Abstract
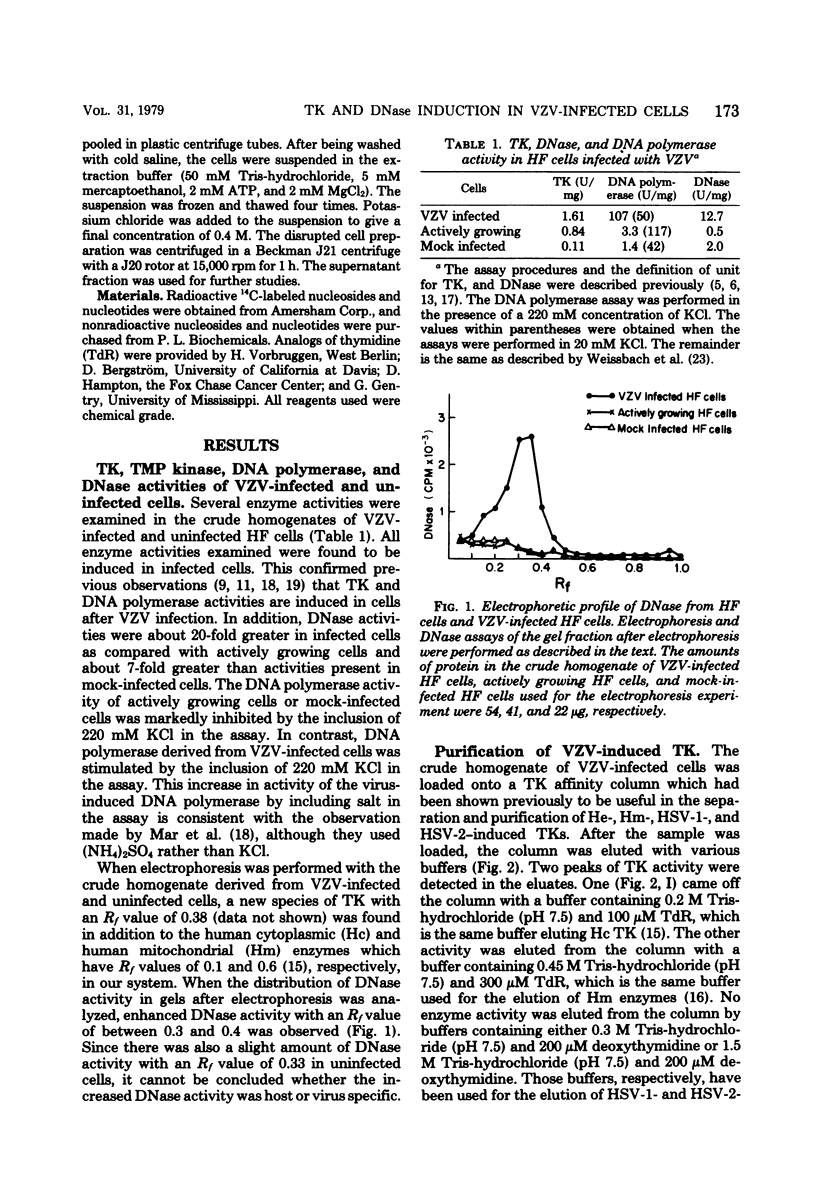
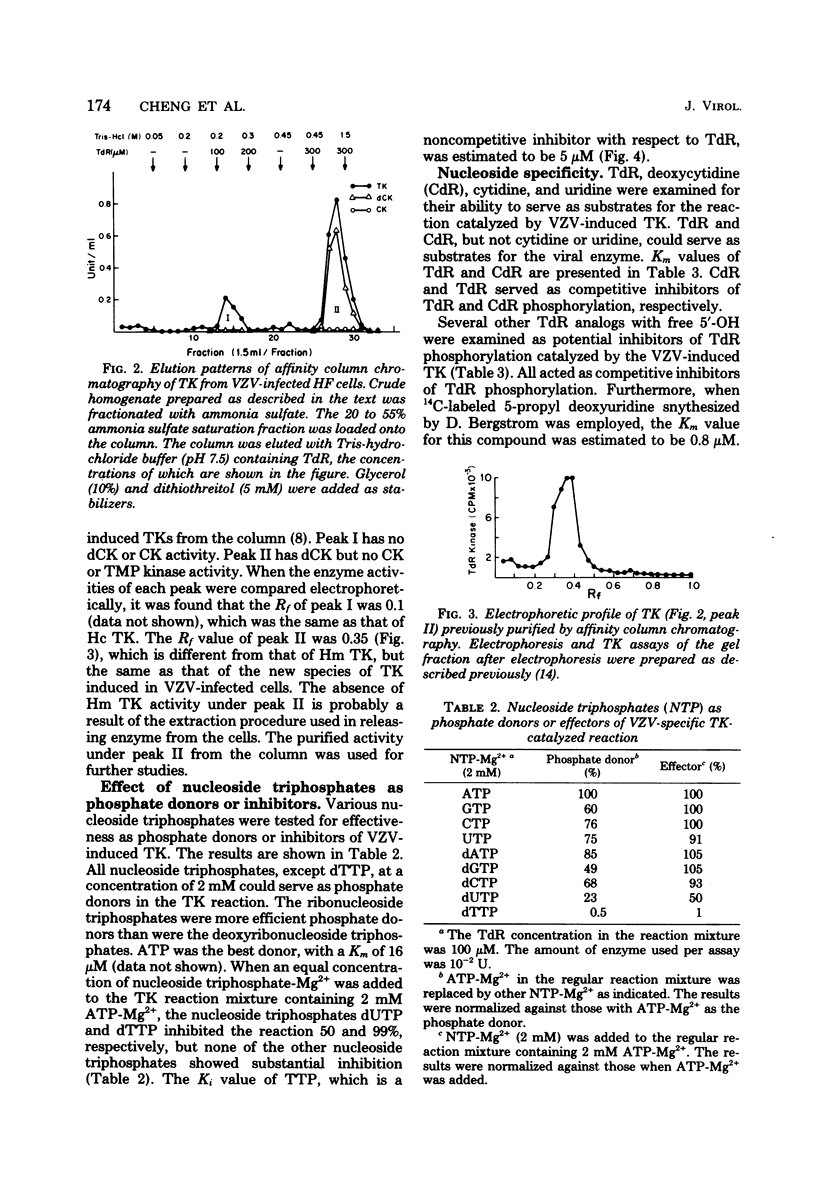
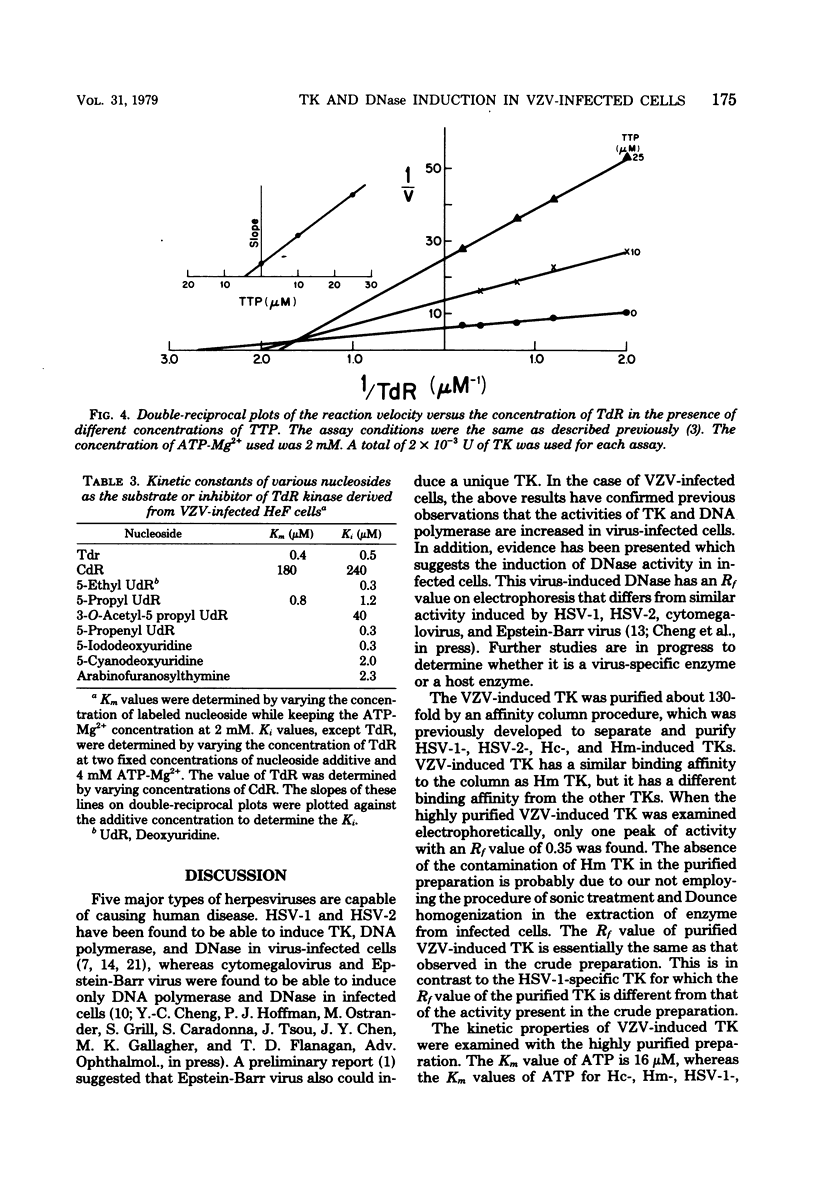
Thymidine kinase (TK), DNA polymerase, and DNase activities were induced in human foreskin fibroblasts after varicella-zoster virus infection. The induced TK and DNase activities have electrophoretic mobilities different from the corresponding host enzymes. Varicella-zoster virus-induced TK was purified and separated from the host enzyme by affinity column chromatography. This enzyme has been shown to have a broader substrate specificity with respect to either the phosphate donor or acceptor as compared with human cytoplasmic and mitochondrial TKs. The best phosphate donor is ATP, with a Km of 16 microM. The Km values of thymidine, deoxycytidine, and 5-propyl deoxyuridine were estimated to be 0.4, 180, and 0.8 microM, respectively. The Ki values for several analogs of thymidine such as 5-iododeoxyuridine, arabinofuranosylthymine, 5-ethyl deoxyuridine, and 5-cyanodeoxyuridine were also examined. TTP acted as a noncompetitive inhibitor with respect to thymidine with a Ki of 5 microM. The kinetic behavior of varicella-zoster virus-induced TK is different from human cytoplasmic, human mitochondrial, and herpes simplex virus type 1- and 2-induced TKs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen S. T., Estes J. E., Huang E. S., Pagano J. S. Epstein-Barr virus-associated thymidine kinase. J Virol. 1978 Apr;26(1):203–208. doi: 10.1128/jvi.26.1.203-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C. A rational approach to the development of antiviral chemotherapy: alternative substrates of herpes simplex virus Type 1 (HSV-1) and Type 2 (HSV-2) thymidine kinase (TK). Ann N Y Acad Sci. 1977 Mar 4;284:594–598. doi: 10.1111/j.1749-6632.1977.tb21992.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C. Deoxythymidine kinase induced in the HELA TK- cells by herpes simplex virus type I and type II. Substrate specificity and kinetic behavior. Biochim Biophys Acta. 1976 Dec 8;452(2):370–381. doi: 10.1016/0005-2744(76)90186-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Domin B. Behavior of various ribo- and deoxyribonucleosides, nucleoside monophosphate kinases, and nucleoside diphosphokinase on Blue Sepharose affinity columns. Anal Biochem. 1978 Apr;85(2):425–429. doi: 10.1016/0003-2697(78)90238-5. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Domin B., Lee L. S. Human deoxycytidine kinase. Purification and characterization of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia patients. Biochim Biophys Acta. 1977 Apr 12;481(2):481–492. doi: 10.1016/0005-2744(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Goz B., Prusoff W. H. Deoxyribonucleotide metabolism in Herpes simplex virus infected HeLa cells. Biochim Biophys Acta. 1975 May 16;390(3):253–263. doi: 10.1016/0005-2787(75)90346-9. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Cheng Y. C. Thymidine kinase from blast cells of myelocytic leukemia. Methods Enzymol. 1978;51:365–371. doi: 10.1016/s0076-6879(78)51049-5. [DOI] [PubMed] [Google Scholar]
- Dobersen M. J., Jerkofsky M., Greer S. Enzymatic basis for the selective inhibition of varicella-zoster virus by 5-halogenated analogues of deoxycytidine. J Virol. 1976 Nov;20(2):478–486. doi: 10.1128/jvi.20.2.478-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J. E., Huang E. S. Stimulation of cellular thymidine kinases by human cytomegalovirus. J Virol. 1977 Oct;24(1):13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Mallavia L. P. Deoxypyrimidine nucleoside metabolism in varicella-zoster virus-infected cells. J Virol. 1978 Feb;25(2):510–517. doi: 10.1128/jvi.25.2.510-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. The deoxyribonuclease induced after infection of KB cells by herpes simplex virus type 1 or type 2. I. Purification and characterization of the enzyme. J Biol Chem. 1978 May 25;253(10):3557–3562. [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Trkula D., Dubbs D. R. Thymidine kinase isozymes of normal and virus-infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):703–715. doi: 10.1101/sqb.1974.039.01.084. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976 May 10;251(9):2600–2604. [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. c. Human deoxythymidine kinase II: substrate specificity and kinetic behavior of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. Biochemistry. 1976 Aug 24;15(17):3686–3690. doi: 10.1021/bi00662a007. [DOI] [PubMed] [Google Scholar]
- Mar E. C., Huang Y. S., Huang E. S. Purification and characterization of varicella-zoster virus-induced DNA polymerase. J Virol. 1978 May;26(2):249–256. doi: 10.1128/jvi.26.2.249-256.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., Otsuka T., Takahashi M. Induction of deoxypyrimidine kinase activity in human embryonic lung cells infected with varicella-zoster virus. J Virol. 1977 Mar;21(3):1232–1235. doi: 10.1128/jvi.21.3.1232-1235.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F. INHIBITION BY METABOLIC ANALOGUES OF PLAQUE FORMATION BY HERPES ZOSTER AND HERPES SIMPLEX VIRUSES. J Immunol. 1964 Oct;93:643–648. [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]


