Abstract
The intramolecular disulfide bond in human Cu,Zn superoxide dismutase 1 (hSOD1) plays a key role in maintaining the protein’s stability and quaternary structure. In mutant forms of SOD1 that cause familial amyotrophic lateral sclerosis (ALS), this disulfide bond is more susceptible to chemical reduction, which may lead to destabilization of the dimer and aggregation. During hSOD1 maturation, disulfide formation is catalyzed by the copper chaperone CCS1. Previous studies in yeast demonstrate that the yeast glutathione (GSH)/glutaredoxin redox system promotes reduction of the hSOD1 disulfide in the absence of CCS1. Herein, we further probe the interaction between hSOD1, GSH, and glutaredoxins to provide mechanistic insight into the redox kinetics and thermodynamics of the hSOD1 disulfide. We demonstrate that human glutaredoxin 1 (hGrx1) uses a monothiol mechanism to reduce the hSOD1 disulfide, and the GSH/hGrx1 system reduces ALS mutant SOD1 at a faster rate than WT hSOD1. However, redox potential measurements demonstrate that the thermodynamic stability of the disulfide is not consistently lower in ALS mutants compared to WT hSOD1. Furthermore, the presence of the metal cofactors does not influence the disulfide redox potential. Overall, these studies suggest that differences in the GSH/hGrx1 reaction rate with WT vs. ALS mutant hSOD1 and not the inherent thermodynamic stability of the hSOD1 disulfide bond may contribute to the greater pathogenicity of ALS mutant hSOD1.
Keywords: Disulfide; redox potential; glutaredoxins; yeast; Cu,Zn superoxide dismutase; monothiol
INTRODUCTION
Cu- and Zn-containing superoxide dismutase 1 (SOD1) is a highly conserved, ubiquitous enzyme that detoxifies superoxide radicals in both the cytosol and mitochondrial intermembrane space of eukaryotic cells. Nascent SOD1 polypeptides must undergo four post-translational modifications to reach the mature form: insertion of zinc, insertion of copper, formation of an intramolecular disulfide bond between Cys57 and Cys146, and dimerization [1, 2]. The mechanism for insertion of the zinc ion is unknown; however, copper is inserted by the copper chaperone for SOD1 (CCS1) [3]. In addition to transferring copper, CCS1 also catalyzes formation of the SOD1 disulfide bond [4]. Human SOD1 can also acquire copper and form the intramolecular disulfide through a CCS1-independent pathway that requires glutathione (GSH), although this process is not completely understood [5]. Biophysical studies on purified hSOD1 demonstrate that both metal occupancy and disulfide redox state play major roles in influencing the structure and stability of the hSOD1 polypeptide. Holo, disulfide-oxidized hSOD1 forms unusually stable, active homodimers, while the inactive apo, reduced form is found predominantly as a monomer at physiological concentrations [6–8].
Despite its role as a protective enzyme, over 100 mutations in hSOD1 have been causally linked to the familial form of the common neurodegenerative disease amyotrophic lateral sclerosis (ALS) [9]. The molecular mechanism for SOD1-linked ALS pathology is still unclear; however, in vivo studies in mouse models and human cell culture have demonstrated that ALS mutant forms of hSOD1 are prone to form insoluble aggregates [10–14], which may be toxic to motor neurons. The biophysical/biochemical properties of WT and ALS mutants of hSOD1 have been compared in a number of studies in order to explain the greater aggregation propensity of ALS mutants [8, 15–20]. Collectively, these studies demonstrate that ALS mutants are more prone to disulfide reduction, unfolding/misfolding, and metal loss than WT hSOD1. These structural aberrations are proposed to promote non-native interactions between SOD1 monomers or other cellular components that lead to aggregation [15, 21]. The apo, reduced forms of ALS mutant hSOD1 are especially vulnerable to destabilization and readily aggregate under mild oxidative stress conditions [20].
Given the importance of the intramolecular disulfide in maintaining the structure and stability of hSOD1, we sought to identify factors that influence the hSOD1 disulfide redox state in vivo. Previous studies have demonstrated that the disulfide bond in human SOD1 is reduced by yeast glutaredoxin 2 (yGrx2) when expressed heterologously in a Saccharomyces cerevisiae ccs1Δ strain. Furthermore, in vitro enzyme assays indicated that hSOD1 ALS mutants are more vulnerable to disulfide reduction by yeast Grx2 than WT hSOD1, suggesting a possible role for Grxs in redox-dependent destabilization of ALS mutants [22]. Since members of the Grx family exhibit subtle structural and mechanistic differences [23, 24], we extended these studies to characterize the in vivo and in vitro interactions between human SOD1 and the human homologue of yGrx2, namely human Grx1. Human Grx1 and hSOD1 both co-localize to the cytosol and intermembrane space [25, 26], thus hGrx1 is poised to have a direct impact on the redox state of hSOD1 under physiological conditions. However, the molecular interactions between human Grx1 and WT and mutant forms of human SOD1 have not previously been addressed. The studies reported herein demonstrate that hGrx1 facilitates reduction of the disulfide bond of hSOD1 in a similar manner to yGrx2. Furthermore, we determined that hGrx1 uses a monothiol mechanism to reduce the disulfide bond in vivo and in vitro, suggesting that at least one of the disulfide cysteines of hSOD1 forms a transient mixed disulfide with GSH prior to reduction by hGrx1. We compared the reactivity and thermodynamic stability of the disulfide bond in holo and apo WT and ALS mutants A4V and G93A in vitro. These studies demonstrate that hGrx1 displays higher reactivity towards mutant hSOD1 than WT hSOD1. However, the thermodynamic stabilities of the disulfide bond in ALS mutant forms are not consistently lower than WT hSOD1. Overall, these studies suggest that hGrx1 may play a significant role in destabilization of ALS mutant hSOD1 in vivo by selectively reducing the kinetic barrier for disulfide reduction.
EXPERIMENTAL
Yeast strains and growth conditions
The yeast S. cerevisiae strains used in these studies are derived from the parental strain CY4 (MATa ura3-52 leu2-3 trp1-1 ade2-1 his3-11 can1-100). Strains Y117 (grx1Δ::LEU2 grx2Δ::HIS3), MC108 (ccs1Δ::ADE2), and MC120 (grx1Δ::LEU2 grx2Δ::HIS3 ccs1Δ::ADE2) were described previously [22]. Strains were maintained at 30 °C on synthetic defined medium (SD) supplemented with 2% glucose and the appropriate amino acids (US Biological). Anaerobic cultures were maintained by growth in an O2-depleted culture jar (BBL Gas Pak).
Plasmids
Human SOD1 was expressed in yeast cells under the control of the PGK1 promoter using plasmids pLC1 (WT hSOD1), pLC2 (A4V hSOD1), and pLC3 (G41D hSOD1) (2µ URA3) described previously [27]. Site-directed mutagenesis of WT hSOD1 was carried out to generate a G93A hSOD1 expression plasmid (pMD100) using the QuikChange Site-Directed Mutagenesis kit (Stratagene). Yeast expression vectors for human Grx1 (hGrx1) were created by PCR amplification of hGrx1 cDNA (Open Biosystems) and insertion of the PCR product into the NdeI and SnaBI sites in pLS108 (CEN LEU2) [28, 29], allowing for insertion of hGrx1 between the promoter and terminator for ySOD1 to create pCO202. Digestion of pCO202 with SalI and BamHI allowed for insertion of the ySOD1 promoter, the hGrx1coding sequence, and the ySOD1 terminator into pRS414 (CEN TRP1), yielding pCO204. A plasmid for overexpression of recombinant hGrx1 in Escherichia coli was created by PCR amplification of the hGrx1 cDNA with primers that introduced NcoI and EcoRI sites at the start and stop sites, respectively. The hGrx1 coding sequence was cloned into the overexpression vector pET24d to create pSB100. Site-directed mutagenesis of hGrx1 expression plasmids pCO204 and pSB100 was conducted according to the QuikChange Site-Directed Mutagenesis kit (Stratagene).
Recombinant hGrx1 and hSOD1 purification
For production of recombinant hGrx1, pSB100 was transformed into the Escherichia coli strain BL21-CodonPlus®(DE3)-RIL (Stratagene). The cells were grown in 500 ml of ZYP-5052 autoinduction media at 37 °C for approximately five hours followed by growth overnight at 30 °C. Following centrifugation, the cell pellet was subjected to 3 freeze-thaw cycles and soluble protein was extracted with 20 mM Tris-HCl, pH 8.0, 5 mM dithiothreitol (DTT). Human Grx1 was precipitated with 40–85% ammonium sulfate and the pellet resuspended in 20 mM Tris-HCl pH 8.0 and subsequently loaded onto a desalting column followed by a HiPrep 16/10 DEAE FF column (GE Healthcare) both equilibrated with 50 mM Tris-HCl, pH 7.5, 5 mM DTT. Since human Grx1 does not bind to the DEAE column, the flow-through was collected and concentrated using an Amicon ultrafiltration apparatus. Mutant forms of hGrx1 were purified using the same protocol. The activity of purified hGrx1 was tested using a coupled enzyme assay monitoring reduction of the model substrate 2-hydroxyethyl disulfide (HED) as previously described [30].
WT and ALS mutant forms of recombinant hSOD1 were expressed in S. cerevisiae and purified as previously described [8]. WT, A4V, and G93A protein prepared by this method typically contained ~ 50% Zn and 50% Cu in the Cu binding site and 100% Zn in the Zn binding site. Preparation of apo-SOD1 with the intramolecular disulfide intact was conducted according to published methods [8]. The apo forms prepared by this method typically contained < 0.05 Cu and Zn per monomer as determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES).
Non-reducing SDS-PAGE and immunoblotting techniques
Protein concentrations were determined by the Bradford assay using bovine serum albumin as the standard. Disulfide oxidation of hSOD1 in yeast cells was monitored by non-reducing SDS-PAGE and immunoblotting using iodoacetimide (IAM) or N-ethylmaleimide (NEM) to alkylate free thiols as previously described (also known as a redox western blot) [31–33]. Briefly, yeast strains were grown at 30 °C to mid-log phase in glucose SD medium without shaking. For IAM alkylation, the cells are washed and lysed with glass beads in 600 mM sorbitol, 10 mM HEPES, pH 7.5, 100 mM IAM, containing 1:100 dilution of protease inhibitor cocktail (Sigma). Oxidized and reduced proteins were separated using non-reducing SDS-PAGE by loading 50–100 µg cell extracts on pre-cast 14% Tris-glycine gels (Invitrogen). For NEM alkylation, 5 OD600 units of cells were acid-quenched with trichloroacetic acid (TCA) (15% w/v final concentration) and incubated on ice for 20 minutes. Pellets were resuspended in 1 ml 10% TCA and lysed by glass beads. The supernatant was aspirated off and the pellet was resuspended in 500 µl 1 SDS loading buffer containing 40 mM NEM and incubated on ice for 10 minutes prior to non-reducing SDS-PAGE. We found that NEM and IAM alkylation produced similar results. After electrophoresis, separated proteins were subjected to in-gel reduction prior to transfer to nitrocellulose membranes as previously described [33]. For analysis of hGrx1 levels in yeast cells, glass bead extracts were prepared as described above for hSOD1 in the absence of IAM. Total cell lysate (50 – 100 µg) was separated by reducing SDS-PAGE on 14% Tris-glycine gels (Invitrogen) and transferred to nitrocellulose for western blotting. Human SOD1 was detected using an anti-hSOD1 antibody (1:5000) kindly provided by Valeria Culotta [33], while hGrx1 was detected using an anti-Grx1 antibody (1:10000) from Abcam. For both hGrx1 and hSOD1, a secondary anti-rabbit IRDye 800CW antibody diluted to 1:30000 was used for detection via the Odyssey Infrared Imaging System according to the manufacturer’s instructions (LI-COR, Lincoln, NE).
GRX activity assays in yeast cell extracts
GRX activity is yeast cell lysates was measured using published protocols [22]. Briefly, yeast cell lysates were prepared by glass bead lysis and heated to 85 °C to inactivate glutathione reductase and thioredoxin reductase. GRX activity in the heat-treated lysates was then measured using a coupled enzyme-HED assay.
In vitro hSOD1 reduction assays
An in vitro assay to monitor reduction of hSOD1 by hGrx1 was adapted from previous methods [22]. Briefly, disulfide-oxidized hSOD1 was diluted to 3 µM in a 300-µl reaction mixture that included 100 mM Tris-HCl, pH 8.0, 2.0 mM EDTA, 1 mM GSH, 0.2 mM NADPH, and 6 µg/mL glutathione reductase. Varying concentrations of GSH were also tested from 0 to 5.0 mM GSH. Human Grx1 (WT or mutant form) was added (50 nM final concentration) to the reaction mixture and incubated at 30 °C for different time periods. At each allotted time, 15-µl aliquots are removed from the stock and 15 µl of 2X sample buffer (160 mM Tris-HCl pH 6.8, 20% glycerol, 5% SDS, 1.0 mg bromophenol blue) with 100 mM IAM are added and incubated at 37 °C for 30 minutes prior to non-reducing SDS-PAGE. Reduced and oxidized forms of hSOD1 were analyzed by quantitative immunoblot with anti-hSOD1 antibodies or Coomassie staining using an Odyssey Infrared Imaging System.
Measurement of hSOD1 disulfide redox potential
The redox potential of the disulfide bond of WT and ALS mutant hSOD1 was determined by incubating the disulfide-oxidized protein in buffers poised at defined redox potential values as previously described [34]. Briefly, 10 µg/ml apo, oxidized SOD1 was incubated with mixtures of reduced DTT and the oxidized form (trans-4,5-dihydroxy-1,2-dithiane) at a total concentration of 2 mM in 50 mM MOPS pH 7.0 (purged with nitrogen) in a Coy anaerobic chamber at 30 °C. The samples were incubated for 12-60 hrs in order for the redox state of the protein to reach equilibrium with the buffer. After incubation, 20% TCA was added for a final concentration of 10% and the samples precipitated on ice for 30 minutes under anaerobic conditions. Following centrifugation, the pellet was resuspended in 2X SDS sample buffer with 100 mM IAM. The samples were incubated at room temperature (25 °C) for 30 minutes, diluted to 1X with water, and separated by non-reducing SDS-PAGE. The redox potential values were calculated by fitting the data to the Nernst equation for a two-electron process using non-linear least squares fitting [35].
RESULTS
Expression of human Grx1 correlates with increased reduction of the disulfide bond in WT and ALS mutant human SOD1
To determine whether hGrx1 influences the disulfide bond of hSOD1 in vivo, we used a yeast expression system to manipulate intracellular redox factors by gene deletion and heterologous protein expression. The disulfide-oxidized and reduced forms of hSOD1 have different mobilities by non-reducing SDS-PAGE, allowing efficient separation and quantification of the two forms (Figure 1). In WT yeast cells expressing the yeast copper chaperone for SOD1 (CCS1) and both yeast dithiol glutaredoxins (GRX1 and GRX2), the intramolecular disulfide in WT hSOD1 is primarily oxidized (Figure 1A, lane 2). Deletion of GRX1 and GRX2 has little effect on the redox state of hSOD1 (lane 3). However, in a ccs1Δ strain, the pool of WT hSOD1 is shifted to the reduced form (Figure 1A, lane 4), confirming that yCCS1 plays an important role in controlling the redox state of hSOD1 as previously reported [22, 32] (Fig 1B). In the grx1Δ grx2Δ ccs1Δ triple mutant strain (Fig 1A, lane 5), hSOD1 is slightly more oxidized than in the ccs1Δ strain (lane 2), which is consistent with previous reports that yCCS1 and the dithiol yGrxs play opposing roles in controlling the redox state of hSOD1 [22]. To test whether hGrx1 influences the redox state of hSOD1, grx1Δ grx2Δ ccs1Δ cells were transformed with a low copy plasmid expressing hGrx1. We confirmed that recombinant WT hGrx1 is active in yeast extracts using a standard assay for Grx activity (Figure 2B). Our data show that expression of hGrx1 (Figure 1A, lane 6) correlates with a shift towards the reduced form of WT hSOD1 as demonstrated for yGrx2 [22]. However, this effect is only observed in a ccs1Δ strain since expression of hGrx1 had no effect on the steady-state redox state of hSOD1 in WT and grx1Δ grx2Δ yeast strains (data not shown).
Figure 1. Human Grx1 reduces the disulfide bond of WT and mutant human superoxide dismutase 1 in vivo.
Yeast strains expressing WT or ALS mutant hSOD1 were grown to mid-log phase in SC –Ura – Trp glucose media. Cells were lysed in buffer with 100 mM IAM to trap free thiols. (A) Redox western blot of 50 µg cell extracts separated by non-reducing SDS-PAGE and immunoblotted with anti-hSOD1 antibodies. Conventional western blotting using anti-hGrx1 antibodies (no IAM treatment, reducing SDS-PAGE) is shown below each hSOD1 redox western blot to demonstrate in vivo expression of hGrx1. Lane 1, WT hSOD1 expressed in WT cells treated with 100 mM IAM and 600 mM DTT. Strains used were: WT, CY4; grx1/2Δ, Y117; ccs1Δ, MC108; ccs1Δ grx1/2Δ, MC120. These strains were all co-transformed with empty vector pRS414 and a hSOD1 expression plasmid (pLC1 (WT), pLC2 (A4V), pLC3 (G41D) or pMD100 (G93A). The strain ccs1Δ grx1/2Δ + hGrx1 is MC120 co-transformed with the hGrx1 expression plasmid pCO204 and a hSOD1 expression plasmid (pLC1, pLC2, pLC3 or pMD100). (B) Reduced (red) and oxidized (ox) forms of hSOD1 were quantified using an Odyssey Infrared Imaging System. The average % oxidized hSOD1 is reported for three to four independent experiments with error bars representing the standard deviation.
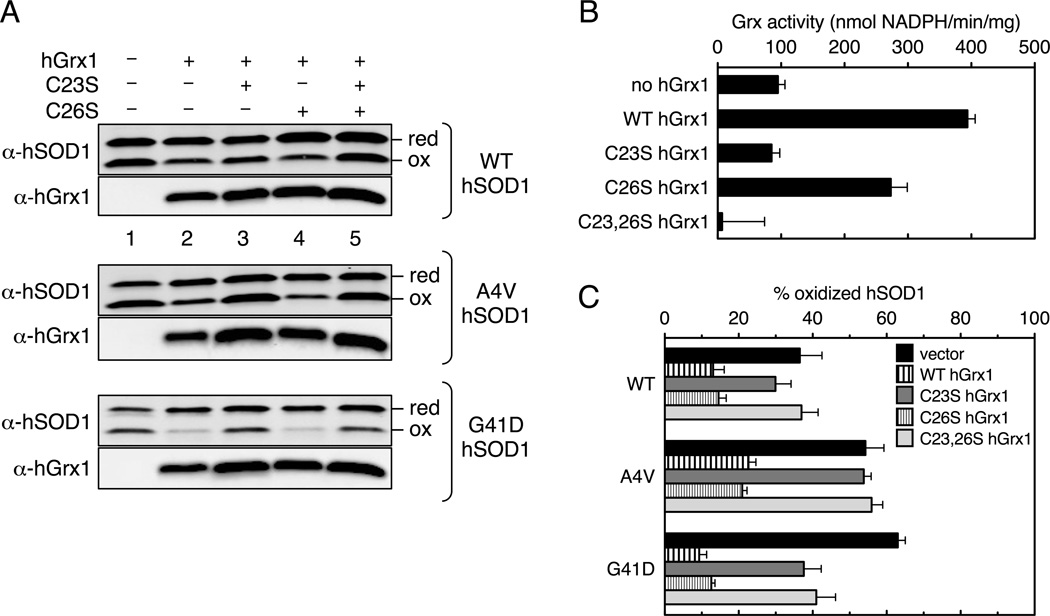
Figure 2. Human Grx1 reduces hSOD1 via a monothiol mechanism in vivo.
Yeast strains expressing WT or ALS mutant hSOD1 and WT or Cys mutants for hGrx1 were grown to mid-log phase in SC –Ura –Trp glucose media. (A) Redox western blotting using anti-hSOD1 antibodies a n d conventional western blotting using anti-hGrx1 antibodies were performed as described in Figure 1. Strains used were ccs1Δ grx1/2Δ (MC120) co-transformed with a hSOD1 expression plasmid (pLC1, pLC2, or pLC3) and a plasmid expressing WT and Cys mutants of hGrx1 (pCO204, pSB101, pSB102, or pSB103) or empty vector pRS414. (B) Glutaredoxin activity assays for MC120 cells expressing the indicated hGrx1 constructs. Activity is reported as nmol NADPH oxidized per min per mg protein in the cell extract. (C) Quantification of redox western blots in A was performed as described in Figure 1. For (B,C), the error bars represent the standard deviation for three independent experiments.
We next tested how hGrx1 expression influenced the disulfide bond in ALS mutant forms of hSOD1. A4V, G41D, and G93A hSOD1 mutants are all enzymatically active “β-barrel mutants,” and A4V and G93A have been shown to produce an ALS phenotype in transgenic rodent models [36–38]. Unlike WT hSOD1, the disulfide of A4V hSOD1 is largely reduced in WT yeast cells (Figure 1A,B). Interestingly, deletion of the dithiol Grxs has an obvious effect on the redox state of this disulfide, shifting it to the oxidized form. In contrast, G41D and G93A hSOD1 are largely oxidized in WT cells and deletion of GRX1/2 has little effect on the redox state of the disulfide. The three mutant forms, A4V, G41D, and G93A hSOD1, display a similar pattern of redox state changes as WT hSOD1 in response to deletion of ccs1 alone and in combination with grx1/2. However, G93A hSOD1 is more reduced under these conditions in comparison to A4V and G41D (Figure 1A, lanes 4,5). Furthermore, expression of hGrx1 in the triple ccs1Δ grx1Δ grx2Δ mutant was found to shift A4V, G41D, and G93A hSOD1 to more reduced states (Figure 1A, lane 6). These data suggest that hGrx1 acts similarly to yGrx2 by promoting reduction of the disulfide in both WT and ALS mutant hSOD1 in the absence of CCS1 activation.
Human Grx1 reduces the disulfide bond of human SOD1 via a monothiol mechanism
After determining that expression of hGrx1 impacts the in vivo redox status of the hSOD1 intramolecular disulfide, we tested whether one or both active site cysteines in hGrx1 were essential for reduction. Dithiol Grxs have two cysteines in the CXXC active site that display different reactivities. Grxs that utilize a dithiol mechanism to reduce intramolecular disulfide bonds require both cysteines, while Grxs that catalyze glutathionylation/deglutathionylation reactions via a monothiol mechanism require only one active site cysteine [39]. Using a variety of model substrates, human Grx1 was shown to favor reduction of glutathione-containing mixed disulfides via a monothiol mechanism [40]. The N-terminal Cys23 is essential for this catalytic function while the C-terminal Cys26 is dispensable [41]. Human Grx1 has no intramolecular disulfide targets identified thus far, and hSOD1 was proposed to be one of those targets [22]. To test whether a dithiol or monothiol mechanism is required for reduction of hSOD1 in vivo, we expressed active site hGrx1 Cys mutants in yeast and monitored the Grx activity and redox state of WT and ALS mutant hSOD1 (Figure 2). The redox state of both WT and ALS mutant hSOD1 is more reduced in strains expressing hGrx1 constructs (WT and C26S) that are active in the enzyme coupled-HED Grx assay (Figure 2A, lanes 2,4). These data indicate that only the N-terminal active site cysteine of hGrx1 (Cys23) is required for reduction of hSOD1, consistent with a monothiol mechanism.
To more directly demonstrate that hGrx1 reduces the disulfide bond of hSOD1 via a monothiol mechanism, we used an in vitro assay to monitor the redox state of purified hSOD1 upon incubation with purified hGrx1 and GSH. Previous studies have shown that yGrx2 specifically reduces the disulfide bond in the apo form of A4V hSOD1, but not holo A4V hSOD1 or apo or holo WT hSOD1 [22]. We have extended these studies to examine the effects of WT and Cys mutants of hGrx1 on WT, A4V, and G93A hSOD1. Recombinant WT and active site mutants of hGrx1 were overexpressed and purified from E. coli and their respective activities verified (Supplemental Figure S1). The assay mixture containing GSH, purified hGrx1, and WT or ALS mutants of hSOD1 was removed at different time points and the hSOD1 disulfide redox state assessed by non-reducing SDS-PAGE (Figure 3A). These results demonstrate that GSH/hGrx1 exhibits no activity towards the Cu/Zn forms of WT and G93A mutant hSOD1 (Figure 3B–D, top), and low activity toward Cu/Zn A4V hSOD1, as previously demonstrated for yeast Grx2 [22]. In contrast, the apo forms of WT, A4V, and G93A all displayed increased reduction by GSH/hGrx1 (Figure 3B–D, bottom) in comparison to the holo forms. In each case we found that WT and C26S hGrx1 exhibited similar reactivity towards the hSOD1 disulfide, while the C23S and C23,26S hGrx1 mutants were similar to the control with no hGrx1 added. Thus, hGrx1 uses a monothiol mechanism involving Cys23 to reduce the disulfide in both WT and ALS mutants of hSOD1.
Figure 3. Reduction of WT and ALS mutant hSOD1 by GSH and WT and Cys mutants of recombinant hGrx1.
An in vitro assay for monitoring reduction of WT and ALS mutant hSOD1 by GSH/hGrx1 was conducted as described in the Experimental section. (A) Purified holo and apo disulfide-oxidized WT hSOD1 incubated in the presence of GSH and WT and Cys mutants of hGrx1. The oxidation state of hSOD1 was monitored by non-reducing SDS-PAGE as described in Figure 1. + DTT = purified hSOD1 incubated with hGrx1 and 600 mM DTT. The control samples include hSOD1 protein incubated in the assay mixture without recombinant hGrx1 added. (B–D) Quantification of reduced and oxidized Cu,Zn (top) and apo (bottom) WT hSOD1 (B), A4V hSOD1 (C), and G93A hSOD1 (D) was performed as described in Figure 1. Error bars represent the standard deviation for three to four independent experiments. Lowercase letters (a–e) are shown next to the plot traces to help identify corresponding data sets shown in the legend.
By comparing the rate of reduction of the apo forms of WT vs. ALS mutant SOD1 (Figure 3B–D, bottom), it is clear that the ALS mutants are reduced at a faster rate than WT hSOD1. These differences are apparent in the first 15 minutes of the assay in which WT hSOD1 is only ~10% reduced, while A4V and G93A are 50% and 65% reduced, respectively. Even with inactive hGrx1 (C23S, C23,26S) and no hGrx1 added (control), the intramolecular disulfide in A4V and G93A hSOD1 is more susceptible to reduction by GSH alone, which is consistent with previous reports [17]. The greater susceptibility of apo mutant hSOD1 to GSH reduction may reflect higher reactivity or greater accessibility of the hSOD1 disulfide to nucleophilic attack by GSH (see Discussion). Here we demonstrate that hGrx1 with a single active site Cys accelerates reduction of hSOD1 in the presence of GSH. To confirm the dependence of disulfide reduction on GSH, the assay for apo G93A hSOD1 was also performed with varying GSH concentrations (Supplemental Figure S2). The results indicate a hyperbolic dependence of hSOD1 reduction on [GSH] as reported for other redox reactions catalyzed by hGrx1 [24, 41].
Measurement of the disulfide bond redox potential for WT and ALS mutant hSOD1
In addition to kinetic factors, the favorability of a redox reaction is also dictated by the reaction thermodynamics [42]. After determining that WT and ALS mutant SOD1 exhibit differential rates of reduction by GSH/hGrx1, we next investigated the thermodynamic stability of the intramolecular disulfide bond of apo and holo WT, A4V, and G93A hSOD1. We measured the redox potential of the disulfide bond by incubating the proteins in redox buffers poised at defined redox potential values under anaerobic conditions [32, 34]. Surprisingly, the redox potential of WT hSOD1 (−301 mV) is virtually identical with and without the metal cofactors as shown in Figure 4 and Table 1. A similar pattern is observed for the apo and holo forms of A4V and G93A hSOD1 (Figure 4B, Table 1). However, we noted that the disulfide redox potentials for the two ALS mutant hSOD1 proteins are significantly different from WT hSOD1, but not similar to each other. The redox potentials of the apo and holo forms of G93A hSOD1 (−315 mV) are lower than WT hSOD1, while the redox potentials of the A4V hSOD1 forms (−282 mV) are higher (Table 1). Thus, the disulfide bond in G93A hSOD1 is more thermodynamically stable than WT hSOD1, while the A4V disulfide is less stable. Since A4V and G93A hSOD1 are both implicated in ALS development, these data suggest that differences in the redox potential of the hSOD1 disulfide may not be a significant factor contributing to the toxicity of hSOD1 mutants. Rather it appears that the more favorable redox kinetics of the mutant forms (i.e. the higher reactivity of the disulfide bond) may be more influential.
Figure 4. Disulfide redox potential measurements for apo and holo WT and ALS mutant hSOD1.
Recombinant, disulfide-oxidized apo and holo WT and ALS mutant hSOD1 were incubated in buffer containing varying concentrations of reduced and oxidized DTT at defined redox potentials as described in the Experimental section. (A) Representative Coomassie-stained non-reducing SDS-PAGE gel for Cu,Zn WT hSOD1 disulfide redox potential measurement. (B) Quantitation of % oxidized WT, A4V, and G41D hSOD1 was determined as described in Figure 1 and plotted against the red-DTT/ox-DTT redox potential E (mV). Open symbols represent data points for the apo proteins and closed symbols represent the holo proteins. The solid lines represent the best fit to the Nernst equation (for a 2 e− reaction) for the holo proteins, while the dotted lines are the best fits for the apo proteins.
Table 1. Disulfide bond redox potential measurements for apo and holo WT, A4V, and G93A hSOD1.
The reported values are the means for two to four independent experiments with +/− representing the standard deviation. The redox potentials were calculated from the Nernst equation with E°DTT = −308 mV [64].
| hSOD1 | apo E° | Cu, Zn E° |
|---|---|---|
| WT | −301 mV +/− 2.9 | −302 mV +/− 3.3 |
| A4V | −282 mV +/− 0.4 | −283 mV +/− 2.2 |
| G93A | −315 mV +/− 4.1 | −318 mV +/− 1.0 |
DISCUSSION
The redox state of the disulfide bond in hSOD1 plays a critical role in the stability and oligomeric state of the protein. Increased levels of apo, disulfide-reduced SOD1 correlates with increased misfolding and aggregation of SOD1 in both in vitro and in vivo studies [8, 17, 18, 20, 22, 43–45]. Thus we sought to identify intracellular factors that influence oxidation/reduction of this disulfide bond. A previous study demonstrated that overexpression of Grx1 in mouse cells lines increases the solubility of ALS mutant hSOD1, presumably via reduction of intermolecular disulfides found within SOD1 aggregates [46]. Although in that case, Cys111 is implicated in formation of these disulfide-linked oligomers rather than the two cysteines that form the intramolecular disulfide bond (Cys57 and Cys146). Here we provide in vivo and in vitro evidence that the SOD1 intramolecular disulfide is a substrate for human Grx1. Our results demonstrate that hGrx1 specifically reduces the intramolecular disulfide bond of WT, A4V, G41D, and G93A ALS mutants in a yeast expression system. For WT, G41D, and G93A hSOD1, this effect requires the absence of CCS1, confirming that CCS1 and Grxs play opposing roles in redox control of the SOD1 disulfide [22]. However, control of the hSOD1 redox state is dominated by CCS1 for these forms since Grx expression has little effect on steady-state levels of the hSOD1 disulfide in the presence of CCS1 in vivo. This result is consistent with our in vitro redox kinetics experiments demonstrating that holo-hSOD1 is more resistant to reduction by hGrx1 than apo-hSOD1. In CCS1+ cells, a higher percentage of hSOD1 is Cu-loaded [32, 47], since CCS1 catalyzes both Cu insertion and disulfide bond formation [48]. Thus, a larger pool of holo-hSOD1 will be kinetically inert to reduction by GSH/hGrx1 in CCS1+ cells. However, we do note that A4V hSOD1 is predominantly reduced in the presence of CCS1 and the dithiol Grxs, suggesting that the dithiol Grxs can successfully compete with CCS1 in controlling the A4V hSOD1 redox state in vivo. Interestingly, this mutant is the only one tested that showed some reactivity with hGrx1 in the holo form in vitro (Figure 4C), providing a possible explanation for its more reduced state in vivo, even in the presence of Cu-loading by CCS1. In addition, the in vivo redox state differences between WT hSOD1 and the various ALS mutants tested likely also reflect differences in the ability of CCS1 to associate and facilitate copper insertion and disulfide formation in the variant forms of hSOD1 [9].
Interestingly, we find that hGrx1 has similar apparent effects on the steady-state redox status of both WT and mutant hSOD1 in vivo, since all four hSOD1 forms tested are shifted to a more reduced state with the addition of hGrx1 in ccs1Δ grx1/2Δ cells (Figure 1). However, the in vitro redox kinetic assays clearly demonstrate that GSH/hGrx1 reduces WT hSOD1 at a much slower rate than the ALS mutant forms (Figure 3). These seemingly conflicting results may be explained by that fact that the in vivo redox state of hSOD1 captured by the thiol-trapping method is a snapshot of the steady-state redox status of the protein. In addition to the rate of disulfide reduction, a number of other factors may influence the steady state redox status of hSOD1. These include the rate of CCS1 oxidation and Cu insertion, the rate of metal loss from the active sites, and the rate of protein turnover for oxidized vs. reduced forms. Previous studies have demonstrated that A4V and G41D hSOD1 proteins are more susceptible to degradation when expressed in the yeast model system than WT hSOD1 [22]. In particular, the reduced form of A4V hSOD1 is more rapidly degraded than the oxidized form, while both the oxidized and reduced forms of WT hSOD1 exhibit much slower turnover rates in vivo [22]. Thus, the pool of reduced mutant hSOD1 generated via GSH/hGrx1 reduction or deficient CCS1-catalyzed maturation may be rapidly degraded in vivo and thus underrepresented in the steady-state redox state measurements. Nevertheless, these results clearly demonstrate that GSH and hGrx1 are additional intracellular factors that have a significant impact on the disulfide redox state of hSOD1 in vivo.
As mentioned above, the in vitro studies strongly indicate that the ALS mutant forms of hSOD1 are more susceptible to reduction by the GSH/hGrx1 system than WT hSOD1, and furthermore that loss of the metal cofactors greatly accelerates this reaction. In general, Grx proteins catalyze thiol-disulfide exchange reactions that utilize one or both cysteines in the active site CXXC motif. These reactions can proceed via a dithiol mechanism, which reduces intramolecular disulfides, or a monothiol mechanism reducing a mixed disulfide bond with GSH [39]. Our results indicate that hGrx1 reduces the disulfide bond of hSOD1 via a monothiol mechanism that only requires the N-terminal active site cysteine of hGrx1. Thus, reduction of the hSOD1 disulfide first proceeds via attack of the hSOD1 disulfide by GSH followed by deglutathionylation catalyzed by hGrx1 (Scheme 1). We note that GSH alone can also facilitate complete reduction of apo A4V and G93A hSOD1 (Figure 3C,D). In the absence of hGrx1, glutathionylated hSOD1 may be fully reduced via reaction with a second GSH molecule, or the hSOD1 disulfide may re-form via nucleophilic attack by the free cysteine in hSOD1. Since reduction of hSOD1 is faster in the presence of hGrx1, our data suggest that hGrx1 is more efficient at reducing the hSOD1-SSG mixed disulfide than GSH itself. Glutathionylation of the hSOD1 disulfide prior to reduction by GSH or hGrx1 is likely a transient modification since a stable GSH adduct with either of the disulfide cysteines (Cys57 and Cys146) has not been detected in vivo [22, 49]. However, we do note that Cys111, which is solvent exposed in WT and mutant holo hSOD1, has been shown to form stable GSH mixed disulfides [49]. In addition, all four cysteines in hSOD1 have been implicated in formation of intermolecular disulfide bonds in higher order hSOD1 aggregates [11, 37, 45, 46, 50, 51].
Scheme 1.
Monothiol mechanism for reduction of hSOD1 by hGrx1 and GSH
The accessibility of the disulfide bond may be a significant factor controlling the differences in disulfide reactivity of apo vs. holo, WT vs. ALS mutant hSOD1. Both disulfide formation and metal insertion drive dimerization of hSOD1 [6–8, 52]. Once formed, the disulfide bond in WT hSOD1 is partially buried near the dimer interface [43]. In the case of the holo proteins, only A4V hSOD1 showed some reactivity with GSH/hGrx1 in the experimental time frame. This mutation is located near the dimer interface and is thus suspected to weaken the dimer interaction [38, 53]. Increased monomerization will likely expose the disulfide to attack by GSH and subsequent deglutathionylation by hGrx1. In addition, A4V hSOD1 has a 30-fold lower Zn(II) affinity that WT hSOD1 and is thus more prone to demetallation [54, 55]. Loss of zinc binding is another factor that promotes monomerization and has been shown to increase the lability of the disulfide bond [56]. Thus it is not surprising that all three apo proteins tested showed some reactivity towards GSH alone that was accelerated with the addition of hGrx1. For WT hSOD1, removal of the metals loosens the structure by increasing the mobility of the electrostatic and zinc-binding loops [57, 58], but does not disrupt dimer formation if the disulfide is intact [6, 8]. This greater structural flexibility may allow limited access to the disulfide located near the dimer interface. However, unlike apo, oxidized WT hSOD1, apo, oxidized A4V and G93A ALS mutants are prone to monomerization [38], which may explain the even greater reactivity of the disulfide bond in these demetallated, mutant forms.
In addition to cysteine reactivity, another important factor that influences thiol-disulfide redox regulation is thermodynamic stability. How does the thermodynamic stability of the hSOD1 disulfide differ for apo and holo forms of WT and ALS mutant hSOD1? To answer this question, we measured the midpoint redox potentials for apo and holo forms of recombinant WT, A4V and G93A hSOD1. We found that apo and holo WT hSOD1 had virtually identical redox potentials (−301 and −302 mV), demonstrating that the metallation state of hSOD1 does not influence the redox potential. Interestingly, this value is significantly lower than the reported value for WT hSOD1 purified from E. coli (−248 mV) [32], indicating higher thermodynamic stability for the disulfide in yeast-purified hSOD1. There is one notable structural difference between hSOD1 purified from yeast vs. E. coli that may account for these different potentials: recombinant hSOD1 expressed in yeast is acetylated at the N-terminus similar to native hSOD1, while hSOD1 expressed in E. coli is not [59]. Although the acetylation site is ~ 20 Å from the disulfide bond, it is possible that this modification has a subtle effect on the protein structure that significantly influences the redox potential; for example, by altering the local electrostatic environment of the disulfide, reducing strain within the disulfide bond, or reducing strain within the overall protein structure. In addition, the N-terminus is located at the dimer interface and thus N-terminal acetylation may impact the quaternary structure, which in turn may affect the strength of the disulfide bond.
Similar to WT hSOD1, our results also demonstrate that the metallation state of ALS mutants does not significantly alter the redox potential for these proteins. However, the measured redox potentials for A4V hSOD1 (−282 mV) and G93A hSOD1 (−315 mV) were higher and lower than WT hSOD1, respectively (Table 1). Thus, the presence of ALS mutations in hSOD1 does not uniformly destabilize the disulfide bond. Our results investigating the kinetic and thermodynamic properties of the disulfide bond in WT and ALS mutant hSOD1 parallel recent studies comparing the thermodynamic stability and folding kinetics of the overall protein structure. Not all ALS mutants are more unstable than WT hSOD1 in the apo state, since some mutants exhibit similar or even higher thermodynamic stability than WT hSOD1 [60]. However, the folding kinetics for ALS mutants are consistently slower than WT hSOD1 [61]. A similar pattern emerges for the redox state of the disulfide bond. We find that the thermodynamic stability of the disulfide in ALS mutants is not consistently lower that WT hSOD1. However, the reduction rate of the disulfide is consistently faster for ALS mutants. Although we have only tested two ALS mutants in this study, our results are in line with previous reports demonstrating that a large variety of ALS mutants are more susceptible to disulfide reduction by GSH than WT hSOD1 [17]. We also demonstrate that hGrx1 plays a significant role in accelerating this reduction via a monothiol catalytic mechanism. Overall, these results suggest that the reaction kinetics between hSOD1 and GSH/Grxs may be a critical factor influencing the pathogenicity of ALS mutant hSOD1. However, in order for the reduction of hSOD1 to proceed via the GSH/Grx system in vivo, the reaction must be thermodynamically favorable. In vivo redox measurements in yeast and mammalian cells confirm that the redox potential of the cytosolic GSH:GSSG pool (−290 to −300 mV) [31, 62, 63] is very similar to the hSOD1 disulfide redox potential, and thus poised to facilitate at least partial reduction of both WT and ALS mutant hSOD1 under physiological conditions.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Valeria Culotta of the Johns Hopkins University Bloomberg School of Public Health (Baltimore, MD) for the hSOD1 antibody as well as helpful discussions and critical reading of the manuscript.
FUNDING
This work was supported by the National Institutes of Health [grant numbers K22 ES013780 and R01 GM086619 to C.E.O., and R01 NS039112 to P.J.H] and the Judith and Jean Pape Adams Charitable Foundation (to P.J.H.).
ABBREVIATIONS USED
- hSOD1
Cu,Zn human superoxide dismutase
- ALS
amyotrophic lateral sclerosis
- CCS1
copper chaperone for SOD1
- Grx
glutaredoxin
- HED
2-hydroxyethyl disulfide
- IAM
iodoacetimide
- NEM
N-ethylmaleimide
- TCA
trichloroacetic acid
- DTT
dithiothreitol
Footnotes
AUTHOR CONTRIBUTION
Samantha Bouldin and Maxwell Darch both designed the research and performed the experimental work. Samantha Bouldin wrote the first draft of the paper and Maxwell Darch worked on subsequent drafts. P. John Hart provided the purified hSOD1 proteins and edited the manuscript. Caryn Outten supervised the research, analyzed the data, and prepared the manuscript.
REFERENCES
- 1.Culotta VC, Yang M, O'Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim. Biophys. Acta. 2006;1763:747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furukawa Y, O'Halloran TV. Posttranslational modifications in Cu,Zn-superoxide dismutase and mutations associated with amyotrophic lateral sclerosis. Antioxid. Redox Signal. 2006;8:847–867. doi: 10.1089/ars.2006.8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J. Biol. Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 4.Brown NM, Torres AS, Doan PE, O'Halloran TV. Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu,Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA. 2004;101:5518–5523. doi: 10.1073/pnas.0401175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leitch JM, Yick PJ, Culotta VC. The right to choose: multiple pathways for activating copper,zinc superoxide dismutase. J. Biol. Chem. 2009;284:24679–24683. doi: 10.1074/jbc.R109.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnesano F, Banci L, Bertini I, Martinelli M, Furukawa Y, O'Halloran TV. The unusually stable quaternary structure of human Cu,Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J. Biol. Chem. 2004;279:47998–48003. doi: 10.1074/jbc.M406021200. [DOI] [PubMed] [Google Scholar]
- 7.Lindberg MJ, Normark J, Holmgren A, Oliveberg M. Folding of human superoxide dismutase: disulfide reduction prevents dimerization and produces marginally stable monomers. Proc. Natl. Acad. Sci. USA. 2004;101:15893–15898. doi: 10.1073/pnas.0403979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doucette PA, Whitson LJ, Cao X, Schirf V, Demeler B, Valentine JS, Hansen JC, Hart PJ. Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability. J. Biol. Chem. 2004;279:54558–54566. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 9.Seetharaman SV, Prudencio M, Karch C, Holloway SP, Borchelt DR, Hart PJ. Immature copper-zinc superoxide dismutase and familial amyotrophic lateral sclerosis. Exp. Biol. Med. 2009;234:1140–1154. doi: 10.3181/0903-MR-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 2000;97:12571–12576. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, Lukas T, Dal Canto MC, O'Halloran TV, Siddique T. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc. Natl. Acad. Sci. USA. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karch CM, Borchelt DR. A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. J. Biol. Chem. 2008;283:13528–13537. doi: 10.1074/jbc.M800564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR. Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum. Mol. Genet. 2003;12:2753–2764. doi: 10.1093/hmg/ddg312. [DOI] [PubMed] [Google Scholar]
- 14.Shinder GA, Lacourse MC, Minotti S, Durham HD. Mutant Cu/Zn-superoxide dismutase proteins have altered solubility and interact with heat shock/stress proteins in models of amyotrophic lateral sclerosis. J. Biol. Chem. 2001;276:12791–12796. doi: 10.1074/jbc.M010759200. [DOI] [PubMed] [Google Scholar]
- 15.Tiwari A, Liba A, Sohn SH, Seetharaman SV, Bilsel O, Matthews CR, Hart PJ, Valentine JS, Hayward LJ. Metal deficiency increases aberrant hydrophobicity of mutant superoxide dismutases that cause amyotrophic lateral sclerosis. J. Biol. Chem. 2009;284:27746–27758. doi: 10.1074/jbc.M109.043729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwari A, Xu Z, Hayward LJ. Aberrantly increased hydrophobicity shared by mutants of Cu,Zn-superoxide dismutase in familial amyotrophic lateral sclerosis. J. Biol. Chem. 2005;280:29771–29779. doi: 10.1074/jbc.M504039200. [DOI] [PubMed] [Google Scholar]
- 17.Tiwari A, Hayward LJ. Familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase are susceptible to disulfide reduction. J. Biol. Chem. 2003;278:5984–5992. doi: 10.1074/jbc.M210419200. [DOI] [PubMed] [Google Scholar]
- 18.Kayatekin C, Zitzewitz JA, Matthews CR. Disulfide-reduced ALS variants of Cu Zn superoxide dismutase exhibit increased populations of unfolded species. J.Mol. Biol. 2010;398:320–331. doi: 10.1016/j.jmb.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khare SD, Ding F, Dokholyan NV. Folding of Cu Zn superoxide dismutase and familial amyotrophic lateral sclerosis. J. Mol. Biol. 2003;334:515–525. doi: 10.1016/j.jmb.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa Y, O'Halloran TV. Amyotrophic lateral sclerosis mutations have the greatest destabilizing effect on the apo- and reduced form of SOD1, leading to unfolding and oxidative aggregation. J. Biol. Chem. 2005;280:17266–17274. doi: 10.1074/jbc.M500482200. [DOI] [PubMed] [Google Scholar]
- 21.Elam JS, Taylor AB, Strange R, Antonyuk S, Doucette PA, Rodriguez JA, Hasnain SS, Hayward LJ, Valentine JS, Yeates TO, Hart PJ. Amyloid-like filaments and water-filled nanotubes formed by SOD1 mutant proteins linked to familial ALS. Nat. Struct. Biol. 2003;10:461–467. doi: 10.1038/nsb935. [DOI] [PubMed] [Google Scholar]
- 22.Carroll MC, Outten CE, Proescher JB, Rosenfeld L, Watson WH, Whitson LJ, Hart PJ, Jensen LT, Cizewski Culotta V. The effects of glutaredoxin and copper activation pathways on the disulfide and stability of Cu,Zn superoxide dismutase. J. Biol. Chem. 2006;281:28648–28656. doi: 10.1074/jbc.M600138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Discola KF, de Oliveira MA, Rosa Cussiol JR, Monteiro G, Barcena JA, Porras P, Padilla CA, Guimaraes BG, Netto LE. Structural aspects of the distinct biochemical properties of glutaredoxin 1 and glutaredoxin 2 from Saccharomyces cerevisiae. J. Mol. Biol. 2009;385:889–901. doi: 10.1016/j.jmb.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 24.Gallogly MM, Starke DW, Mieyal JJ. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid. Redox Signal. 2009;11:1059–1081. doi: 10.1089/ars.2008.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pai HV, Starke DW, Lesnefsky EJ, Hoppel CL, Mieyal JJ. What is the functional significance of the unique location of glutaredoxin 1 (GRx1) in the intermembrane space of mitochondria? Antioxid. Redox Signal. 2007;9:2027–2033. doi: 10.1089/ars.2007.1642. [DOI] [PubMed] [Google Scholar]
- 26.Kawamata H, Manfredi G. Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria. Hum. Mol. Genet. 2008;17:3303–3317. doi: 10.1093/hmg/ddn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corson LB, Strain JJ, Culotta VC, Cleveland DW. Chaperone-facilitated copper binding is a property common to several classes of familial amyotrophic lateral sclerosis-linked superoxide dismutase mutants. Proc. Natl. Acad. Sci. USA. 1998;95:6361–6366. doi: 10.1073/pnas.95.11.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen LT, Culotta VC. Activation of CuZn superoxide dismutases from Caenorhabditis elegans does not require the copper chaperone CCS. J. Biol. Chem. 2005;280:41373–41379. doi: 10.1074/jbc.M509142200. [DOI] [PubMed] [Google Scholar]
- 29.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone CCS localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 30.Holmgren A, Aslund F. Glutaredoxin. Methods Enzymol. 1995;252:283–292. doi: 10.1016/0076-6879(95)52031-7. [DOI] [PubMed] [Google Scholar]
- 31.Hu J, Dong L, Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J. Biol. Chem. 2008;283:29126–29134. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leitch JM, Jensen LT, Bouldin SD, Outten CE, Hart PJ, Culotta VC. Activation of Cu,Zn-superoxide dismutase in the absence of oxygen and the copper chaperone CCS. J. Biol. Chem. 2009;284:21863–21871. doi: 10.1074/jbc.M109.000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proescher JB, Son M, Elliott JL, Culotta VC. Biological effects of CCS in the absence of SOD1 enzyme activation: implications for disease in a mouse model for ALS. Hum. Mol. Genet. 2008;17:1728–1737. doi: 10.1093/hmg/ddn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason JT, Kim SK, Knaff DB, Wood MJ. Thermodynamic basis for redox regulation of the Yap1 signal transduction pathway. Biochemistry. 2006;45:13409–13417. doi: 10.1021/bi061136y. [DOI] [PubMed] [Google Scholar]
- 35.Brown AM. A step-by-step guide to non-linear regression analysis of experimental data using a Microsoft Excel spreadsheet. Comput. Meth. Prog. Bio. 2001;65:191–200. doi: 10.1016/s0169-2607(00)00124-3. [DOI] [PubMed] [Google Scholar]
- 36.Borchelt DR, Lee MK, Slunt HS, Guarnieri M, Xu ZS, Wong PC, Brown RH, Jr, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. USA. 1994;91:8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furukawa Y, Fu R, Deng HX, Siddique T, O'Halloran TV. Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu Zn-superoxide dismutase aggregates in spinal cords of model mice. Proc. Natl. Acad. Sci. USA. 2006;103:7148–7153. doi: 10.1073/pnas.0602048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galaleldeen A, Strange RW, Whitson LJ, Antonyuk SV, Narayana N, Taylor AB, Schuermann JP, Holloway SP, Hasnain SS, Hart PJ. Structural and biophysical properties of metal-free pathogenic SOD1 mutants A4V and G93A. Arch. Biochem. Biophys. 2009;492:40–47. doi: 10.1016/j.abb.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid. Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 40.Gravina SA, Mieyal JJ. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry. 1993;32:3368–3376. doi: 10.1021/bi00064a021. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Jao S, Nanduri S, Starke DW, Mieyal JJ, Qin J. Reactivity of the human thioltransferase (glutaredoxin) C7S, C25S, C78S, C82S mutant and NMR solution structure of its glutathionyl mixed disulfide intermediate reflect catalytic specificity. Biochemistry. 1998;37:17145–17156. doi: 10.1021/bi9806504. [DOI] [PubMed] [Google Scholar]
- 42.Jensen KS, Hansen RE, Winther JR. Kinetic and thermodynamic aspects of cellular thiol-disulfide redox regulation. Antioxid. Redox Signal. 2009;11:1047–1058. doi: 10.1089/ars.2008.2297. [DOI] [PubMed] [Google Scholar]
- 43.Banci L, Bertini I, Cantini F, D'Amelio N, Gaggelli E. Human SOD1 before harboring the catalytic metal: solution structure of copper-depleted, disulfide-reduced form. J. Biol. Chem. 2006;281:2333–2337. doi: 10.1074/jbc.M506497200. [DOI] [PubMed] [Google Scholar]
- 44.Jonsson PA, Graffmo KS, Andersen PM, Brannstrom T, Lindberg M, Oliveberg M, Marklund SL. Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129:451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 45.Niwa J, Yamada S, Ishigaki S, Sone J, Takahashi M, Katsuno M, Tanaka F, Doyu M, Sobue G. Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1. J. Biol. Chem. 2007;282:28087–28095. doi: 10.1074/jbc.M704465200. [DOI] [PubMed] [Google Scholar]
- 46.Cozzolino M, Amori I, Pesaresi MG, Ferri A, Nencini M, Carri MT. Cysteine 111 affects aggregation and cytotoxicity of mutant Cu,Zn-superoxide dismutase associated with familial amyotrophic lateral sclerosis. J. Biol. Chem. 2008;283:866–874. doi: 10.1074/jbc.M705657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll MC, Girouard JB, Ulloa JL, Subramaniam JR, Wong PC, Valentine JS, Culotta VC. Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc. Natl. Acad. Sci. USA. 2004;101:5964–5969. doi: 10.1073/pnas.0308298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furukawa Y, Torres AS, O'Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. EMBO. J. 2004;23:2872–2881. doi: 10.1038/sj.emboj.7600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilcox KC, Zhou L, Jordon JK, Huang Y, Yu Y, Redler RL, Chen X, Caplow M, Dokholyan NV. Modifications of superoxide dismutase (SOD1) in human erythrocytes: a possible role in amyotrophic lateral sclerosis. J. Biol. Chem. 2009;284:13940–13947. doi: 10.1074/jbc.M809687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banci L, Bertini I, Durazo A, Girotto S, Gralla EB, Martinelli M, Valentine JS, Vieru M, Whitelegge JP. Metal-free superoxide dismutase forms soluble oligomers under physiological conditions: a possible general mechanism for familial ALS. Proc. Natl. Acad. Sci. USA. 2007;104:11263–11267. doi: 10.1073/pnas.0704307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banci L, Bertini I, Boca M, Girotto S, Martinelli M, Valentine JS, Vieru M. SOD1 and amyotrophic lateral sclerosis: mutations and oligomerization. PLoS One. 2008;3:e1677. doi: 10.1371/journal.pone.0001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hornberg A, Logan DT, Marklund SL, Oliveberg M. The coupling between disulphide status, metallation and dimer interface strength in Cu/Zn superoxide dismutase. J. Mol. Biol. 2007;365:333–342. doi: 10.1016/j.jmb.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 53.Hough MA, Grossmann JG, Antonyuk SV, Strange RW, Doucette PA, Rodriguez JA, Whitson LJ, Hart PJ, Hayward LJ, Valentine JS, Hasnain SS. Dimer destabilization in superoxide dismutase may result in disease-causing properties: structures of motor neuron disease mutants. Proc. Natl. Acad. Sci. USA. 2004;101:5976–5981. doi: 10.1073/pnas.0305143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crow JP, Sampson JB, Zhuang Y, Thompson JA, Beckman JS. Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J. Neurochem. 1997;69:1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- 55.Lyons TJ, Liu H, Goto JJ, Nersissian A, Roe JA, Graden JA, Cafe C, Ellerby LM, Bredesen DE, Gralla EB, Valentine JS. Mutations in copper-zinc superoxide dismutase that cause amyotrophic lateral sclerosis alter the zinc binding site and the redox behavior of the protein. Proc. Natl. Acad. Sci. USA. 1996;93:12240–12244. doi: 10.1073/pnas.93.22.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts BR, Tainer JA, Getzoff ED, Malencik DA, Anderson SR, Bomben VC, Meyers KR, Karplus PA, Beckman JS. Structural characterization of zinc-deficient human superoxide dismutase and implications for ALS. J. Mol. Biol. 2007;373:877–890. doi: 10.1016/j.jmb.2007.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banci L, Bertini I, Cramaro F, Del Conte R, Viezzoli MS. Solution structure of Apo Cu,Zn superoxide dismutase: role of metal ions in protein folding. Biochemistry. 2003;42:9543–9553. doi: 10.1021/bi034324m. [DOI] [PubMed] [Google Scholar]
- 58.Strange RW, Antonyuk S, Hough MA, Doucette PA, Rodriguez JA, Hart PJ, Hayward LJ, Valentine JS, Hasnain SS. The structure of holo and metal-deficient wild-type human Cu Zn superoxide dismutase and its relevance to familial amyotrophic lateral sclerosis. J. Mol. Biol. 2003;328:877–891. doi: 10.1016/s0022-2836(03)00355-3. [DOI] [PubMed] [Google Scholar]
- 59.Hallewell RA, Mills R, Tekampolson P, Blacher R, Rosenberg S, Otting F, Masiarz FR, Scandella CJ. Amino terminal acetylation of authentic human Cu,Zn superoxide-dismutase produced in yeast. Bio-Technol. 1987;5:363–366. [Google Scholar]
- 60.Rodriguez JA, Shaw BF, Durazo A, Sohn SH, Doucette PA, Nersissian AM, Faull KF, Eggers DK, Tiwari A, Hayward LJ, Valentine JS. Destabilization of apoprotein is insufficient to explain Cu,Zn-superoxide dismutase-linked ALS pathogenesis. Proc. Natl. Acad. Sci. USA. 2005;102:10516–10521. doi: 10.1073/pnas.0502515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruns CK, Kopito RR. Impaired post-translational folding of familial ALS-linked Cu Zn superoxide dismutase mutants. EMBO J. 2007;26:855–866. doi: 10.1038/sj.emboj.7601528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Østergaard H, Tachibana C, Winther JR. Monitoring disulfide bond formation in the eukaryotic cytosol. J. Cell Biol. 2004;166:337–345. doi: 10.1083/jcb.200402120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- 64.Rothwarf DM, Scheraga HA. Equilibrium and kinetic constants for the thiol-disulfide interchange reaction between glutathione and dithiothreitol. Proc. Natl. Acad. Sci. USA. 1992;89:7944–7948. doi: 10.1073/pnas.89.17.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.