Abstract
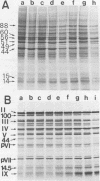
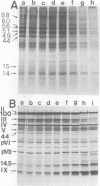
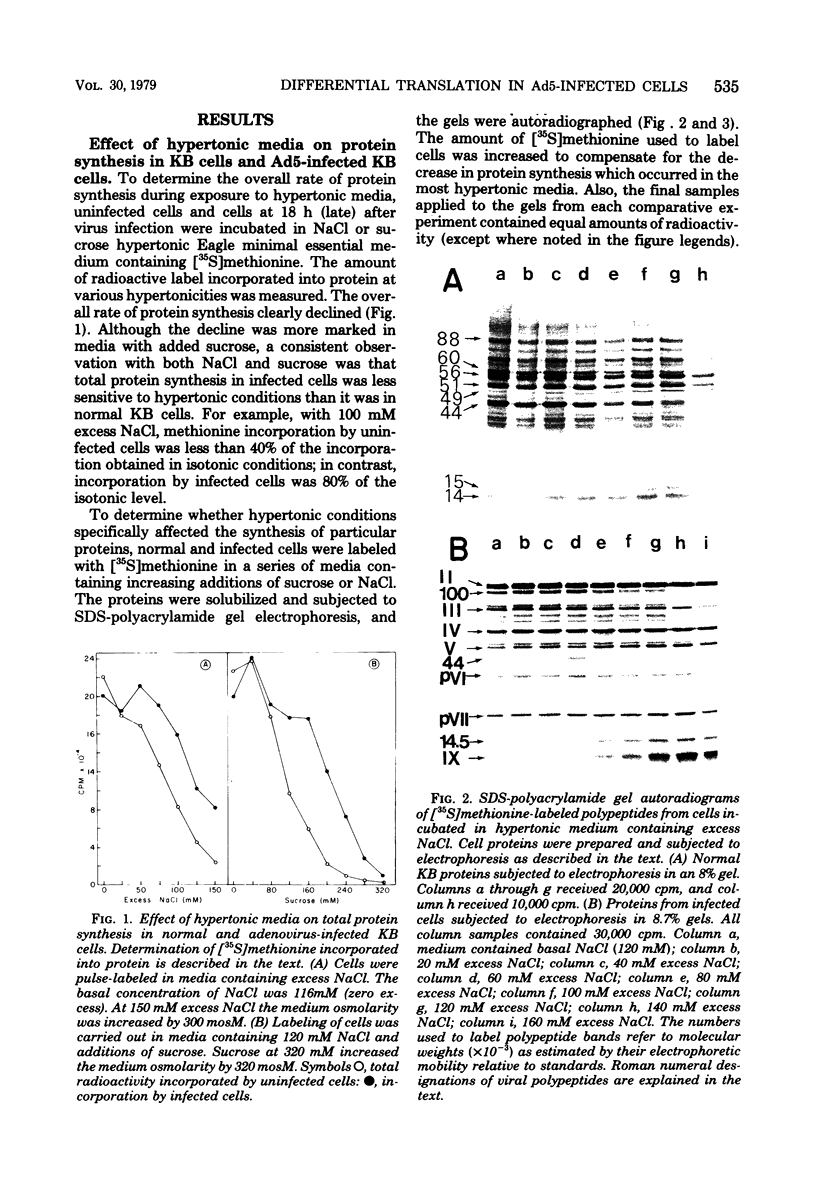
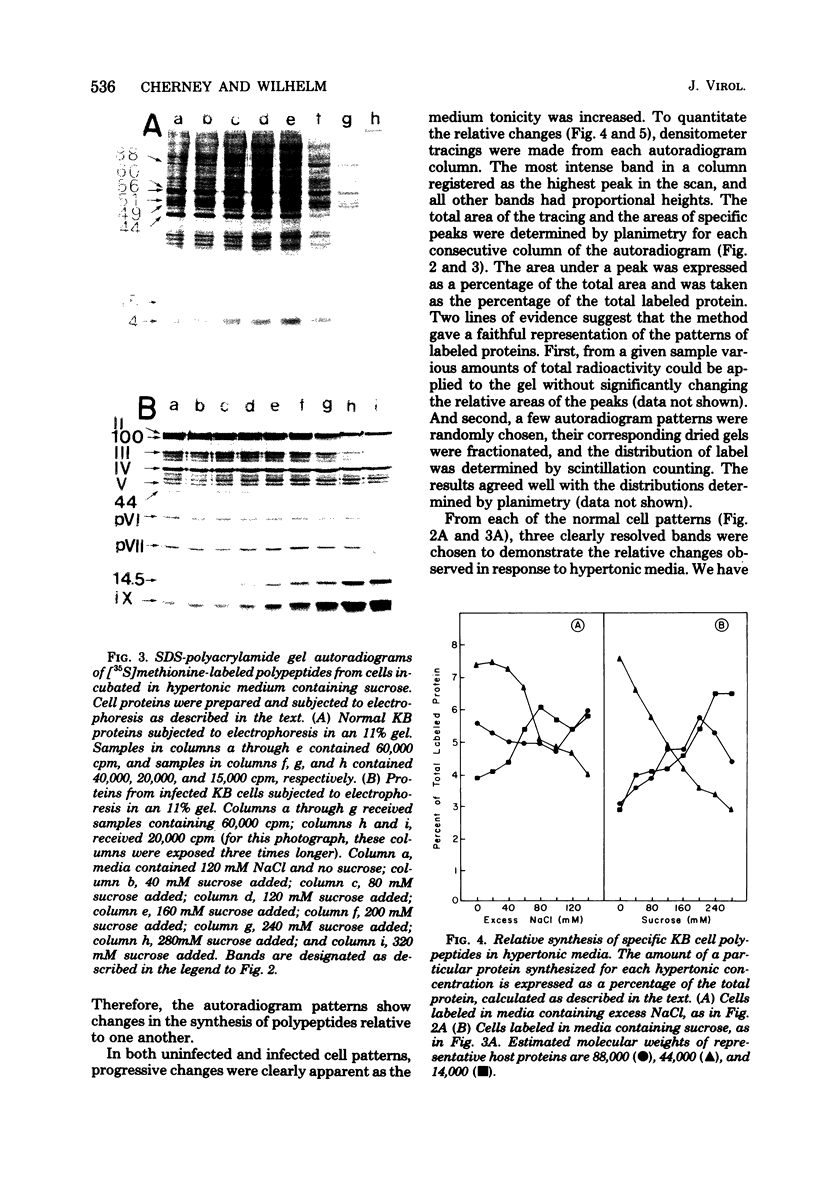
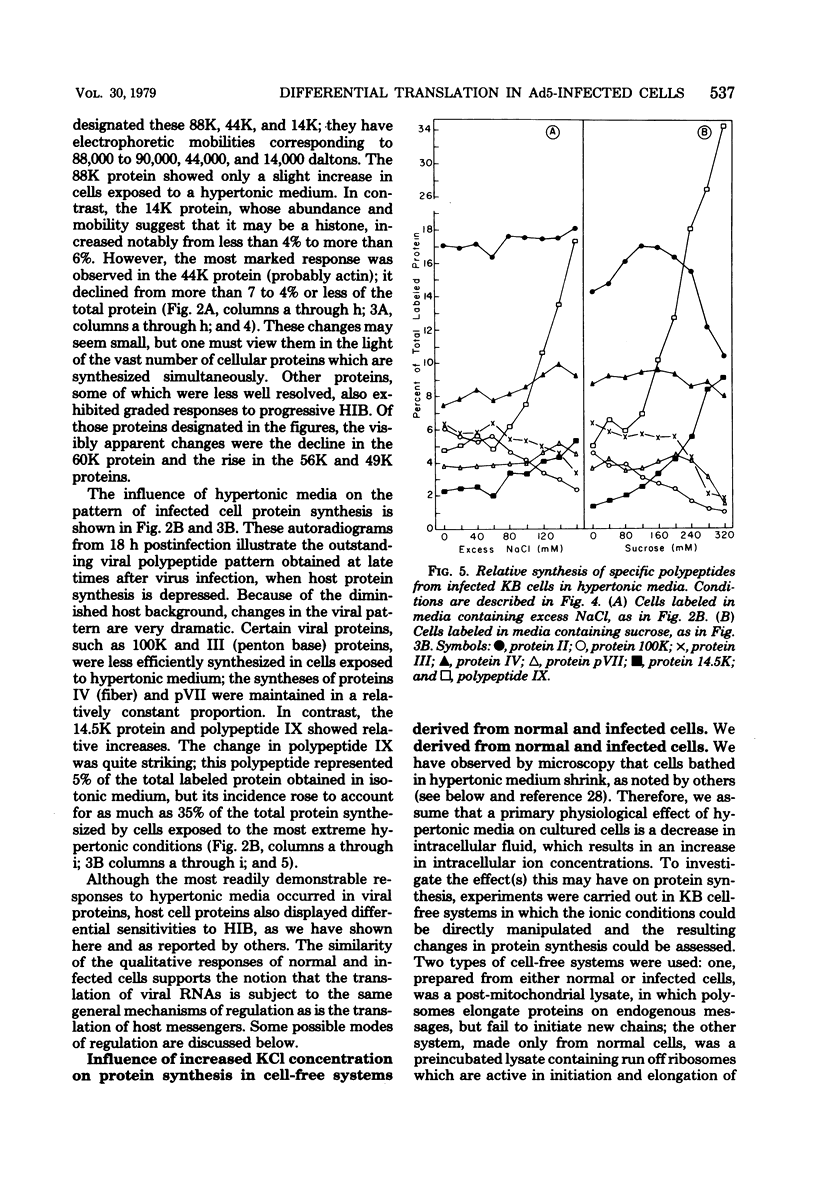
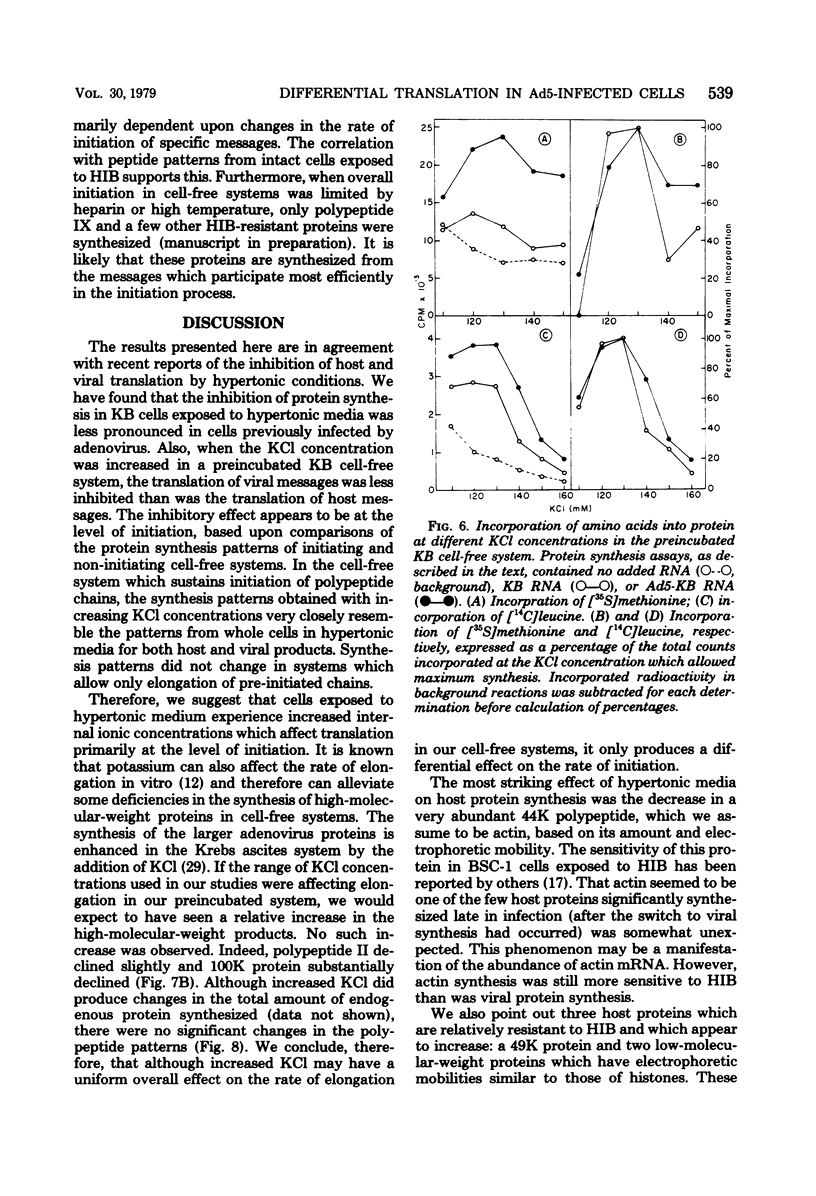
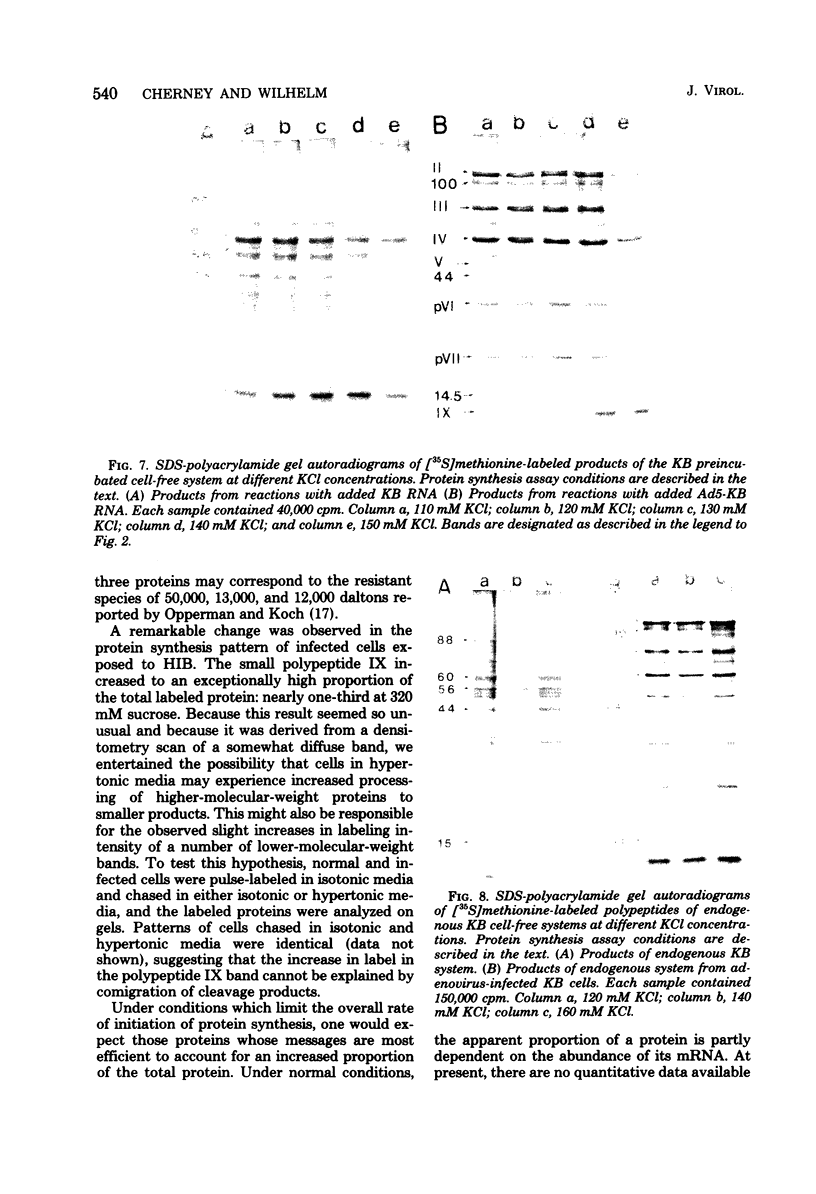
When uninfected or adenovirus 5-infected KB cells are exposed to hypertonic medium, the incorporation of radioactive amino acids into protein decreases in both, but more severely in the uninfected cells. Although the effect of hypertonic medium on the synthesis of specific polypeptides varies, the translation of viral polypeptides as a class is less inhibited. The same patterns of proteins are synthesized regardless of the solute used in the hypertonic medium. The mechanism by which hypertonic conditions exert their effect on whole cells was investigated in K cell-free systems. It was possible to simulate the differential patterns of protein synthesis obtained in whole cells in hypertonic medium by increasing ion concentrations in cell-free extracts which are capable of initiating polypeptide chains on exogenous templates. However, in cell lysates which only elongate proteins, the same patterns were not obtained. Certain host and viral polypeptides displayed striking responses to increased ionic conditions in whole cells and cell-free systems. The synthesis of a host 44K protein, actin, appeared to be most sensitive; lower-molecular-weight proteins were fairly resistant. Among the viral proteins, the synthesis of 100K was inhibited, but most notable was the marked resistance of the synthesis of polypeptide IX. Possible mechanisms for differential synthesis and their significance are considered.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bello L. J., Ginsberg H. S. Relationship between deoxyribonucleic acid-like ribonucleic acid synthesis and inhibition of host protein synthesis in type 5 adenovirus-infected KB cells. J Virol. 1969 Feb;3(2):106–113. doi: 10.1128/jvi.3.2.106-113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L., Smith A. E. Sodium ions and the shut-off of host cell protein synthesis by picornaviruses. Nature. 1976 Dec 23;264(5588):807–809. doi: 10.1038/264807a0. [DOI] [PubMed] [Google Scholar]
- Carrasco L. The inhibition of cell functions after viral infection. A proposed general mechanism. FEBS Lett. 1977 Apr 1;76(1):11–15. doi: 10.1016/0014-5793(77)80110-5. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Sharp P. A. Adenovirus transcription. V. Quantitation of viral RNA sequences in adenovirus 2-infected and transformed cells. J Mol Biol. 1976 Sep 25;106(3):749–774. doi: 10.1016/0022-2836(76)90263-1. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Koch G., Oppermann H., Bilello P., Koch F., Nuss D. Control of peptide chain initiation in uninfected and virus infected cells by membrane mediated events. Hamatol Bluttransfus. 1976;19:541–555. doi: 10.1007/978-3-642-87524-3_51. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Lodish H. F. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974 Oct 4;251(5474):385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Osborn M. The rate of polypeptide chain elongation in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1974 Mar 8;340(2):147–152. doi: 10.1016/0005-2787(74)90107-5. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Koch G. Differential inhibition of vesicular stomatitis virus polypeptide synthesis by hypertonic initiation block. J Virol. 1975 Jan;17(1):283–286. doi: 10.1128/jvi.17.1.283-286.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Koch G. Variation in the relative synthesis of immunoglobulin G and non-immunoglobulin G proteins in cultured MPC-11 cells with changes in the overall rate of polypeptide chain initiation and elongation. J Mol Biol. 1976 Apr 15;102(3):601–612. doi: 10.1016/0022-2836(76)90337-5. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Koch G. Individual translational efficiencies of SV40 and cellular mRNAs. Arch Virol. 1976;52(1-2):123–134. doi: 10.1007/BF01317871. [DOI] [PubMed] [Google Scholar]
- Persson H., Oberg B., Philipson L. In vitro translation with adenovirus polyribosomes. J Virol. 1977 Jan;21(1):187–198. doi: 10.1128/jvi.21.1.187-198.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Pettersson U., Mathews M. B. Synthesis of a structural adenovirus polypeptide in the absence of viral DNA replication. Virology. 1978 Oct 1;90(1):67–79. doi: 10.1016/0042-6822(78)90334-3. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mathews M. B. The gene and messenger RNA for adenovirus polypeptide IX. Cell. 1977 Nov;12(3):741–750. doi: 10.1016/0092-8674(77)90274-4. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Nucleotide sequences of ribosome recgonition sites in messenger RNAs of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3672–3676. doi: 10.1073/pnas.74.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., McDowell M., Baltimore D., Lodish H. F. Translation of reovirus mRNA, poliovirus RNA and bacteriophage Qbeta RNA in cell-free extracts of mammalian cells. Methods Enzymol. 1974;30:709–723. doi: 10.1016/0076-6879(74)30068-7. [DOI] [PubMed] [Google Scholar]
- Waldman A. A., Goldstein J. Inhibition by heparin of globin messenger rbinucleic acid translation in a mammalian cell-free system. Biochemistry. 1973 Jul 3;12(14):2706–2711. doi: 10.1021/bi00738a025. [DOI] [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Nuss D. L., Baglioni C. 5'-Terminal 7-methylguanosine and mRNA function: influence of potassium concentration on translation in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3254–3258. doi: 10.1073/pnas.74.8.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Medium hypertonicity and polyribosome structure in Hela cells. The influence of hypertonicity of the growth medium on polyribosomes in Hela cells. Eur J Biochem. 1972 May;27(1):162–173. doi: 10.1111/j.1432-1033.1972.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Westphal H., Eron L., Ferdinand F. J., Callahan R., Lai S. P. Analysis of adenovirus type 2 gene functions by cell-free translation of viral messenger RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):575–579. doi: 10.1101/sqb.1974.039.01.071. [DOI] [PubMed] [Google Scholar]
- Wilhelm J. M., Jessop J. J., Pettitt S. E. Aminoglycoside antibiotics and eukaryotic protein synthesis: stimulation of errors in the translation of natural messengers in extracts of cultured human cells. Biochemistry. 1978 Apr 4;17(7):1149–1153. doi: 10.1021/bi00600a002. [DOI] [PubMed] [Google Scholar]
- Wu G. J., Zubay G. Prolonged transcription in a cell-free system involving nuclei and cytoplasm. Proc Natl Acad Sci U S A. 1974 May;71(5):1803–1807. doi: 10.1073/pnas.71.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]