0.05 versus PNU-120596 (4 mg/kg). PNU, PNU-120596; Veh, vehicle.
Tolerance to PNU-120596’s Effects in the Formalin Test Did Not Develop after Subchronic Exposure.
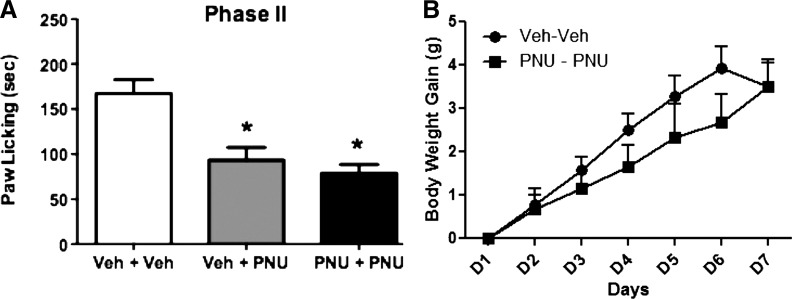
We investigated whether tolerance to PNU-120596’s antinociceptive effects develops after subchronic exposure to the drug. The dosing protocol of PNU-120596 was based on the time course study of the drug shown earlier (24 hours’ long). Animals were treated with either vehicle or PNU-120596 (4 mg/kg i.p.) once a day (8:00 AM) for 6 days. On day 7, mice were challenged with PNU (4 mg/kg i.p.) and tested 15 minutes later. As seen in Fig. 9A, tolerance did not develop [F(2,15) = 17.89, P < 0.0001] after subchronic exposure to PNU-120596 during phase 2 of the formalin test. Furthermore, the subchronic treatment with PNU-120596 did not significantly change the weight gain of mice compared with the vehicle-treated group (Fig. 9B).
Fig. 9.
Lack of tolerance to PNU-120596–induced antinociception in the formalin test after subchronic administration of the drug. (A) Mice were treated with either vehicle or PNU-120596 (4 mg/kg i.p.) once a day for 6 days. On day 7, mice were challenged with PNU (4 mg/kg i.p.) and tested 15 minutes later during phase 2 of the formalin test. Control group received vehicle for 6 days, were challenged on day 7 with vehicle, and were tested 15 minutes later in the formalin test. (B) Lack of significant effect on the body weight change of mice after subchronic injection of PNU-120596. Body weight change (body weight on injection day − initial body weight before treatment) in the two treatment groups was recorded daily at the same time. Each bar represents the mean ± S.E.M. for each group of six to eight mice. *P < 0.05 versus vehicle-vehicle group. PNU, PNU-120596; Veh, vehicle.
Measurement of α7 Types I and II PAM Levels in the Brain and Plasma.
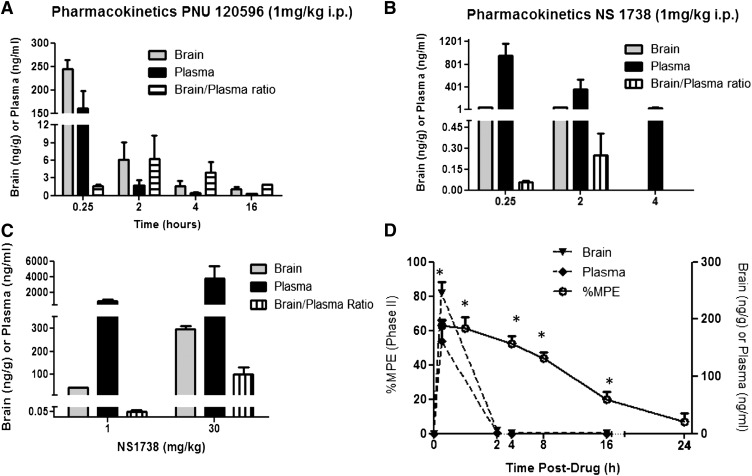
To estimate the ability of PAMs to permeate the blood–brain barrier, mice were administered a concentration of 1 mg/kg of either NS1738 or PNU-120596 i.p. Brain and plasma concentrations of PNU-120596 were measured at 0.25, 2, 4, and 16 hours after dosing. At the 0.25-hour time point, plasma and brain concentrations corresponded to 150 to 250 ng/ml respectively, and decreased thereafter. Four hours after PNU-120596 injection, brain and plasma levels were close to 2 ng/ml (Fig. 10A). Brain and plasma concentrations were measured at 0.25, 2, and 4 hours after injection of 1 mg/kg NS1738 i.p., which yielded only 1 ng/ml in brain versus 1000 ng/ml in plasma samples (Fig. 10B). To assess whether pharmacokinetic factors are responsible for the lack of pharmacological effects for NS1738, a 30 mg/kg i.p. dose was also assessed, and brain/plasma ratio was estimated at the 15-minute time point. As shown in Fig. 10C, sufficient NS1738 (close to 300 ng/ml) crosses the blood-brain barrier to further support pharmacodynamic differences between type I and II PAMs with regard to antinociceptive efficacy. Finally, when we examined the time course relationship of plasma and brain levels associated with antinociceptive efficacy, a discordance between the pharmacokinetic-pharmacodynamic properties of PNU-120596 was observed. As depicted in Fig. 10D, a large antinociceptive effect was observed during phase 2 at 4 hours, despite the substantial reduction in its plasma concentration at this time point compared with the peak levels. Moreover, at 16 hours, significant effects were still observed, although the plasma and brain concentrations were below the detection limit.
Fig. 10.
Pharmacokinetics of NS1738 and PNU-120596. (A) Mice (n = 3) were dosed with 1 mg/kg PNU-120596 i.p. at time 0; both brain and plasma samples were collected at 0.25, 2, 4, and 16 hours and analyzed for PNU-120596 content by mass spectrometry to estimate the brain/plasma ratio at each time point. (B) Mice (n = 3) were dosed i.p. with 1 mg/kg NS1738 at time 0, and both brain and plasma samples were collected at 0.25, 2, and 4 hours. (C) To parallel in vivo efficacious doses, mice (n = 3) were dosed with either 1 or 30 mg/kg NS1738 i.p. at time 0, and both brain and plasma samples were collected at 15 minutes to compare levels. (D) Pharmacokinetic-pharmacodynamic relationship of PNU-120596. Time course of efficacy and relation to plasma and brain concentrations of PNU-120596. Antinociception (%MPE) was assessed at 0.25, 2, 4, 8, 16, and 24 hours postdose of 4 mg/kg (left y-axis). Plasma and brain levels of PNU-120596 were determined in a satellite group of mice (plotted along the right y-axis). Although plasma and brain concentrations of PNU-120596 decreased to <5 ng/ml by 2 hours, a significant effect persists in the formalin test (P < 0.05 versus vehicle controls). All data are mean ± S.E.M. *P < 0.05 from time zero.
Discussion
In the present study, we evaluated the effects of α7 nAChRs type I and II PAMs in animal models of acute and tonic pain. Our studies show that, similar to α7 nAChR agonists, α7 nAChR PAMs are not active in acute thermal pain tests (hot plate and tail-flick) and mechanical sensitivity tests. Type II α7 nAChR (PNU-120596), but not type I PAMs, attenuated pain behavior in the early and late phases of the formalin test. Indeed, PNU-120596 had a long-lasting antinociceptive effect, with a greater potency (6-fold) in the late (inflammatory) phase of the test. Importantly, no changes were seen in motor locomotion or coordination with antinociceptive doses of PNU-120596 in mice. In line with our data, Munro et al. (2012) recently showed that PNU-120596 produces antihyperalgesic and anti-inflammatory effects in the carrageenan or complete Freund’s adjuvant tests in rats. However, the potency of PNU-120596 was greater in the mouse formalin test than in the rat one. Furthermore, Munro et al. (2012) did not investigate the effects of type I PAMs in their models. In our mouse studies, NS1738, a type I α7 nAChR PAM, failed to exhibit antinociceptive effects in the late phase of the formalin test after systemic (i.p.) and central (i.t.) administration, but it showed a modest decrease in the early phase of the test. The lack of effect after NS1738 administration is not due to poor drug distribution to the brain after systemic injection. Indeed, measuring brain concentrations of NS1738 after i.p. injection (30 mg/kg) yield drug levels (∼0.80 µM) close to those reported to enhance ACh in expressed α7 nAChRs (Timmermann et al., 2007). In addition, the concentration of NS1738 in the mouse brain is similar to that seen in the rats after i.p. injection (Timmermann et al., 2007). Similarly, after a dose of 1 mg/kg PNU-120596 (∼0.25 µM), brain levels fall near the EC50 for potentiating effects of PNU-120596 (EC50 ∼0.2–1.5 µM) in various in vitro expressed and native α7 nAChRs preparations (Hurst et al., 2005; Gronlien et al., 2007; Barron et al., 2009; Gusev and Uteshev, 2010; Kalappa et al., 2010). Therefore, our data strongly suggest that type I and II α7 nAChR PAMs differentially modulate nociceptive behavior in the formalin test.
The difference in efficacy might be related to differences in α7 nAChR regulation by these two drugs. Type I PAMs predominantly affect the apparent peak current, with little effect on desensitization kinetics, whereas type II PAMs increase the apparent peak current and evoke a distinct weakly decaying current, causing dramatic slowing of receptor desensitization (Hurst et al., 2005). Indeed, PNU-120596 was shown to activate α7 nAChRs that would otherwise be desensitized (Papke et al., 2009).
In contrast to the type II α7 nAChR PAM, the selective α7 agonist PHA-543613 had a very narrow window of antinociceptive effect, as reflected by its U-shaped dose-effect curve in the formalin test. Although drug distribution and metabolism factors could account for PHA-543613’s and PNU-120596’s different dose-response profiles, differences in α7 nAChR activation and desensitization properties may play a more important role. Similar to other α7 nAChR agonists, PHA-543613 induces receptor desensitization after an initial phase of receptor activation.
The inflammatory tonic pain model consists of two distinct phases. The first phase (immediately after formalin injection) seems to be caused by the direct effect of formalin on sensory C-fibers. The second phase (starting later after formalin injection), known as the inflammatory phase, is associated with the development of a delayed inflammatory response and spinal dorsal horn sensitization (Abbott et al., 1995; Davidson and Carlton, 1998). We observed that PNU-120596 attenuated pain behavior in the early and late phases of the formalin test, suggesting that PNU-120596 acts both centrally and peripherally to reduce tonic pain.
The long-acting effect of PNU-120596 that we observed in the mouse formalin test is in line with PNU-12096's effects reported in the rat carrageenan test (Munro et al., 2012), in which the drug significantly blunted mechanical hyperalgesia for up to 4 h. This effect cannot be simply explained by the pharmacokinetic profile of the drug. Indeed, as shown in Fig. 10, most of the PNU-120596 is eliminated from plasma and brain of the animals 4 hours after injection of the drug. In this regard, PNU-120596’s properties are similar to those reported for α7 nAChR agonists, such as ABT-107 (Bitner et al., 2010). PNU-120596 appears to offer prolonged efficacy that may be associated with activation of signaling pathways, leading to lasting secondary functional changes linked to synaptic plasticity. Our results with the MEK inhibitor U0126 suggest that the ERK1/2 pathway is one possible postreceptor signaling mechanism. The ERK1/2 pathway regulates a diverse array of cellular functions, such as cell growth, differentiation, and survival, which may underlie the synaptic plasticity required for persistent pain processes (Alter et al., 2010). Furthermore, El Kouhen et al. (2009) observed a robust increase in ERK1/2 phosphorylation induced by α7 agonists in the presence of type II PAMs in PC12 cells. It is also possible that the prolonged effects of PNU-120596 could be mediated by an α7-dependent regulation of anti-inflammatory chemokines, such as TNF-α, through an NF-κB pathway (Bernik et al., 2002). In line with this possibility, Munro et al. (2012) recently showed that PNU-120596’s anti-inflammatory effects in rats might be mediated through a decrease in the levels of peripheral TNF-α and IL-6.
PAMs are compounds that facilitate endogenous neurotransmission and/or enhance the efficacy and potency of agonists, without directly stimulating the agonist binding sites. Our PNU-120596 data suggest the presence of a proantinociceptive endogenous tone mediated by α7 nAChRs. PNU-120596 may be acting in the formalin test through the enhancement of subthreshold concentrations (i.e., 5–10 μM) of the endogenous α7 agonists choline and/or ACh (Sarter and Parikh, 2005; Parikh and Sarter, 2006). Supporting this possibility of choline, PNU-120596 was recently reported to enhance the effects of subthreshold, physiologic concentrations of choline on native α7 nAChR in hypothalamic neurons (Gusev and Uteshev, 2010). Although the enhancement seen using in vitro α7 nAChRs preparations is greatly decreased when experimental temperatures increase to near physiologic levels (Sitzia et al., 2011; Williams et al., 2012), apparently it is not completely eliminated given that PNU-120596 required enhanced endogenous α7 nicotinic activation to produce the antinociceptive effects in our tests. In addition, Williams et al., (2012) showed that, although the effects of PNU-120596 are strongly temperature dependent, physiologic factors, such as serum albumens, reverse this temperature dependency. Alternatively, the pharmacological effects of PNU-120596 could be mediated independently of α7 channel activation. Indeed, a growing body of literature suggests that α7 receptors have low intrinsic open probability and high propensity toward the induction of nonconducting ligand-bound states (Williams et al., 2011; 2012). These properties imply that some pharmacological effects of α7 ligands could be mediated independently of ion-channel activation. This channel-independent regulation of signal-transduction pathways by α7 nAChRs is well documented in immune cells regulating inflammation (for review see Marrero et al., 2011). Our results with the MEK inhibitor suggest such a possibility in an in vivo animal model.
In our studies, MLA, an α7 nAChR antagonist, significantly blocked PNU-120596’s antinociceptive effect in the early and late phases of the formalin test, whereas DHBE, a β2* antagonist, did not. Using both pharmacological (i.e., naloxone) and genetic approaches (i.e., α7 KO mice), we confirmed that PNU-120596’s effect is mediated by α7 nAChRs and not by opioid receptors. Similarly, α7 nAChRs seem to mediate the antihyperalgesic effects of PNU-120596 in the rat (Munro et al., 2012).
Furthermore, our results show that tolerance did not develop following subchronic exposure to the type II α7 nAChR PAM. Importantly, no change in body weight gain was seen after subchronic administration of PNU-120596 in mice. Munro et al. (2012) reported effects of PNU-120596 only after acute administration in the rat carrageenan test.
It seems that multiple sites are involved in PNU-120596’s antinociceptive effect. Indeed, the data associated with i.p. and i.t. MLA provide evidence of central and peripheral involvement, respectively. Our results support the possibility that the spinal cord is an important site of action for PNU-120696 in the formalin test. However, a role for peripheral and local α7 nAChRs cannot be discounted. In the periphery, α7 nAChRs are expressed on T cells, macrophages, and other immune cells that are capable of producing ACh (Kawashima and Fujii, 2003; Wang and Wang, 2003; Fujii et al., 2008). Additionally, in the rat hind paw, α7 nAChRs were identified on skin keratinocytes and resident macrophages but not on peripheral nerve endings (Kurzen et al., 2007).
Finally, our data from phase 2 of the formalin test show a blockade of PNU-120596’s effect by NS1738. Supporting our finding, Williams et al. (2011) recently reported that the type I PAM 5HI reduced PNU-120596’s in vitro effects in oocytes expressing human α7 nAChRs. These results suggest that types I and II PAMs may compete for a common allosteric transmembrane site in the α7 nAChRs. Indeed, Collins et al. (2011) recently showed that both type I PAM NS1738 and type II PAM PNU-120596 bind competitively at a shared or overlapping allosteric transmembrane site on the α7 nAChR.
Acknowledgments
The authors thank Tie Han for technical assistance. Kennan Marsh (Abbott Laboratories) performed the pharmacokinetic studies.
Abbreviations
- ACh
acetylcholine
- AR-R17779
(2)-spiro[1-azabicyclo[2.2.2]octane-3,59-oxazolidin]-29-one
- DHβE
dihydro-β-erythroidine
- ERK
extracellular signal–regulated kinase
- JN 403
(S)-(1-azabicyclo[2.2.2]oct-3-yl)-carbamic acid (S)-1-(2-fluoro-phenyl)-ethyl ester
- KO
knockout
- MEK
mitogen-activated protein kinase kinase
- MLA
methyllycaconitine citrate
- %MPE
percentage maximum possible effect
- nAChR
nicotinic acetylcholine receptor
- NS1738
N-(5-chloro-2-hydroxyphenyl)-N′-[2-chloro-5-(trifluoromethyl)phenyl
- PAM
positive allosteric modulator
- PHA-543613
N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide
- PNU-120596
1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)
- U0126
1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene monoethanolate
- WT
wild type
Authorship Contributions
Participated in research design: Freitas, Damaj.
Conducted experiments: Freitas.
Performed data analysis: Freitas, Damaj.
Wrote or contributed to the writing of the manuscript: Carroll, Freitas, Damaj.
Footnotes
This work was supported by National Institutes of Health [Grants DA-019377] (to M.I.D.) and [DA12001] (to F.I.C.).
References
- Abbott FV, Franklin KB, Westbrook RF. (1995) The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain 60:91–102 [DOI] [PubMed] [Google Scholar]
- Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. (2001) Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci 21:6933–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BJ, Zhao C, Karim F, Landreth GE, Gereau RW., 4th (2010) Genetic targeting of ERK1 suggests a predominant role for ERK2 in murine pain models. J Neurosci 30:11537–11547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron SC, McLaughlin JT, See JA, Richards VL, Rosenberg RL. (2009) An allosteric modulator of alpha7 nicotinic receptors, N-(5-Chloro-2,4-dimethoxyphenyl)-N’-(5-methyl-3-isoxazolyl)-urea (PNU-120596), causes conformational changes in the extracellular ligand binding domain similar to those caused by acetylcholine. Mol Pharmacol 76:253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Gopalakrishnan M. (2007) Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol 74:1155–1163 [DOI] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Decker MW, et al. (2010) In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer’s disease. J Pharmacol Exp Ther 334:875–886 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63 [DOI] [PubMed] [Google Scholar]
- Collins T, Young GT, Millar NS. (2011) Competitive binding at a nicotinic receptor transmembrane site of two α7-selective positive allosteric modulators with differing effects on agonist-evoked desensitization. Neuropharmacology 61:1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Changeux JP. (2001) Tonic nicotinic modulation of serotoninergic transmission in the spinal cord. Proc Natl Acad Sci USA 98:2803–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Pons S, Faure P, Changeux JP. (2004) Nicotine differentially activates inhibitory and excitatory neurons in the dorsal spinal cord. Pain 109:308–318 [DOI] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79 [Google Scholar]
- Damaj MI, Meyer EM, Martin BR. (2000) The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology 39:2785–2791 [DOI] [PubMed] [Google Scholar]
- Davidson EM, Carlton SM. (1998) Intraplantar injection of dextrorphan, ketamine or memantine attenuates formalin-induced behaviors. Brain Res 785:136–142 [DOI] [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L. (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151:915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey WL, Harris LS, Howes JF, Nuite JA. (1970) The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J Pharmacol Exp Ther 175:435–442 [PubMed] [Google Scholar]
- Dixon W. (1965) The up-and-down method for small samples. J Am Stat Assoc 60:967–978 [Google Scholar]
- Duncia JV, Santella JB, 3rd, Higley CA, et al. (1998) MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett 8:2839–2844 [DOI] [PubMed] [Google Scholar]
- El Kouhen R, Hu M, Anderson DJ, Li J, Gopalakrishnan M. (2009) Pharmacology of alpha7 nicotinic acetylcholine receptor mediated extracellular signal-regulated kinase signalling in PC12 cells. Br J Pharmacol 156:638–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerbach D, Lingenhoehl K, Olpe HR, et al. (2009) The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology 56:254–263 [DOI] [PubMed] [Google Scholar]
- Fujii T, Takada-Takatori Y, Kawashima K. (2008) Basic and clinical aspects of non-neuronal acetylcholine: expression of an independent, non-neuronal cholinergic system in lymphocytes and its clinical significance in immunotherapy. J Pharmacol Sci 106:186–192 [DOI] [PubMed] [Google Scholar]
- Gao B, Hierl M, Clarkin K, et al. (2010) Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain 149:33–49 [DOI] [PubMed] [Google Scholar]
- Gillberg PG, Aquilonius SM. (1985) Cholinergic, opioid and glycine receptor binding sites localized in human spinal cord by in vitro autoradiography. Changes in amyotrophic lateral sclerosis. Acta Neurol Scand 72:299–306 [DOI] [PubMed] [Google Scholar]
- Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, Role L. (1999) Heteromeric complexes of a5 and/or a7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann N Y Acad Sci 868:578–590 [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. (2006) Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27:482–491 [DOI] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. (2007) Distinct profiles of α7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72:715–724 [DOI] [PubMed] [Google Scholar]
- Gurun MS, Parker R, Eisenach JC, Vincler M. (2009) The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of alpha7 nicotinic acetylcholine receptor. Anesth Analg 108:1680–1687 [DOI] [PubMed] [Google Scholar]
- Gusev AG, Uteshev VV. (2010) Physiological concentrations of choline activate native α7-containing nicotinic acetylcholine receptors in the presence of PNU-120596 [1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)-urea]. J. Pharmacol Exp Ther 332:588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Gopalakrishnan M, Li J. (2009) Positive allosteric modulation of α7 neuronal nicotinic acetylcholine receptors: lack of cytotoxicity in PC12 cells and rat primary cortical neurons. Br J Pharmacol 158:1857–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, et al. (2005) A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25:4396–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. (1980) Intrathecal morphine in mice: A new technique. Eur J Pharmacol 67:313–316 [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Gusev AG, Uteshev VV. (2010) Activation of functional α7-containing nAChRs in hippocampal CA1 pyramidal neurons by physiological levels of choline in the presence of PNU-120596. PLoS ONE 5:e13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. (2003) The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci 74:675–696 [DOI] [PubMed] [Google Scholar]
- Khan IM, Taylor P, Yaksh TL. (1994) Stimulatory pathways and sites of action of intrathecally administered nicotinic agents. J Pharmacol Exp Ther 271:1550–1557 [PubMed] [Google Scholar]
- Kominato Y, Tachibana T, Dai Y, Tsujino H, Maruo S, Noguchi K. (2003) Changes in phosphorylation of ERK and Fos expression in dorsal horn neurons following noxious stimulation in a rat model of neuritis of the nerve root. Brain Res 967:89–97 [DOI] [PubMed] [Google Scholar]
- Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. (2007) The non-neuronal cholinergic system of human skin. Horm Metab Res 39:125–135 [DOI] [PubMed] [Google Scholar]
- Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, Chessell IP. (2008) Activation of the alpha7-nicotinic acetylcholine receptor reverses complete freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J Pain 9:580–587 [DOI] [PubMed] [Google Scholar]
- van Maanen MA, Lebre MC, van der Poll T, LaRosa GJ, Elbaum D, Vervoordeldonk MJ, Tak PP. (2009) Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum 60:114–122 [DOI] [PubMed] [Google Scholar]
- Marrero MB, Bencherif M, Lippiello PM, Lucas R. (2011) Application of alpha7 nicotinic acetylcholine receptor agonists in inflammatory diseases: an overview. Pharm Res 28:413–416 [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56:237–246 [DOI] [PubMed] [Google Scholar]
- Munro G, Hansen RR, Erichsen HK, Timmermann DB, Christensen JK, Hansen HH. (2012) The α7 nicotinic ACh receptor agonist compound B and positive allosteric modulator PNU-120596 both alleviate inflammatory hyperalgesia and cytokine release in the rat. Br J Pharmacol 167:421–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA. (2009) Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther 329:791–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. (2006) Cortical choline transporter function measured in vivo using choline-sensitive microelectrodes: clearance of endogenous and exogenous choline and effects of removal of cholinergic terminals. J Neurochem 97:488–503 [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. (2000) Neuronal nicotinic receptors in the human brain. Prog Neurobiol 61:75–111 [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. (2004) Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci 61:2322–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. (2010) Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth 105:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V. (2005) Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci 6:48–56 [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzia F, Brown JT, Randall AD, Dunlop J. (2011) Voltage- and Temperature-Dependent Allosteric Modulation of α7 Nicotinic Receptors by PNU120596. Front Pharmacol. 2:81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. (2010) Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des 16:323–343 [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Grønlien JH, Kohlhaas KL, et al. (2007) An allosteric modulator of the α7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther 323:294–307 [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter JIM, Deneris E, Heinemann S, Patrick JIM, and Swanson LW (1989) Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol 284:314–335. [DOI] [PubMed]
- Wang LX, Wang ZJ. (2003) Animal and cellular models of chronic pain. Adv Drug Deliv Rev 55:949–965 [DOI] [PubMed] [Google Scholar]
- Wang Y, Su DM, Wang RH, Liu Y, Wang H. (2005) Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience 132:49–56 [DOI] [PubMed] [Google Scholar]
- Williams DK, Peng C, Kimbrell MR, Papke RL. (2012) Intrinsically Low Open Probability of α7 Nicotinic Acetylcholine Receptors Can Be Overcome by Positive Allosteric Modulation and Serum Factors Leading to the Generation of Excitotoxic Currents at Physiological Temperatures. Mol Pharmacol 82:746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. (2011) Investigation of the molecular mechanism of the α7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol Pharmacol 80:1013–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]