Abstract
Protein inclusion is a prominent feature of neurodegenerative diseases including frontotemporal lobar degeneration (FTLD) that is characterized by the presence of ubiquitinated TDP-43 inclusion. Presence of protein inclusions indicates an interruption to protein degradation machinery or the overload of misfolded proteins. In response to the increase in misfolded proteins, cells usually initiate a mechanism called unfolded protein response (UPR) to reduce misfolded proteins in the lumen of endoplasmic reticules. Here we examined the effects of mutant TDP-43 on the UPR in transgenic rats that express mutant human TDP-43 restrictedly in the neurons of the forebrain. Overexpression of mutant TDP-43 in rats caused prominent aggregation of ubiquitin and remarkable fragmentation of Golgi complexes prior to neuronal loss. While ubiquitin aggregates and Golgi fragments were accumulating, neurons expressing mutant TDP-43 failed to upregulate chaperones residing in the endoplasmic reticules and failed to initiate the UPR. Prior to ubiquitin aggregation and Golgi fragmentation, neurons were depleted of X-box binding protein 1 (XBP1), a key player of UPR machinery. While it remains to determine how mutation of TDP-43 leads to the failure of the UPR, our data demonstrate that failure of the UPR is implicated in TDP-43 pathogenesis.
Keywords: TDP-43, XBP1, ubiquitin aggregation, Golgi fragmentation, rats
Introduction
Frontotemporal lobar degeneration (FTLD) is a common cause of pre-senile dementia. A subset of FTLD is defined by presence of ubiquitin-positive inclusions in affected cells (Neumann et al., 2006). While mutation of Tar DNA binding protein 43 (TDP-43) is causative of familial FTLD and amyotrophic lateral sclerosis (ALS) (Kabashi et al., 2008, Rutherford et al., 2008, Sreedharan et al., 2008), wildtype TDP-43 protein is hyper-phosphorylated, ubiquitinated, and aggregated in the cytoplasm and nuclei of affected cells in sporadic FTLD and ALS (Neumann et al., 2006). The intermediates of protein aggregates are considered cytotoxic (Bucciantini et al., 2002, Walsh et al., 2002), but long fibrils of protein aggregates might be bystanders (Walsh et al., 2002). In FTLD, it is not known how ubiquitin inclusions are developed in response to disease factors such as mutant TDP-43.
Presence of ubiquitin inclusions is indicative of the overload of ubiquitin-proteasome degradation system and also is suggestive of the accumulation of inappropriately folded proteins inside the cells (Ito and Suzuki, 2009). In response to the increase in unfolded or misfolded proteins, cells initiate a mechanism called unfolded protein response (UPR) to reduce misfolded proteins in the lumen of endoplasmic reticules (ER) (Ito and Suzuki, 2009, Lowe, 2011). Three classes of transducer proteins are known to mediate UPR pathway and these transducers include inositol-requiring protein 1 (IRE1), activating transcription factor 6 (ATF6), and PKR-related ER kinase (Tudor et al.) (Tudor et al., Lowe, 2011). The transducer proteins recognize misfolded protein as a common input, but their output signals are different. While IRE1 activates the splicing of X-box binding protein 1 (XBP1) mRNA, activated ATF6 promotes the transcription of XBP1 mRNA (Yoshida et al., 2001). Unspliced XBP1 mRNA (XBP1u) undergoes an unusual process of splicing that is promoted by IRE1 on the surface of ER and only the protein products of spliced XBP1 (XBP1s) mRNA gain transcriptional activities (Yoshida et al., 2001). XBP1s upregulates the expression of ER chaperone proteins to reduce misfolded or unfolded proteins in the ER lumen and thus to protect cells against the toxicity of misfolded proteins (Casas-Tinto et al., 2011). When cell-protective UPR is compromised, cells may suffer from the accumulation of misfolded or unfolded proteins, of which some proteins may be ubiquitinated and designated for degradation (Casas-Tinto et al., 2011). It is not known how UPR machinery responds to mutation or alteration in TDP-43.
TDP-43 is a ribonucleoprotein able to bind DNA and RNA molecules (Polymenidou et al., 2011, Sephton et al., 2011, Tollervey et al., 2011). TDP-43 regulates thousands of genes by affecting RNA splicing and stability (Buratti et al., 2001, Polymenidou et al., 2011, Sephton et al., 2011, Tollervey et al., 2011). TDP-43 tunes its own expression through affecting its RNA stability (Ayala et al., 2011, Cohen et al., 2011, Igaz et al., 2011). Mutation of TDP-43 may lead to alteration in UPR machinery in affected neurons. Here we examined the effects of mutant TDP-43 on UPR machinery in transgenic rats expressing mutant TDP-43. Overexpression of mutant TDP-43 in neurons caused progressive loss of neurons and memory that was preceded by abundant formation of ubiquitin aggregates and by remarkable fragmentation of Golgi apparatuses. While ubiquitinated proteins were accumulating, the three UPR transducers including PERK, ATF6, and IRE1 were not upregulated in the neurons. In neurons expressing mutant TDP-43, both spliced and unspliced XBP1 proteins were depleted and ER chaperone proteins were not upregulated throughout the disease courses in transgenic rats. Overexpression of mutant TDP-43 in rats led to UPR repression and XBP1 depletion.
Material and Methods
Ethics statement
Animal utility followed NIH guidelines and was approved by the Institutional Animal Care and Use Committees (IACUC) at Thomas Jefferson University.
Animal experiments
Creation of TRE-TDP-43M337V and Camk2α-tTA transgenic rats was reported (Zhou et al., 2010, Huang et al., 2012a, Huang et al., 2012b). TRE-TDP-43M337V and Camk2α-tTA transgenic rats were bred to double hemizygote and transgenic offspring was identified by PCR analysis of rat’s tail DNA as described previously (Huang et al., 2012a, Huang et al., 2012b). All transgenic rats were maintained on Sprague-Dawley genomic background. Rat’s spatial learning and memory were examined with a Barnes Maze (Med Associates) as described previously (Huang et al., 2011). Mutant TDP-43 transgenic rats were trained to locate escaping hole on a Barnes maze three times per day, for three consecutive days. After three training sections, rats were examined of spatial memory once per week for determining the progression of disease phenotypes. Disease onset was defined as the unrecoverable increase in time spent to locate escaping hole in a Barnes maze and disease end-stage was defined as the inability to locate escaping hole in a Barnes maze within 60 seconds.
TDP-43 transgene is driven by tetracycline-regulated promoter (TRE) and is subjected to the regulation by Doxycycline (Dox). To suppress TDP-43 transgene from expression during embryonic and postnatal development, Doxycycline (Dox, Sigma) was dissolved in drinking water (50 µg/ml) and given to breeding rats and their offspring. To induce disease phenotype in adult rats, Dox was withdrawn from TRE-TDP-43M337V and Camk2α-tTA double transgenic rats at the age of 35 days such that the mutant rats developed disease phenotype (loss of spatial memory) by age of 60 days.
Histology and immunostaining
As described previously (Huang et al., 2011, Huang et al., 2012b), rats were deeply anesthetized and transcardially perfused with 4% paraformaldehyde (Gibbs et al.) dissolved in phosphate buffer. After perfusion, rat’s tissues were dissected and were dehydrated as described previously (Huang et al., 2011, Huang et al., 2012b). Tissue sections of 12 µm were immunostained with the following primary antibodies as described (Huang et al., 2011, Huang et al., 2012b): mouse monoclonal antibody to human TDP-43 (Abnova, clone 2E2-D3), chicken antibody to ubiquitin (Sigma), mouse monoclonal antibodies against Iba-1 (Wako Chemical) or GFAP (Millipore), mouse monoclonal antibody to GM130 (BD bioscience), rabbit polyclonal antibody to GLG1 (Abjent), and mouse monoclonal antibody to NeuN (Millipore). For histochemistry, immunostained sections were visualized with an ABC kit in combination with diaminobenzidine (Vector) and counterstained with hematoxylin to display nuclei. For immunofluorescent staining, tissue sections were incubated with specific primary antibodies and then with secondary antibodies labeled with fluorescent dyes (Jackson Immunoresearch). The primary antibodies were incubated overnight at 4°C and the secondary antibodies were incubated for 2 hours at room temperature. Confocal microscopy was used to detect colocalization of two related proteins on tissue sections and single-layer image was scanned with a Zeiss LSM510 META confocal system (Imaging Facility of Kimmel Cancer Center at Jefferson). To reveal the integrity of Golgi Trans and Cis complexes, z-stacks of confocal images (at 1 µm of intervals) were projected to reconstruct Golgi structure.
Total number of neurons was estimated by unbiased stereological cell counting as described previously (Huang et al., 2012a, Huang et al., 2012b). The frontal cortex was defined as the area from the apical forebrain to the first occurrence of corpus callosum. Rat’s forebrains were cut into serial coronal sections (20 µm) and every 12th section (a total of 15 to 18 sections) was counted of neurons in the defined brain regions. Tissue sections were stained with Cresyl violet and mounted in sequential order (rostral-caudal). The number of targeted neurons was estimated using a procedure described previously (Huang et al., 2011, Huang et al., 2012b).
Golgi staining
Golgi impregnation method was used to visualize the dendrites and dendritic spines of rat’s cortical neurons and was done per manufacturer’s instruction (FD NeuroTechnologies). Five neurons in selected brain region of individual rats were examined of dendrite branches and dendritic spine density (Woolley et al., 1990). Dendritic spine density was calculated by dividing the number of spines with the length of dendrite. Both apical and basal dendrites were examined of spine density.
Immunoblotting
As described previously (Zhou et al., 2010), expression of TDP-43 transgene and rat’s endogenous genes was estimated by immunoblotting. Rat’s frontal cortex was dissected and homogenized and the solution fraction of tissue homogenates was collected for immunoblotting. Twenty micrograms of total proteins in tissue lysates were resolved in SDS-PAGE and specified proteins were probed with primary antibodies recognizing PERK, spliced XBP1 (XBP1s), unspliced XBP1 (XBP1u), BiP, PDI, calnexin, ERO1L, or VCP. All the primary antibodies except XBP1s were purchased from Abcam. XBP1s was bought from Novus.
Electromicroscopy
Ultrastructure of cellular organelles was visualized by electromicroscopy as described previously (Zhou et al., 2010, Huang et al., 2011). In brief, fixed tissues were embedded in Epon 812 and cut into thin sections (50nm). Sections were stained with uranyl acetate and lead citrate and were examined under a transmission electron microscope (EM facility affiliated to the Department of Pathology, Thomas Jefferson University).
Statistical analysis
The number of neurons in the defined region was compared between groups of rats and comparison among experimental groups was performed by one-way ANOVA followed by Tukey’s post-hoc test. The null hypothesis was rejected at the level of 0.05.
Results
Temporal expression of mutant TDP-43 in adult rats causes a progressive loss of neurons and spatial memory
While mutation of TDP-43 is linked to inherited ALS and FTLD (Kabashi et al., 2008, Rutherford et al., 2008, Sreedharan et al., 2008), ubiquitin-positive TDP-43 inclusion is a hallmark of sporadic ALS and FTLD (Neumann et al., 2006). To study the pathogenesis of TDP-43 in the cortical dementia, we crossed inducible TRE-TDP-43M337V transgenic rats with Camk2α-tTA transgenic rats and thus restricted the expression of mutant TDP-43 in the forebrain of the double transgenic rats (Huang et al., 2012a, Huang et al., 2012b). In TRE-TDP-43M337V/Camk2α-tTA double transgenic rats, expression of mutant human TDP-43 is dependent on the transcriptional activity of tetracycline-regulated transactivator (tTA) and thus is subjected to the regulation by Doxycycline (Dox) (Zhou et al., 2009, Zhou et al., 2010, Huang et al., 2011, Huang et al., 2012b). To induce disease phenotypes in adult rats, we supplied transgenic rats with Dox during embryonic and postnatal development and withheld Dox when the rats reached the age of 35 days (Figure 1A). Upon Dox withdrawal, expression of TDP-43 transgene was quickly initiated in a tissue-dependent manner (Figure 1A–1C). In TRE-TDP-43M337V/Camk2α-tTA rats, expression of human TDP-43 was restricted in neurons and was undetectable in microglia or astrocytes (Figure 1D–1L). Camk2α-tTA drove expression of mutant human TDP-43 in the neurons of the forebrain in transgenic rats.
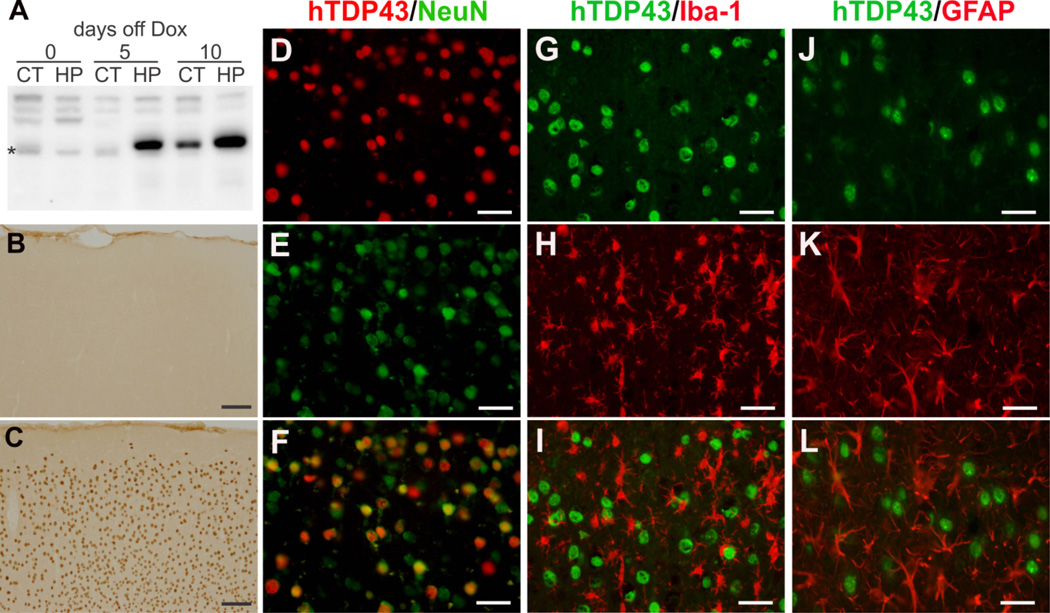
Figure 1.
Restricted expression of mutant TDP-43 in the neurons of rat forebrain. (A) Immunoblotting reveals regulated expression of human TDP-43 in the cortex (CT) and hippocampus [HP] of Camk2a-tTA/TRE-TDP43M337V double transgenic rats. The double transgenic rats were given Dox during development and were deprived of Dox at the age of 35 days. Expression of mutant TDP-43 transgene was quickly recovered upon Dox withdrawal. * indicates a nonspecific band. (B, C) Immunostaining reveals expression of human TDP-43 in the cortex of Camk2a-tTA/TRE-TDP43M337V double transgenic rat (C), but not in the tissues of Camk2a-tTA single transgenic rat (B). (D–L) Double-labeling immunofluorescence staining reveals that mutant human TDP-43 (hTDP43) was colocalized with the neuronal marker NeuN and was not colocalized with either the microglia marker Iba-1 (G–I) or the astrocyte marker GFAP (J–L). Scale bars: B and C, 100 µm; D–L, 30 µm.
Restricted expression of mutant human TDP-43 in neurons caused a progressive loss of neurons in transgenic rats (Figure 2). Expression of mutant TDP-43 was initiated first in the hippocampus and then in the cortex (Figure 2A–2B). Accordingly, neuron loss was observed first in the dentate gyrus and then in the cortex of transgenic rats (Figure 2C–2F). Unbiased stereological cell counting revealed a severe loss of neurons in the hippocampus and a moderate loss of neurons in the cortex in rats at early symptomatic stages (Figure 2E–2F). Progressive loss of cortical and hippocampal neurons in rats caused a deficit in spatial memory that was detected by Barnes maze assay (Figure 2G). Intriguingly, rats reserved certain spatial memory even though most of the hippocampal neurons were lost (Figure 2). Neurons in the other brain regions rather than the dentate gyrus contribute largely to the spatial memory. Restricted expression of mutant human TDP-43 in the neurons of rat’s forebrain recapitulated features of FTLD.
Figure 2.
Temporal expression of mutant TDP-43 causes a progressive loss of neurons and spatial memory in adult rats. (A, B) Immunostaining for human TDP-43 reveals that the restoration of transgene expression was tissue-dependent, with a quicker recovery in the hippocampus (B1–6) compared to the cortex (A1–6). Camk2a-tTA single (tTA) and Camk2atTA/TRE-TDP43M337V double (M337V) transgenic rats were constantly given Dox until they were 35 days old. (C, D) Cresyl violet staining reveals that the onset of neuronal loss was closely correlated with the start of transgene expression in the cortex (C1–6) and hippocampus (D1–6). (E, F) Stereological cell counting reveals a remarkable loss of neurons in the dentate gyrus (E) and frontal cortex (F). Data are means + SEM (n = 6). * p < 0.01. (G) Barnes maze assay reveals a progressive loss of spatial memory in mutant TDP-43 transgenic rats (M337V) compared to the normal controls (tTA). Data are means + SEM (n = 12). Loss of spatial memory is used as a parameter defining the stages of disease progression in mutant TDP-43 transgenic rats. Scale bars: A1–6, B1–6 and D1–D6, 100 µm; C1–6, 30 µm.
Mutant TDP-43 in neurons preferentially reduces dendritic complexity prior to neuron death
To determine whether neurons degenerate in retrograde manner in TDP-43 transgenic rats, we examined the dendritic complexity of rat’s cortex by Golgi staining (Figure 3). Cortical neurons retracted their dendrites before they died (Figure 3A–3J). Neurons in the layer II–III were affected earlier than neurons in the other layers of the cortex (Figure 3C–3D). As the disease progressed in TRE-TDP-43M337V/Camk2α-tTA transgenic rats, most neurons in the cortex displayed retracted dendrites (Figure 3B–3E). We quantified the dendritic complexity of rat’s cortical neurons and observed that the spine density of both apical and basal dendrites was markedly reduced in rats expressing mutant human TDP-43 (Figure 3K). The branch points of apical and basal dendrites were reduced accordingly (Figure 3L). Expression of mutant TDP-43 caused retrograde degeneration of neurons in transgenic rats.
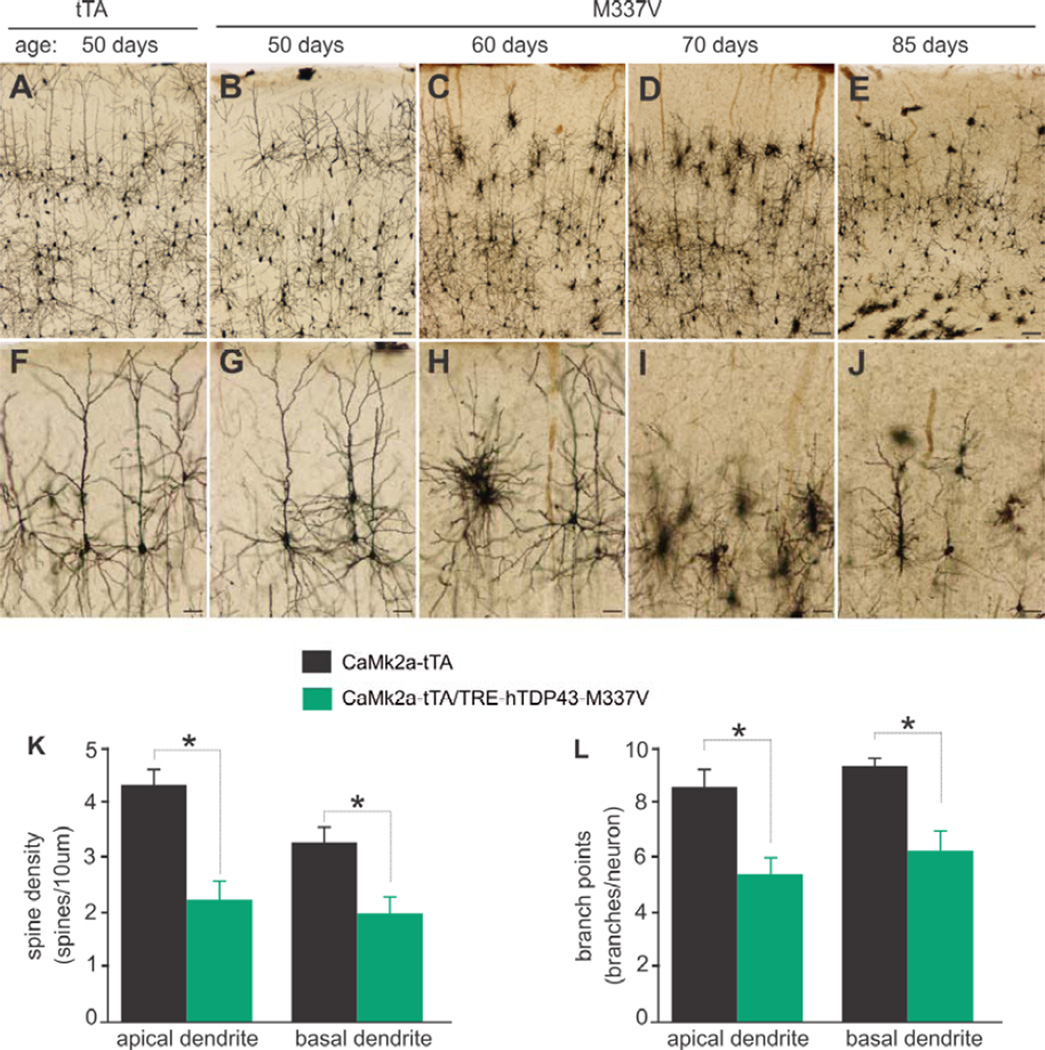
Figure 3.
Neurites and spines are lost prior to neuron loss in mutant TDP-43 transgenic rats. (A–J) Golgi staining reveals that the neurites were retracted before the soma of neurons was lost. The parietal cortex corresponding to the start of hippocampus was examined for Camk2a-tTA single (tTA) and Camk2a-tTA/TRE-TDP43M337V double (M337V) transgenic rats at defined ages. Scale bars: A–E, 100 µm; F–J, 50 µm. (K, L) Spine densities and branch points were quantified for three neurons in the layer V of parietal cortex for individual rats. Data are means + SEM (n = 5). * p < 0.05.
Expression of mutant TDP-43 in neurons induces ubiquitin aggregation and Golgi fragmentation prior to neuron death in transgenic rats
TDP-43 proteinopathy defines a large subset of sporadic FTLD that is characterized by formation of ubiquitin-positive inclusions (Neumann et al., 2006). Abundant ubiquitin aggregates were detected in neurons in the hippocampus and cortex of TRE-TDP-43M337V/Camk2α-tTA transgenic rats (Figures 4 and S1). Occurrence of ubiquitin inclusions corresponded to the start of transgene expression. Expression of mutant TDP-43 was initiated first in the dentate gyrus and accordingly ubiquitin aggregates were detected first in the tissue (Figure S1). As disease was progressing, most neurons in rat’s brain developed ubiquitin aggregates (Figures 4A–4D and S1). We quantified neurons with ubiquitin aggregates in transgenic rats at varying ages and we observed that more than 70% of cortical neurons developed ubiquitin aggregates in symptomatic rats (Figure 4C). Intriguingly, ubiquitin inclusions did not contain mutant TDP-43 (Figure 4E), in consistence with previous findings in transgenic rodents expressing mutant TDP-43 (Zhou et al., 2010). Inducible TRE-TDP-43M337V/Camk2α-tTA transgenic rats recapitulated a core phenotype of FTLD.
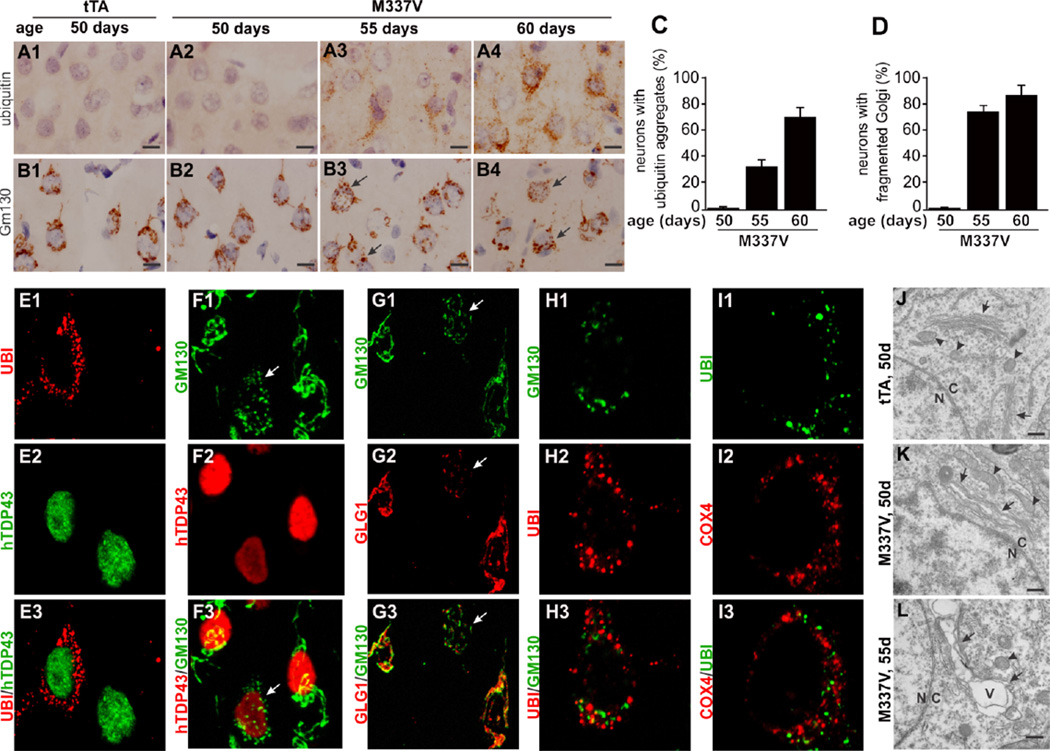
Figure 4.
Ubiquitin forms aggregates and Golgi apparatuses are fragmented in neurons expressing mutant TDP-43. (A, B) Representative photos show a gradual development of ubiquitin aggregates (A1–4) and Golgi fragments (B1–4) in the frontal cortex of Camk2atTA/ TRE-TDP43M337V double transgenic rats (M337V), but not in the tissues of Camk2a-tTA single transgenic rats (tTA). Arrows point to neurons with fragmented Golgi apparatus. Scale bars: 10 µm. (C, D) Cortical neurons with ubiquitin aggregates (C) or fragmented Golgi were quantified for individual rats. Data are means + SEM (n = 5). (E–H) Confocal microscopy reveals that aggregated ubiquitin and fragmented Golgi occurred in neurons expressing mutant TDP-43 and that Cis-(stained of GM130) and Trans (stained of GLG1)-Golgi complexes were simultaneously fragmented and Golgi fragments were not colocalized with ubiquitin aggregates. Arrows point to fragmented Golgi complex. Z-stack images were taken at the same magnification and projected to show the profile of Golgi fragmentation (F–G). (I) Confocal microscopy reveals that aggregated mitochondria were not colocalized with ubiquitin inclusions. (J–L) Electromicroscopy reveals that damaged endoplasmic reticules and Golgi complexes of cortical neurons were swollen to form vacuoles as disease progressed. Nuclear envelope defined the boundary between the nucleus (N) and the cytoplasm (C). Arrows point to endoplasmic reticules and Golgi complexes. Arrowheads point to mitochondria. Vacuoles are marked with “V”. Scale bars: 500 nm.
Golgi fragmentation is reported in patients with TDP-43 inclusion (Fujita et al., 2008). In our transgenic rats, both Cis-and Trans-Golgi complexes were fragmented in neurons expressing mutant human TDP-43 (Figure 4F–4G). Fragmented Golgi complex and aggregated ubiquitin were coincidently detected in the same neurons, although they were not colocalized in affected cells (Figure 4H), indicating that fragmented Golgi apparatus was not a component of ubiquitin inclusions in mutant TDP-43 transgenic rats. Additionally, mitochondria were also aggregated in neurons with ubiquitin inclusions and aggregated mitochondria were not colocalized with ubiquitin in the inclusion (Figure 4, I1–I4). In the cortex, Golgi fragmentation and ubiquitin aggregation were detected in transgenic rats at the same disease stages (Figure S2), suggesting that these two events happened simultaneously in the TDP-43 pathogenesis. Electromicroscopy revealed that the two related organelles—endoplasmic reticules (ER) and Golgi apparatus—were swollen to form vacuoles (Figure 4J–L). In affected cells, ER and Golgi apparatus were severely deformed while mitochondria remained morphologically intact (Figure 4). Compared to mitochondria, ER and Golgi apparatuses appear vulnerable to mutant TDP-43 toxicity.
XBP1 is depleted in neurons expressing mutant human TDP-43 in transgenic rats
In TRE-TDP-43M337V/Camk2α-tTA double transgenic rats, ubiquitin was abundantly aggregated and Golgi was severely fragmented prior to neuronal death (Figure 4), implying that ER stress occurred in affected cells. In response to ER stress, transducer proteins may be activated to upregulate the expression of chaperone proteins that aim to reduce misfolded proteins in ER lumen and to reduce ER stress. Unexpectedly, overexpression of mutant TDP-43 in neurons failed to activate any of the three different transducer proteins including IRE1 (Figure S3), ATF6 (Figure S4), and PERK (Tudor et al.) (Figure 5A). None of the critical chaperone proteins responsive to ER stress was upregulated in the brain of TRE-TDP-43M337V/Camk2α-tTA transgenic rats (Figure 5B). Immunoblotting revealed that spliced and unspliced XBP1 (XBP1s and XBP1u) proteins were increased in the homogenates of rat’s forebrain (Figure 5A). Immunofluorescence staining revealed that XBP1s and XBP1u were progressively depleted in neurons expressing mutant TDP-43 (Figures 5 and S5). By contrast, XBP1 proteins were upregulated in reactive microglia; however, XBP1 was not upregulated in reactive astrocytes (Figure S5). In the normal rats, XBP1 proteins were mainly expressed in neurons and were undetectable in astrocytes and microglia (Figure S5). In transgenic mice expressing mutant SOD1, XBP1 deficiency induces autophagy that clears protein inclusions and ameliorates disease phenotypes (Hetz et al., 2009). We examined autophagy by immunostaining and did not observe upregulation of the autophagy marker LC3 in the neurons expressing mutant TDP-43 (Figure S5). Overexpression of mutant TDP-43 in neurons led to downregulation of both spliced and unspliced XBP1 in transgenic rats.
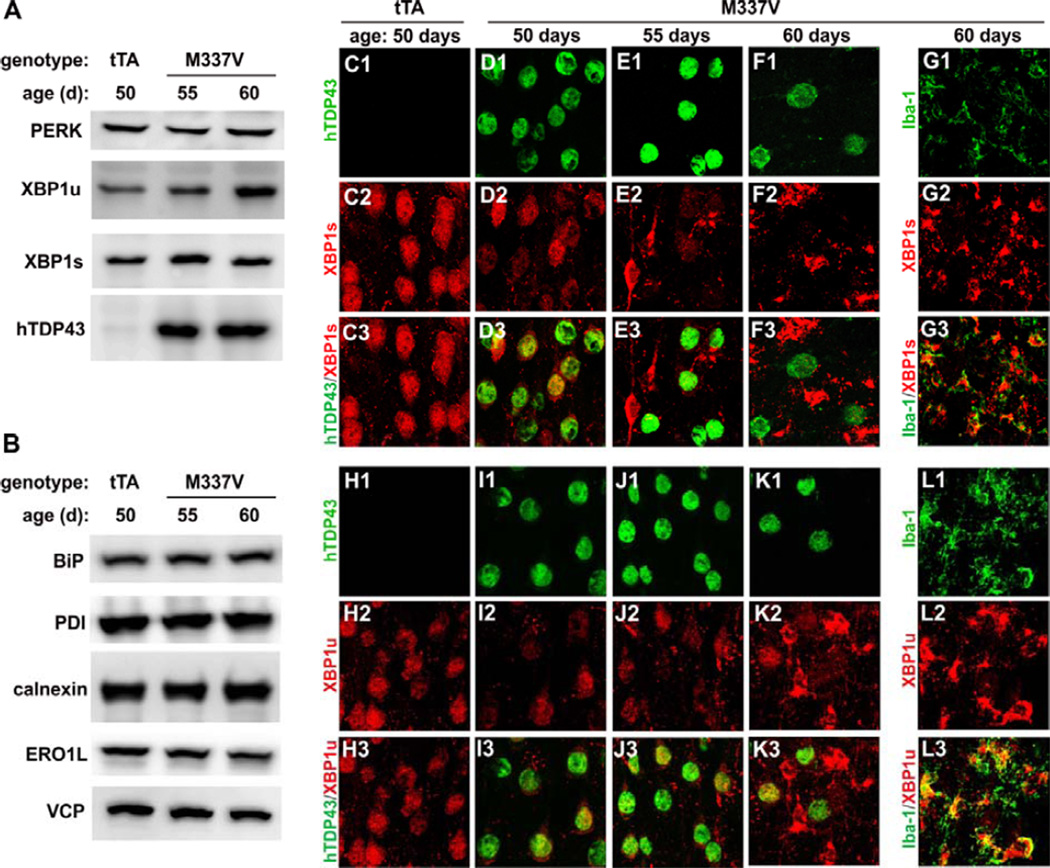
Figure 5.
Expression of XBP1 is downregulated in neurons and upregulated in reactive microglia in mutant TDP-43 transgenic rats. (A, B) Immunoblotting reveals expression of the genes responsive to endoplasmic reticule stress in the cortex of transgenic rats. Each lane was loaded with 20 µg of total proteins. Spliced (XBP1s) and unspliced (XBP1u) forms of XBP1 were upregulated in the total tissue homogenates of Camk2a-tTA/TRE-TDP43M337V double transgenic rats (M337V) as compared to Camk2a-tTA single transgenic rats (tTA). (C1–L3) Confocal microscopy reveals that both XBP1s and XBP1u were downregulated in neurons expressing mutant TDP-43 and were upregulated in reactive microglia. Z-stack images were taken at the same magnification and were projected to show the profile of gene expression.
Glial cells are activated in response to neurodegeneration in mutant TDP-43 transgenic rats
Glial reaction is a common feature of neurodegenerative diseases. In response to neuron death in mutant TDP-43 transgenic rats, astrocytes and microglia underwent morphological changes and became reactive (Figure 6). Reactive microglia was transformed from ramified cells into amoeboid-like cells with fewer ramifications (Figure 6A–6J). By contrast, activated astrocytes extended their processes and increased synthesis of its marker protein GFAP (Figure 6K–6T). Mutant TDP-43 transgene was expressed and neuron loss was detected first in the hippocampus (Figure 2). Accordingly, activated microglia and astrocytes were first detected in the hippocampus (Figure 6). Glial activation was related to neuron death in mutant TDP-43 transgenic rats.
Figure 6.
Glial cells are gradually activated in mutant TDP-43 transgenic rats. (A–J) Immunostaining of the microglial marker Iba-1 reveals remarkable activation of microglia in the tissues of Camk2a-tTA/TRE-TDP43M337V double transgenic rats (M337V) as compared to those of Camk2a-tTA single transgenic rats (tTA). (K–T) Immunostaining of the astrocyte marker GFAP reveals a progressive activation of astrocytes in mutant TDP-43 transgenic rats. Scale bars: A–H and K–N, 100 µm; O–R, 50 µm; I–J and S–T, 25 µm.
Discussion
Compared to other TDP-43 transgenic mice and rats (Wegorzewska et al., 2009, Shan et al., 2010, Stallings et al., 2010, Tsai et al., 2010, Wils et al., 2010, Xu et al., 2010, Igaz et al., 2011, Swarup et al., 2011a, Swarup et al., 2011b), this transgenic rat line expresses mutant human TDP-43 restrictedly in the neurons of rat’s forebrain and develops prominent ubiquitin aggregates in affected neurons. Using this ideal rat model, we examined the effects of mutant TDP-43 on the UPR in the formation of protein inclusion. In the rat model, ubiquitin inclusions developed prior to neuron death that was correlated with the expression of mutant TDP-43 and was related to the progressive loss of spatial memory. Consistent with previous findings (Zhou et al., 2010, Igaz et al., 2011, Uchida et al., 2012), our data show that ubiquitin inclusions did not contain mutant TDP-43 protein. Abundant ubiquitin aggregates imply that ubiquitinated proteins were accumulated in neurons and were delayed in degradation. Accumulation of ubiquitinated proteins may result from the dysfunction of proteasome, the increase in the production of misfolded or unfolded proteins, or the damage to cell-protective UPR within ER. Immunostaining did not reveal an accumulation of mutant TDP-43 in the cytoplasm, suggesting that mutant TDP-43 by itself did not impose a great burden on protein degradation machinery at least in our transgenic rats. As TDP-43 regulates numerous genes (Polymenidou et al., 2011, Sephton et al., 2011, Tollervey et al., 2011), it is not excluded that mutant TDP-43 affects protein degradation machinery through regulating the genes critical to protein degradation machinery.
Accumulation of misfolded or unfolded proteins inside cells may induce ER stress (Fujita et al., 2007). Indeed, overexpression of mutant TDP-43 in neurons caused severe and widespread fragmentation of Golgi apparatus, an organelle that is structurally and functionally related to ER (Hegde and Ploegh, 2010, Park and Blackstone, 2010). Ubiquitin aggregation and Golgi fragmentation were detected in the neurons of transgenic rats at nonsymptomatic stages. Although mitochondrion was affected in TDP-43 transgenic rats (Figure 4I), most mitochondria were morphologically intact when ER and Golgi apparatuses were severely swollen and vacuolated in nonsymptomatic rats (Figure 4). Golgi and ER appeared sensitive to mutation of TDP-43. Unexpectedly, neurons failed to activate UPR machinery even though ubiquitin aggregates were greatly accumulated. Three UPR transducer proteins were not upregulated in neurons and none of the ER chaperone proteins examined was upregulated. Moreover, XBP1 was depleted in neurons expressing mutant TDP-43. XBP1 is a key player in UPR and its suppression would compromise the ability of UPR machinery to upregulate ER chaperones and to decrease misfolded proteins (Casas-Tinto et al., 2011). In TDP-43 transgenic rats, XBP1 depletion may deteriorate cell-protective UPR machinery. In mutant SOD1 mice, deletion of XBP1 activates autophagy, initiating an alternative mechanism to decrease protein aggregates and to protect motor neurons (Hetz et al., 2009). In TDP-43 transgenic rats, XBP1 depletion did not significantly activate autophagy in neurons. While XBP1 was suppressed in neurons expressing mutant TDP-43, XBP1 was remarkably upregulated in reactive microglia. Upregulation of XBP1 in microglia may be related to the increase in cellular activity. Although it remains to determine how mutant TDP-43 leads to UPR failure, XBP1 deficiency and UPR failure likely contributes to TDP-43 pathogenesis in the disease.
Mutation of TDP-43 likely causes cell death by both a gain of toxic properties and a loss of protective functions. While overexpression of human TDP-43 in animals induces disease phenotypes (Wegorzewska et al., 2009, Shan et al., 2010, Stallings et al., 2010, Tsai et al., 2010, Wils et al., 2010, Xu et al., 2010, Igaz et al., 2011, Swarup et al., 2011a, Swarup et al., 2011b), deletion of mouse TDP-43 also causes motor neuron death (Majumder et al., 2012, Wu et al., 2012). TDP-43 tunes its expression through a self-regulatory mechanism (Ayala et al., 2011, Cohen et al., 2011, Igaz et al., 2011). In transgenic cells or animals, exogenous TDP-43 binds to the 3-untranslationed region of endogenous TDP-43 mRNA and thus decreases the stability of the mRNA, reducing the expression of endogenous TDP-43 (Ayala et al., 2011, Cohen et al., 2011, Igaz et al., 2011). Overexpression of mutant human TDP-43 in rats possibly causes disease phenotypes through affecting rat’s TDP-43 expression; however, an exclusive loss of functions is inadequate to explain disease phenotypes in all animal models. Deletion of mouse TDP-43 in the motor neurons causes a moderate loss of motor neurons and a slow progression of ALS phenotypes in conditional knockout mice (Wu et al., 2012); whereas, restricted overexpression of mutant TDP-43 in the motor neurons causes a severe loss of motor neurons and a rapid progression of ALS phenotypes in transgenic rats (Huang et al., 2012b). No matter whether mutation of TDP-43 induces neuron death mainly through a gain or a loss of functions, compromised UPR and defected XBP1 is likely implicated in the neuronal death caused by mutation in TDP-43. Further study should be to examine how mutant TDP-43 leads to XBP1 depletion and UPR failure.
Supplementary Material
Acknowledgement
We thank Ms. Xiao-Tao Wei for technical assistance. This work was supported by the National Institutes of Health (NS073829 to H.Z).
Abbreviation used
- XBP1
X-box binding protein 1
- ALS
amyotrophic lateral sclerosis
- FTLD
frontotemporal lobar degeneration
- ER
endoplasmic reticules
- UPR
unfolded protein response
- TDP-43
Tar DNA binding protein 43
- IRE1
include inositol-requiring protein 1
- ATF6
activating transcription factor 6
- PERK
PKR-related ER kinase
Footnotes
Conflict of interest: The authors declare that no conflict of interest exists.
References
- Ayala YM, De Conti L, Avendano-Vazquez SE, Dhir A, Romano M, D'Ambrogio A, Tollervey J, Ule J, Baralle M, Buratti E, Baralle FE. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011;30:277–288. doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. Embo J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Tinto S, Zhang Y, Sanchez-Garcia J, Gomez-Velazquez M, Rincon-Limas DE, Fernandez-Funez P. The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum Mol Genet. 2011;20:2144–2160. doi: 10.1093/hmg/ddr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Hwang AW, Unger T, Trojanowski JQ, Lee VM. Redox signalling directly regulates TDP-43 via cysteine oxidation and disulphide cross-linking. EMBO J. 2011 doi: 10.1038/emboj.2011.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II) Hum Mol Genet. 2007;16:618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Mizuno Y, Takatama M, Okamoto K. Anterior horn cells with abnormal TDP-43 immunoreactivities show fragmentation of the Golgi apparatus in ALS. Journal of the Neurological Sciences. 2008;269:30–34. doi: 10.1016/j.jns.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, Okwuonu G, Hines S, Lewis L, DeRamo C, Delgado O, Dugan-Rocha S, Miner G, Morgan M, Hawes A, Gill R, Celera, Holt RA, Adams MD, Amanatides PG, Baden-Tillson H, Barnstead M, Chin S, Evans CA, Ferriera S, Fosler C, Glodek A, Gu Z, Jennings D, Kraft CL, Nguyen T, Pfannkoch CM, Sitter C, Sutton GG, Venter JC, Woodage T, Smith D, Lee HM, Gustafson E, Cahill P, Kana A, Doucette-Stamm L, Weinstock K, Fechtel K, Weiss RB, Dunn DM, Green ED, Blakesley RW, Bouffard GG, De Jong PJ, Osoegawa K, Zhu B, Marra M, Schein J, Bosdet I, Fjell C, Jones S, Krzywinski M, Mathewson C, Siddiqui A, Wye N, McPherson J, Zhao S, Fraser CM, Shetty J, Shatsman S, Geer K, Chen Y, Abramzon S, Nierman WC, Havlak PH, Chen R, Durbin KJ, Egan A, Ren Y, Song XZ, Li B, Liu Y, Qin X, Cawley S, Cooney AJ, D'Souza LM, Martin K, Wu JQ, Gonzalez-Garay ML, Jackson AR, Kalafus KJ, McLeod MP, Milosavljevic A, Virk D, Volkov A, Wheeler DA, Zhang Z, Bailey JA, Eichler EE, Tuzun E, Birney E, Mongin E, Ureta-Vidal A, Woodwark C, Zdobnov E, Bork P, Suyama M, Torrents D, Alexandersson M, Trask BJ, Young JM, Huang H, Wang H, Xing H, Daniels S, Gietzen D, Schmidt J, Stevens K, Vitt U, Wingrove J, Camara F, Mar Alba M, Abril JF, Guigo R, Smit A, Dubchak I, Rubin EM, Couronne O, Poliakov A, Hubner N, Ganten D, Goesele C, Hummel O, Kreitler T, Lee YA, Monti J, Schulz H, Zimdahl H, Himmelbauer H, Lehrach H, Jacob HJ, Bromberg S, Gullings-Handley J, Jensen-Seaman MI, Kwitek AE, Lazar J, Pasko D, Tonellato PJ, Twigger S, Ponting CP, Duarte JM, Rice S, Goodstadt L, Beatson SA, Emes RD, Winter EE, Webber C, Brandt P, Nyakatura G, Adetobi M, Chiaromonte F, Elnitski L, Eswara P, Hardison RC, Hou M, Kolbe D, Makova K, Miller W, Nekrutenko A, Riemer C, Schwartz S, Taylor J, Yang S, Zhang Y, Lindpaintner K, Andrews TD, Caccamo M, Clamp M, Clarke L, Curwen V, Durbin R, Eyras E, Searle SM, Cooper GM, Batzoglou S, Brudno M, Sidow A, Stone EA, Payseur BA, Bourque G, Lopez-Otin C, Puente XS, Chakrabarti K, Chatterji S, Dewey C, Pachter L, Bray N, Yap VB, Caspi A, Tesler G, Pevzner PA, Haussler D, Roskin KM, Baertsch R, Clawson H, Furey TS, Hinrichs AS, Karolchik D, Kent WJ, Rosenbloom KR, Trumbower H, Weirauch M, Cooper DN, Stenson PD, Ma B, Brent M, Arumugam M, Shteynberg D, Copley RR, Taylor MS, Riethman H, Mudunuri U, Peterson J, Guyer M, Felsenfeld A, Old S, Mockrin S, Collins F. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Hegde RS, Ploegh HL. Quality and quantity control at the endoplasmic reticulum. Curr Opin Cell Biol. 2010;22:437–446. doi: 10.1016/j.ceb.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tong J, Bi F, Wu Q, Huang B, Zhou H, Xia XG. Entorhinal Cortical Neurons Are the Primary Targets of FUS Mislocalization and Ubiquitin Aggregation in FUS Transgenic Rats. Hum Mol Genet in press. 2012a doi: 10.1093/hmg/dds299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tong J, Bi F, Zhou H, Xia XG. Mutant TDP-43 in motor neurons promotes the onset and progression of ALS in rats. J Clin Invest. 2012b;122:107–118. doi: 10.1172/JCI59130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Zhou H, Tong J, Chen H, DW TW, LY J, Xia XG. FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration. PLOS Genetics. 2011;7:e1002011. doi: 10.1371/journal.pgen.1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Lee EB, Chen-Plotkin A, Swanson E, Unger T, Malunda J, Xu Y, Winton MJ, Trojanowski JQ, Lee VM. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J Clin Invest. 2011;121:726–738. doi: 10.1172/JCI44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Suzuki N. Seipinopathy: a novel endoplasmic reticulum stress-associated disease. Brain. 2009;132:8–15. doi: 10.1093/brain/awn216. [DOI] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Velde CV, Bouchard J-P, Lacomblez L, Pochigaeva K, Salachas F, Pradat P-F, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature Genetics. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Lowe M. Structural organization of the Golgi apparatus. Curr Opin Cell Biol. 2011;23:85–93. doi: 10.1016/j.ceb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Majumder P, Chen YT, Bose JK, Wu CC, Cheng WC, Cheng SJ, Fang YH, Chen YL, Tsai KJ, Lien CC, Shen CK. TDP-43 regulates the mammalian spinogenesis through translational repression of Rac1. Acta Neuropathol. 2012 doi: 10.1007/s00401-012-1006-4. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Park SH, Blackstone C. Further assembly required: construction and dynamics of the endoplasmic reticulum network. EMBO Rep. 2010;11:515–521. doi: 10.1038/embor.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, Kordasiewicz H, Sedaghat Y, Donohue JP, Shiue L, Bennett CF, Yeo GW, Cleveland DW. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14:459–468. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford NJ, Zhang YJ, Baker M, Gass JM, Finch NA, Xu YF, Stewart H, Kelley BJ, Kuntz K, Crook RJ, Sreedharan J, Vance C, Sorenson E, Lippa C, Bigio EH, Geschwind DH, Knopman DS, Mitsumoto H, Petersen RC, Cashman NR, Hutton M, Shaw CE, Boylan KB, Boeve B, Graff-Radford NR, Wszolek ZK, Caselli RJ, Dickson DW, Mackenzie IR, Petrucelli L, Rademakers R. Novel Mutations in TARDBP (TDP-43) in Patients with Familial Amyotrophic Lateral Sclerosis. PLoS Genet. 2008;4:e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton CF, Cenik C, Kucukural A, Dammer EB, Cenik B, Han Y, Dewey CM, Roth FP, Herz J, Peng J, Moore MJ, Yu G. Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J Biol Chem. 2011;286:1204–1215. doi: 10.1074/jbc.M110.190884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Chiang PM, Price DL, Wong PC. Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc Natl Acad Sci U S A. 2010;107:16325–16330. doi: 10.1073/pnas.1003459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings NR, Puttaparthi K, Luther CM, Burns DK, Elliott JL. Progressive motor weakness in transgenic mice expressing human TDP-43. Neurobiol Dis. 2010;40:404–414. doi: 10.1016/j.nbd.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Swarup V, Phaneuf D, Bareil C, Robertson J, Rouleau GA, Kriz J, Julien JP. Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments. Brain. 2011a;134:2610–2626. doi: 10.1093/brain/awr159. [DOI] [PubMed] [Google Scholar]
- Swarup V, Phaneuf D, Dupre N, Petri S, Strong M, Kriz J, Julien JP. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor kappaB-mediated pathogenic pathways. J Exp Med. 2011b;208:2429–2447. doi: 10.1084/jem.20111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, Konig J, Hortobagyi T, Nishimura AL, Zupunski V, Patani R, Chandran S, Rot G, Zupan B, Shaw CE, Ule J. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KJ, Yang CH, Fang YH, Cho KH, Chien WL, Wang WT, Wu TW, Lin CP, Fu WM, Shen CK. Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. J Exp Med. 2010;207:1661–1673. doi: 10.1084/jem.20092164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor EL, Galtrey CM, Perkinton MS, Lau KF, De Vos KJ, Mitchell JC, Ackerley S, Hortobagyi T, Vamos E, Leigh PN, Klasen C, McLoughlin DM, Shaw CE, Miller CC. Amyotrophic lateral sclerosis mutant vesicle-associated membrane protein-associated protein-B transgenic mice develop TAR-DNA-binding protein-43 pathology. Neuroscience. 2010;167:774–785. doi: 10.1016/j.neuroscience.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Uchida A, Sasaguri H, Kimura N, Tajiri M, Ohkubo T, Ono F, Sakaue F, Kanai K, Hirai T, Sano T, Shibuya K, Kobayashi M, Yamamoto M, Yokota S, Kubodera T, Tomori M, Sakaki K, Enomoto M, Hirai Y, Kumagai J, Yasutomi Y, Mochizuki H, Kuwabara S, Uchihara T, Mizusawa H, Yokota T. Non-human primate model of amyotrophic lateral sclerosis with cytoplasmic mislocalization of TDP-43. Brain. 2012;135:833–846. doi: 10.1093/brain/awr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proceedings of the National Academy of Sciences. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wils H, Kleinberger G, Janssens J, Pereson S, Joris G, Cuijt I, Smits V, Ceuterick-de Groote C, Van Broeckhoven C, Kumar-Singh S. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proceedings of the National Academy of Sciences. 2010;107:3858–3863. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LS, Cheng WC, Shen CK. Targeted Depletion of TDP-43 Expression in the Spinal Cord Motor Neurons Leads to the Development of Amyotrophic Lateral Sclerosis (ALS)-like Phenotypes in Mice. J Biol Chem. 2012 doi: 10.1074/jbc.M112.359000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YF, Gendron TF, Zhang YJ, Lin WL, D'Alton S, Sheng H, Casey MC, Tong J, Knight J, Yu X, Rademakers R, Boylan K, Hutton M, McGowan E, Dickson DW, Lewis J, Petrucelli L. Wild-Type Human TDP-43 Expression Causes TDP-43 Phosphorylation, Mitochondrial Aggregation, Motor Deficits, and Early Mortality in Transgenic Mice. Journal of Neuroscience. 2010;30:10851–10859. doi: 10.1523/JNEUROSCI.1630-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Zhou H, Huang C, Chen H, Wang D, Landel CP, Xia PY, Bowser R, Liu YJ, Xia XG. transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLOS Genetics. 2010;6:e1000887. doi: 10.1371/journal.pgen.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Huang C, Yang M, Landel CP, Xia PY, Liu YJ, Xia XG. Developing tTA Transgenic Rats for Inducible and Reversible Gene Expression. Int J Biol Sci. 2009;2:171–181. doi: 10.7150/ijbs.5.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.