Abstract
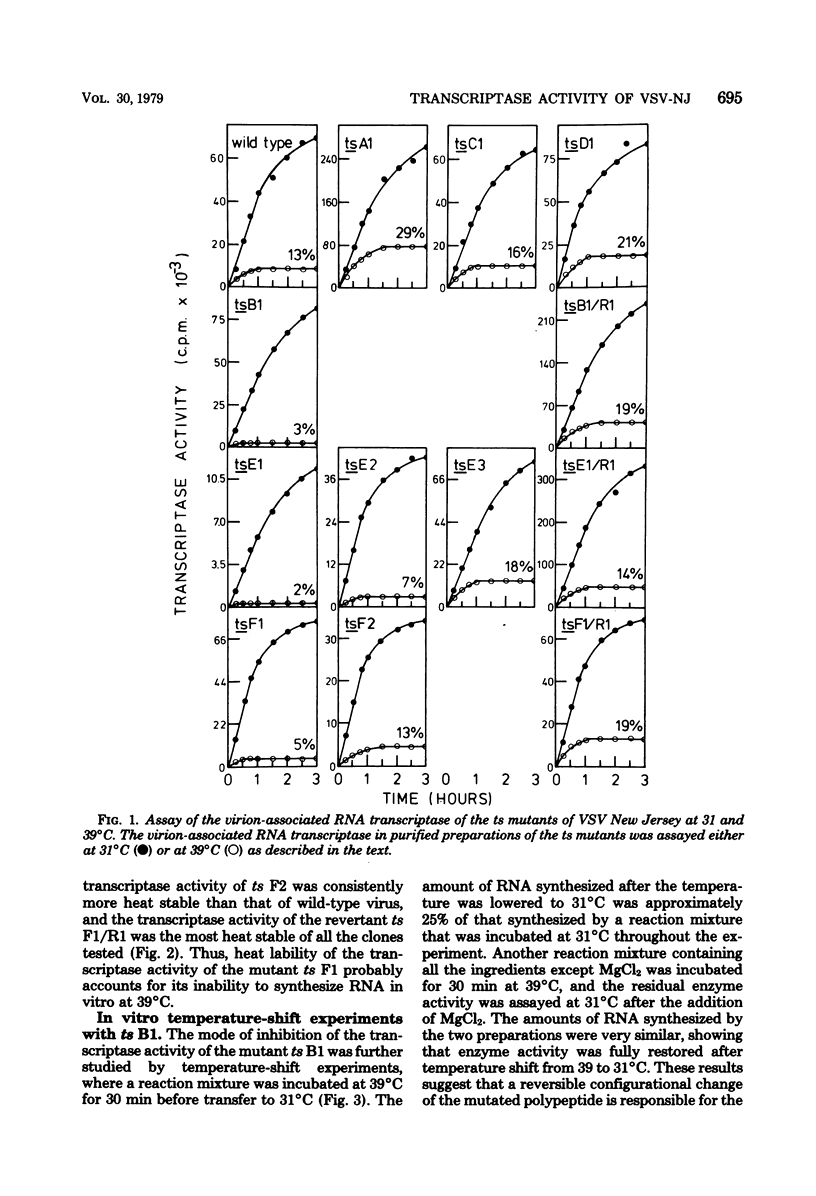
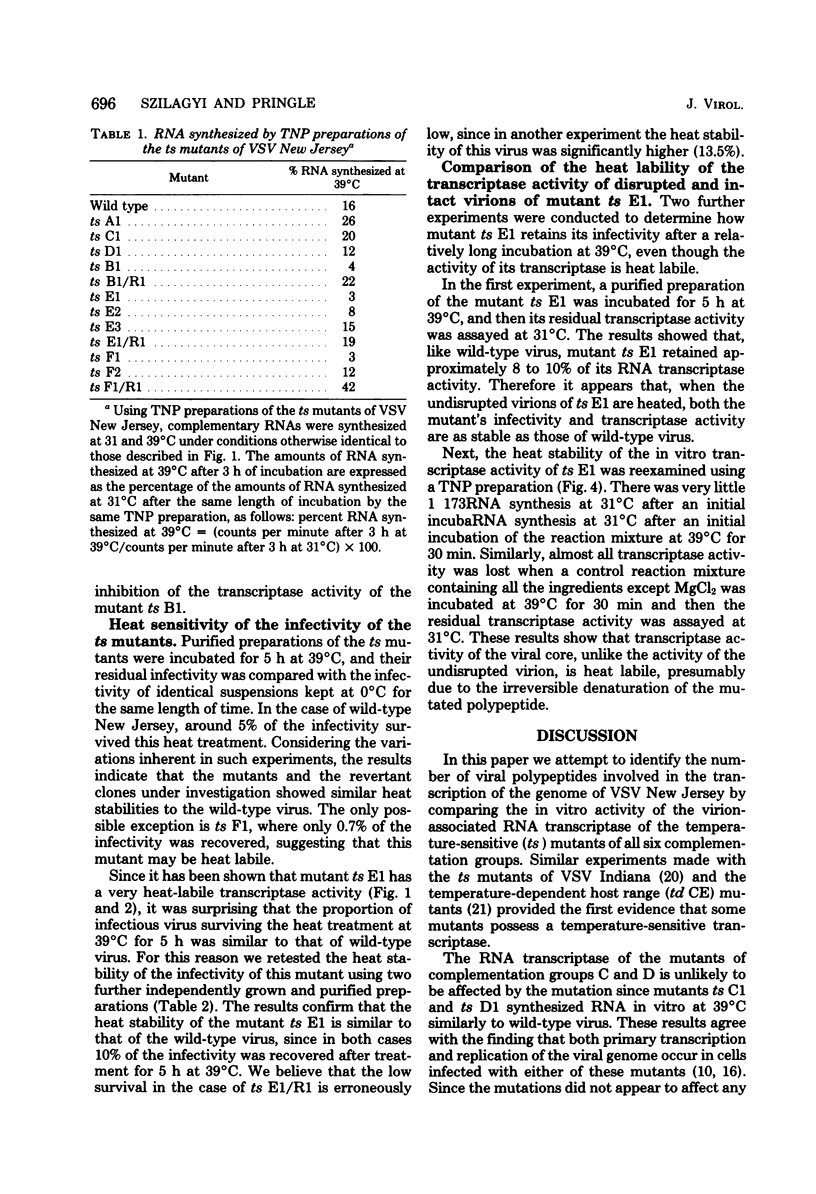
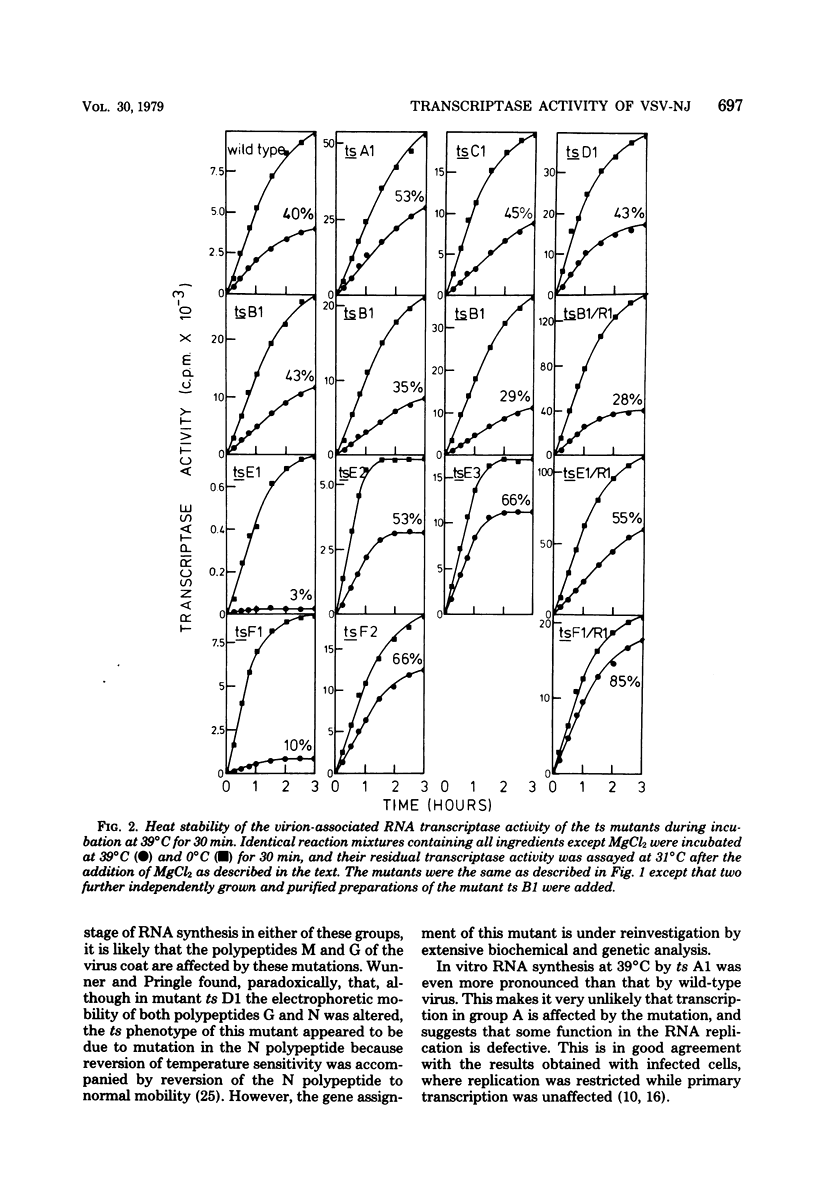
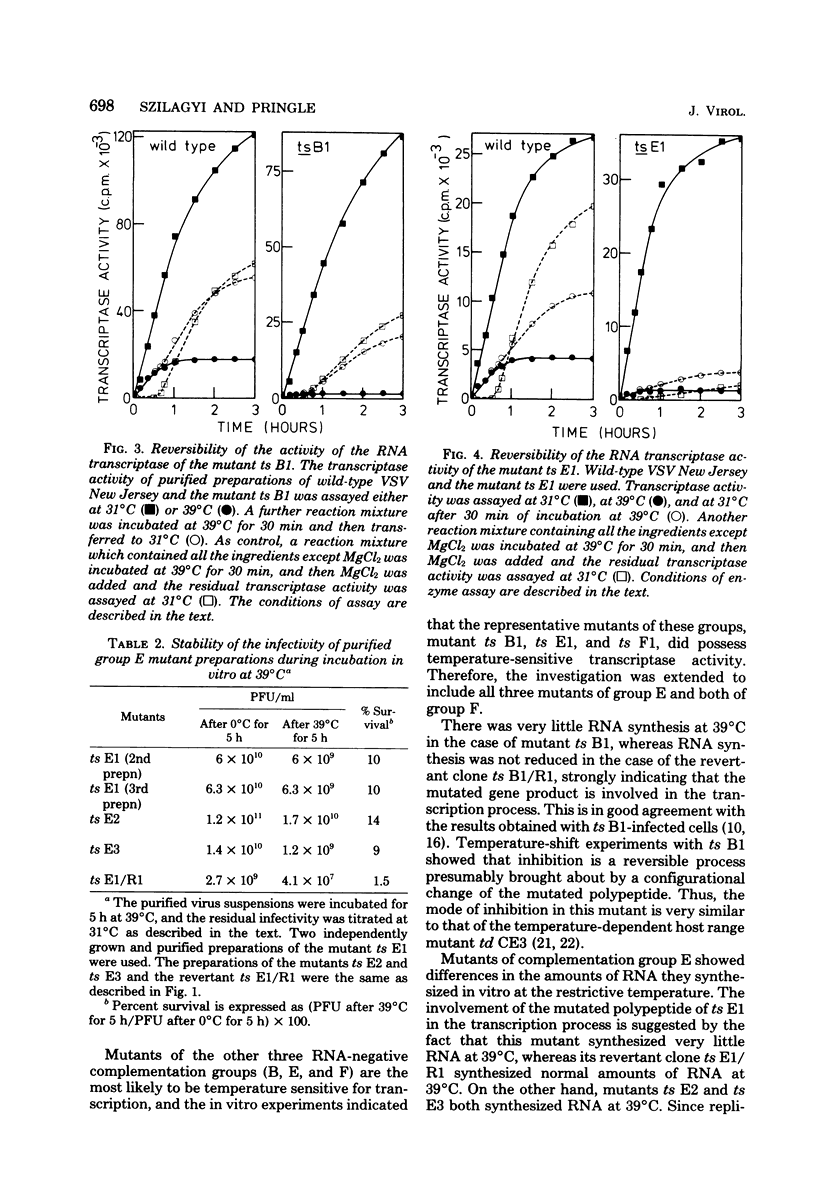
The virion-associated RNA transcriptase activity of vesicular stomatitis virus New Jersey temperature-sensitive (ts) mutants was assayed in vitro at the permissive (31°C) and restrictive (39°C) temperatures. RNA synthesis at 39°C by the RNA-negative ts A1 and the RNA-positive ts C1 and ts D1 mutants was similar to that of wild-type virus. The RNA-negative ts B1 synthesized only small amounts of RNA in vitro at 39°C. The three mutants of complementation group E were dissimilar in the amounts of RNA they synthesized at 39°C: ts E1 synthesized very little RNA, ts E2 synthesized moderate amounts, and RNA synthesis by ts E3 was not inhibited. The two mutants of group F were also dissimilar, since ts F1 synthesized very little RNA at 39°C, whereas ts F2 synthesized as much RNA as wild-type virus. The revertant clones ts B1/R1, ts E1/R1, and ts F1/R1 synthesized RNA at 39°C in amounts comparable to wild-type virus, indicating that the heat sensitivity of the transcriptase activity of the mutants ts B1, ts E1, and ts F1 was associated with temperature sensitivity. Similar heat sensitivities were observed when transcribing nucleoprotein complexes were used in the assays, showing that the mutated polypeptides were part of the viral core. The heat stability of the mutant ts B1 was similar to that of wild-type virus, and in vitro RNA synthesis was fully restored when the temperature was lowered to 31°C after 30 min of preincubation at 39°C, showing that the inhibition was due to reversible configurational change of the mutated polypeptide. When virions of the mutant ts E1 were heated for 5 h at 39°C, their infectivity and transcriptase activity were as stable as those of the wild-type virus, whereas transcriptase activity became very heat labile after disruption of the viral coat with a neutral detergent. This suggests an interaction between the mutated polypeptide and a coat polypeptide which stabilizes the activity of the transcriptase. The RNA transcriptase activity of the mutant ts F1 was also heat labile, although to a lesser extent than that of ts E1. Thus, the defects in transcriptase activity of groups B, E, and F suggest that all three polypeptides of the virus core, polypeptides L, N, and NS, are involved in the transcription. In addition, we postulate that the mutated gene products of groups E and F are multifunctional, being required both in transcription and replication, and that the gene product of group E may also be involved in some late stage of virus development.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Flamand A., Pringle C. R. The homologies of spontaneous and induced temperature-sensitive mutants of vesicular stomatitis virus isolated in chick embryo and BHK 21 cells. J Gen Virol. 1971 May;11(2):81–85. doi: 10.1099/0022-1317-11-2-81. [DOI] [PubMed] [Google Scholar]
- Hunt D. M., Emerson S. U., Wagner R. R. RNA- temperature-sensitive mutants of vesicular stomatitis virus: L-protein thermosensitivity accounts for transcriptase restriction of group I mutants. J Virol. 1976 May;18(2):596–603. doi: 10.1128/jvi.18.2.596-603.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Wagner R. R. Location of the transcription defect in group I temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):28–35. doi: 10.1128/jvi.13.1.28-35.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesnaw J. A., Dickson L. R. In vitro functional analysis of a temperature-sensitive mutant of vesicular stomatitis virus, New Jersey serotype, defective in transcription. Virology. 1978 Nov;91(1):51–59. doi: 10.1016/0042-6822(78)90354-9. [DOI] [PubMed] [Google Scholar]
- Lesnaw J. A., Reichmann M. E. RNA synthesis by temperature-sensitive mutants of vesicular stomatitis virus, New Jersey serotype. Virology. 1975 Feb;63(2):492–504. doi: 10.1016/0042-6822(75)90322-0. [DOI] [PubMed] [Google Scholar]
- Preston C. M., Szilagyi J. F. Cell-free translation of RNA synthesized in vitro by a transcribing nucleoprotein complex prepared from purified vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1002–1009. doi: 10.1128/jvi.21.3.1002-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B. Preliminary physiological characterization of temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Jul;8(1):56–61. doi: 10.1128/jvi.8.1.56-61.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B., Stevenson M. Isolation and characterization of temperature-sensitive mutants of vesicular stomatitis virus, New Jersey serotype. J Virol. 1971 Dec;8(6):836–841. doi: 10.1128/jvi.8.6.836-841.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. The tdCE and hrCE phenotypes: host range mutants of vesicular stomatitis virus in which polymerase function is affected. Cell. 1978 Oct;15(2):597–606. doi: 10.1016/0092-8674(78)90028-4. [DOI] [PubMed] [Google Scholar]
- Printz P., Wagner R. R. Temperature-sensitive mutants of vesicular stomatitis virus: synthesis of virus-specific proteins. J Virol. 1971 May;7(5):651–662. doi: 10.1128/jvi.7.5.651-662.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repik P., Flamand A., Bishop D. H. Synthesis of RNA by mutants of vesicular stomatitis virus (Indiana serotype) and the ability of wild-type VSV New Jersey to complement the VSV Indiana ts G I-114 transcription defect. J Virol. 1976 Oct;20(1):157–169. doi: 10.1128/jvi.20.1.157-169.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Dumont R., Baltimore D. Screening procedure for complementation-dependent mutants of vesicular stomatitis virus. J Virol. 1975 Jan;15(1):41–49. doi: 10.1128/jvi.15.1.41-49.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Effect of temperature-sensitive mutations on the virion-associated RNA transcriptase of vesicular stomatitis virus. J Mol Biol. 1972 Nov 14;71(2):281–291. doi: 10.1016/0022-2836(72)90351-8. [DOI] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R., Macpherson T. M. Temperature-dependent host range mutation in vesicular stomatitis virus affecting polypeptide L. J Virol. 1977 May;22(2):381–388. doi: 10.1128/jvi.22.2.381-388.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Virion trascriptase activity differences in host range mutants of vesicular stomatitis virus. J Virol. 1975 Oct;16(4):927–936. doi: 10.1128/jvi.16.4.927-936.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Uryvayev L. Isolation of an infectious ribonucleoprotein from vesicular stomatitis virus containing an active RNA transcriptase. J Virol. 1973 Feb;11(2):279–286. doi: 10.1128/jvi.11.2.279-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. A temperature-sensitive mutant of vesicular stomatitis virus with two abnormal virus proteins. J Gen Virol. 1974 Apr;23(1):97–106. doi: 10.1099/0022-1317-23-1-97. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Protein synthesis in BHK21 cells infected with vesicular stomatitis virus. I. ts Mutants of the Indiana serotype. Virology. 1972 Apr;48(1):104–111. doi: 10.1016/0042-6822(72)90118-3. [DOI] [PubMed] [Google Scholar]


