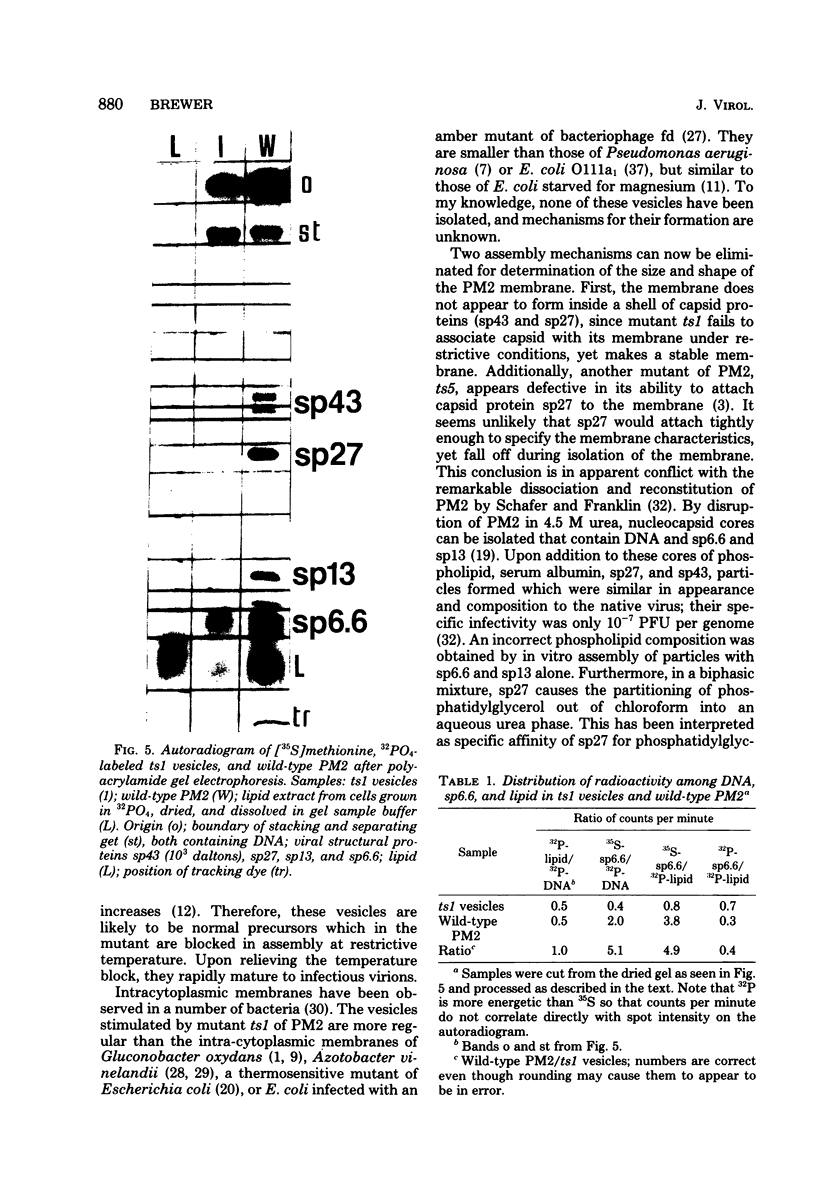
Abstract
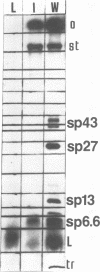
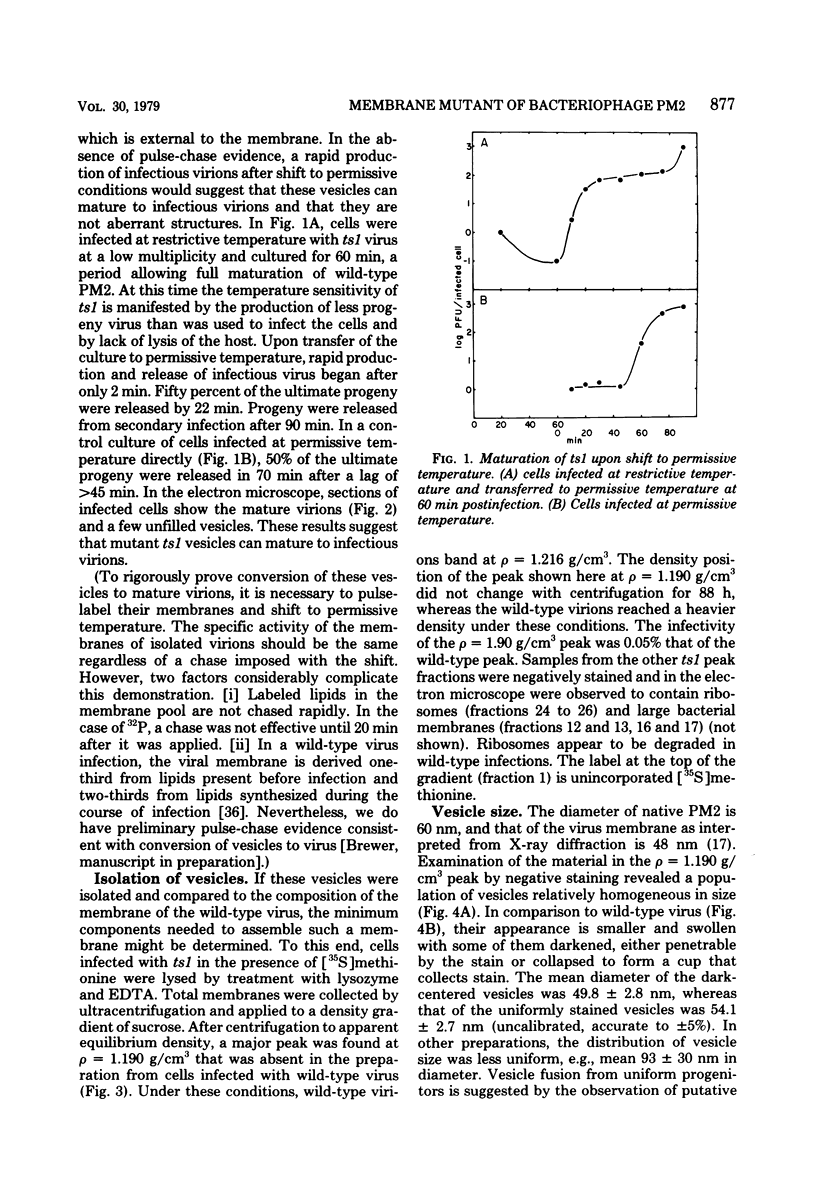
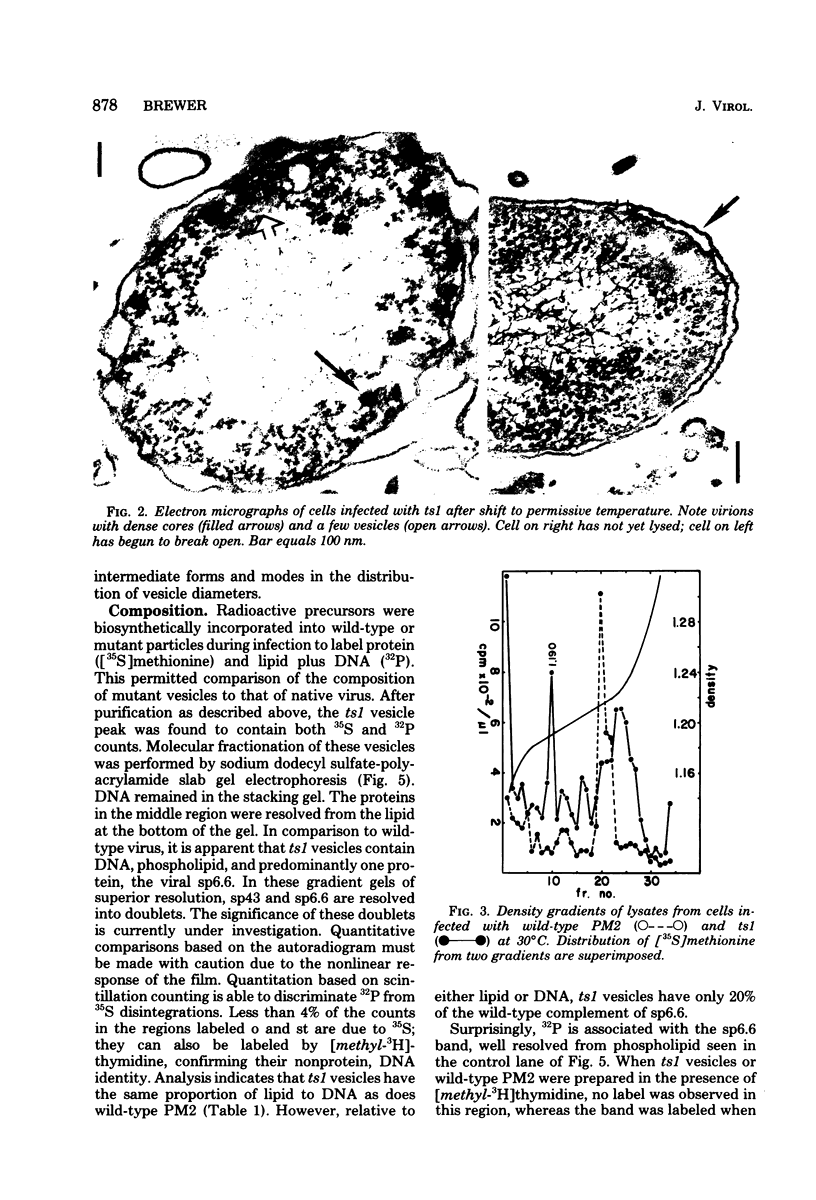
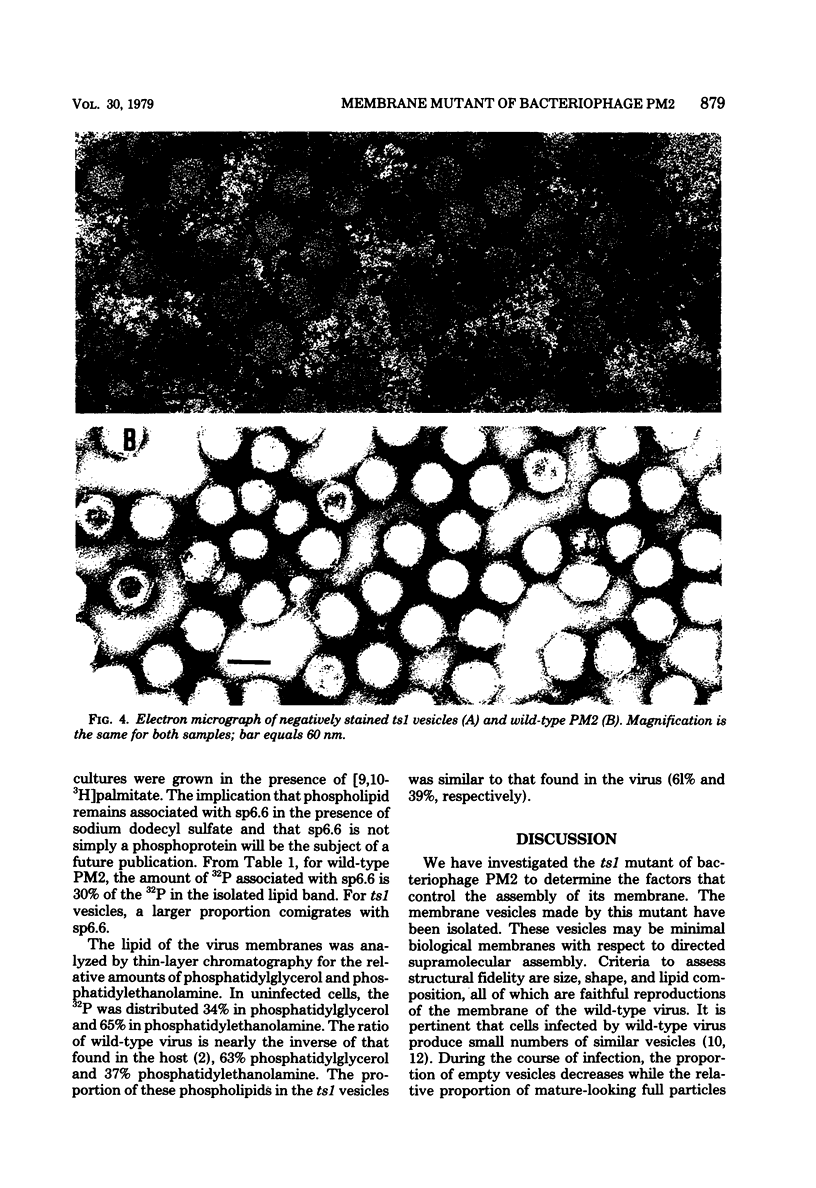
At restrictive temperature, mutant ts1 of bacteriophage PM2 makes membrane vesicles inside infected Alteromonas espejiana. A shift from restrictive to permissive temperature resulted in rapid maturation to infectious virions. The membrane vesicles were isolated from cellular membranes by sucrose density gradient centrifugation. Analysis of the unique peak at rho = 1.190 g/cm3 showed spheres of two diameters, 50 nm and 54 nm. The wild-type virus is icosahedral with an average diameter of 60 nm. Gel electrophoresis indicated the absence in the vesicles of the coat and spike proteins. sp27 and sp43, respectively, and the presence of only one viral structural protein, sp6.6. DNA was also present. The lipid in the vesicles was composed of phosphatidylglycerol and phosphatidylethanolamine in a proportion similar to that of the wild-type virus, whose ratio is nearly the inverse of that found in the host membrane. Thus, membrane vesicles made by mutant ts1 resembled the membrane of the wild-type virus in size, shape, and lipid composition, but contained only one of the four structural proteins of the virus. This hydrophobic protein, sp6.6 may be responsible for stimulating membrane morphogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batzing B. L., Claus G. W. Fine structural changes of Acetobacter suboxydans during growth in a defined medium. J Bacteriol. 1973 Mar;113(3):1455–1461. doi: 10.1128/jb.113.3.1455-1461.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein S. N., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. V. Phospholipids of the host BAL-31 and of the bacteriophage PM2. Virology. 1971 Mar;43(3):685–695. doi: 10.1016/0042-6822(71)90292-3. [DOI] [PubMed] [Google Scholar]
- Brewer G. J. Characterization of temperature-sensitive mutants of bacteriophage PM2: membrane mutants. Mol Gen Genet. 1978 Nov 16;167(1):65–74. doi: 10.1007/BF00270322. [DOI] [PubMed] [Google Scholar]
- Brewer G. J. Control of membrane morphogenesis in bacteriophage PM2. J Supramol Struct. 1976;5(1):73–79. doi: 10.1002/jss.400050108. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Singer S. J. On the disposition of the proteins of the membrane-containing bacteriophage PM2. Biochemistry. 1974 Aug 13;13(17):3580–3588. doi: 10.1021/bi00714a028. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. XII. The fatty acids and lipid content of bacteriophage PM2. Virology. 1972 Aug;49(2):385–393. doi: 10.1016/0042-6822(72)90491-6. [DOI] [PubMed] [Google Scholar]
- Carrick L., Jr, Berk R. S. Membranous inclusions of Pseudomonas aeruginosa. J Bacteriol. 1971 Apr;106(1):250–256. doi: 10.1128/jb.106.1.250-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus G. W., Batzing B. L., Baker C. A., Goebel E. M. Intracytoplasmic membrane formation and increased oxidation of glycerol growth of Gluconobacter oxydans. J Bacteriol. 1975 Sep;123(3):1169–1183. doi: 10.1128/jb.123.3.1169-1183.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota-Robles E. H. Internal membranes in cells of Escherichia coli. J Ultrastruct Res. 1966 Dec;16(5):626–639. doi: 10.1016/s0022-5320(66)80010-2. [DOI] [PubMed] [Google Scholar]
- Cota-Robles E., Espejo R. T., Haywood P. W. Ultrastructure of bacterial cells infected with bacteriophage PM2, a lipid-containing bacterial virus. J Virol. 1968 Jan;2(1):56–68. doi: 10.1128/jvi.2.1.56-68.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. IV. Electron microscopic studies of PM2-infected Pseudomonas BAL-31. Virology. 1970 Dec;42(4):1073–1086. doi: 10.1016/0042-6822(70)90355-7. [DOI] [PubMed] [Google Scholar]
- Datta A., Camerini-Otero R. D., Braunstein S. N., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. VII. Structural proteins of bacteriophage PM2. Virology. 1971 Jul;45(1):232–239. doi: 10.1016/0042-6822(71)90130-9. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties and characterization of the host bacterium of bacteriophage PM2. J Bacteriol. 1968 May;95(5):1887–1891. doi: 10.1128/jb.95.5.1887-1891.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Harrison S. C., Caspar D. L., Camerini-Otero R. D., Franklin R. M. Lipid and protein arrangement in bacteriophage PM2. Nat New Biol. 1971 Feb 17;229(7):197–201. doi: 10.1038/newbio229197a0. [DOI] [PubMed] [Google Scholar]
- Hinnen R., Chassin R., Schäfer R., Franklin R. M., Hitz H., Schäfer D. Structure and synthesis of a lipid-containing bacteriophage. Purification, chemical composition, and partial sequences of the structural proteins. Eur J Biochem. 1976 Sep;68(1):139–152. doi: 10.1111/j.1432-1033.1976.tb10772.x. [DOI] [PubMed] [Google Scholar]
- Hinnen R., Schäfer R., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. Preparation of virus and localization of the structural proteins. Eur J Biochem. 1974 Dec 16;50(1):1–14. doi: 10.1111/j.1432-1033.1974.tb03867.x. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Hsiao C. L., Black L. W. DNA packaging and the pathway of bacteriophage T4 head assembly. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3652–3656. doi: 10.1073/pnas.74.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Plasma membrane lipids and parainfluenza virus assembly. Virology. 1970 Apr;40(4):939–947. doi: 10.1016/0042-6822(70)90140-6. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Teaff N., D'Ambrosia J. Maturation of the head of bacteriophage T4. III. DNA packaging into preformed heads. J Mol Biol. 1974 Oct 5;88(4):749–765. doi: 10.1016/0022-2836(74)90397-0. [DOI] [PubMed] [Google Scholar]
- Onishi Y., Kuwano M. Growth inhibition and appearance of the membranous structure in Escherichia coli infected with bacteriophage fd. J Virol. 1971 May;7(5):673–678. doi: 10.1128/jvi.7.5.673-678.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J., Marcus L. Correlation of ultrastructure in Azotobacter vinelandii with nitrogen source for growth. J Bacteriol. 1970 Jan;101(1):286–291. doi: 10.1128/jb.101.1.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Shah V. K., Brill W. J. Internal membrane control in Azotobacter vinelandii. J Bacteriol. 1973 Jun;114(3):1346–1350. doi: 10.1128/jb.114.3.1346-1350.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. XIX. Reconstitution of bacteriophage PM2 in vitro. J Mol Biol. 1975 Sep 5;97(1):21–34. doi: 10.1016/s0022-2836(75)80019-2. [DOI] [PubMed] [Google Scholar]
- Schäfer R., Hinnen R., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. Properties of the structural proteins and distribution of the phospholipid. Eur J Biochem. 1974 Dec 16;50(1):15–27. doi: 10.1111/j.1432-1033.1974.tb03868.x. [DOI] [PubMed] [Google Scholar]
- Snipes W., Cupp J., Sands J. A., Keith A., Davis A. Calcium requirement for assemby of the lipid-containing bacteriophage PM2. Biochim Biophys Acta. 1974 Mar 29;339(3):311–322. doi: 10.1016/0005-2736(74)90158-8. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Birdwell C. R., Lenches E. M., Staples S. E., Strauss J. H. Mutants of Sindbis virus. II. Characterization of a maturation-defective mutant, ts103. Virology. 1977 Oct 1;82(1):122–149. doi: 10.1016/0042-6822(77)90038-1. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. 13. Studies on the origin of the viral phospholipids. Virology. 1974 Jun;59(2):408–417. [PubMed] [Google Scholar]
- Weigand R. A., Shively J. M., Greenawalt J. W. Formation and ultrastructure of extra membranes in Escherichia coli. J Bacteriol. 1970 Apr;102(1):240–249. doi: 10.1128/jb.102.1.240-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y'Yoshii S., Maeno K., Matsumoto T. Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology. 1976 May;71(1):143–161. doi: 10.1016/0042-6822(76)90101-x. [DOI] [PubMed] [Google Scholar]