Abstract
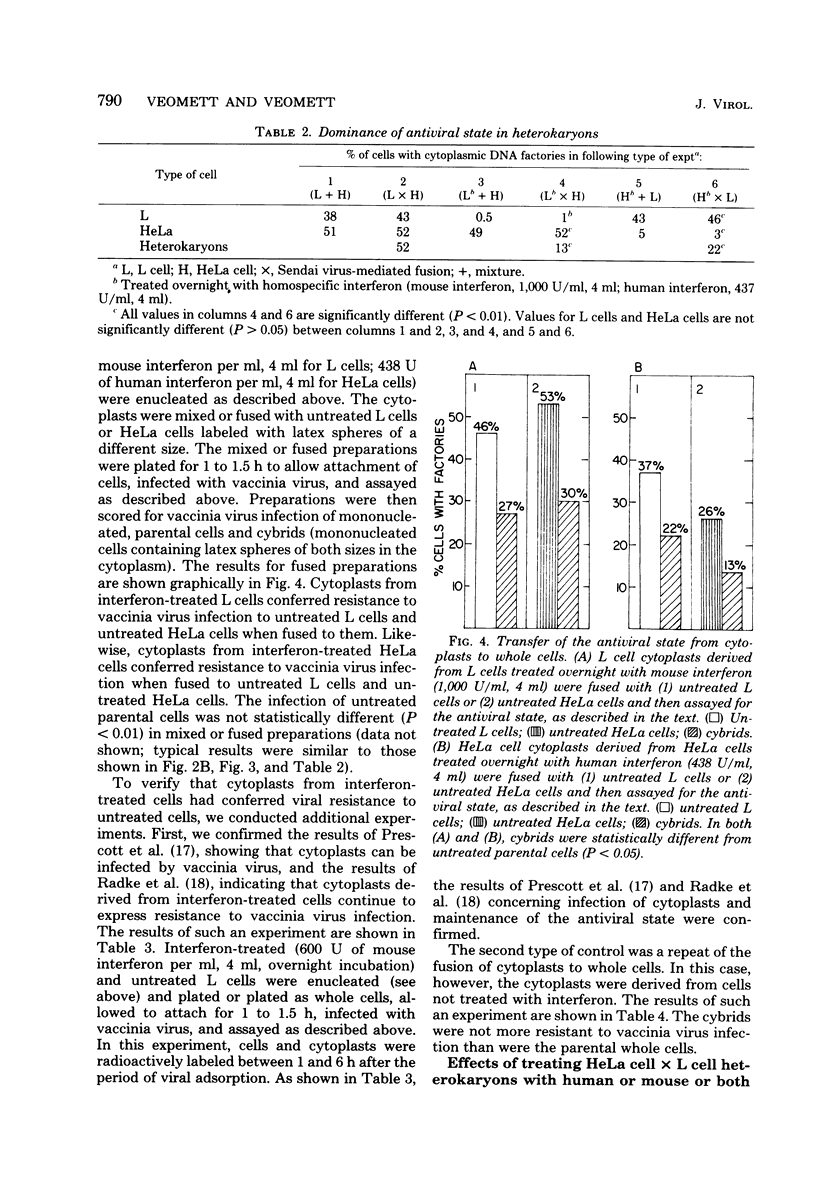
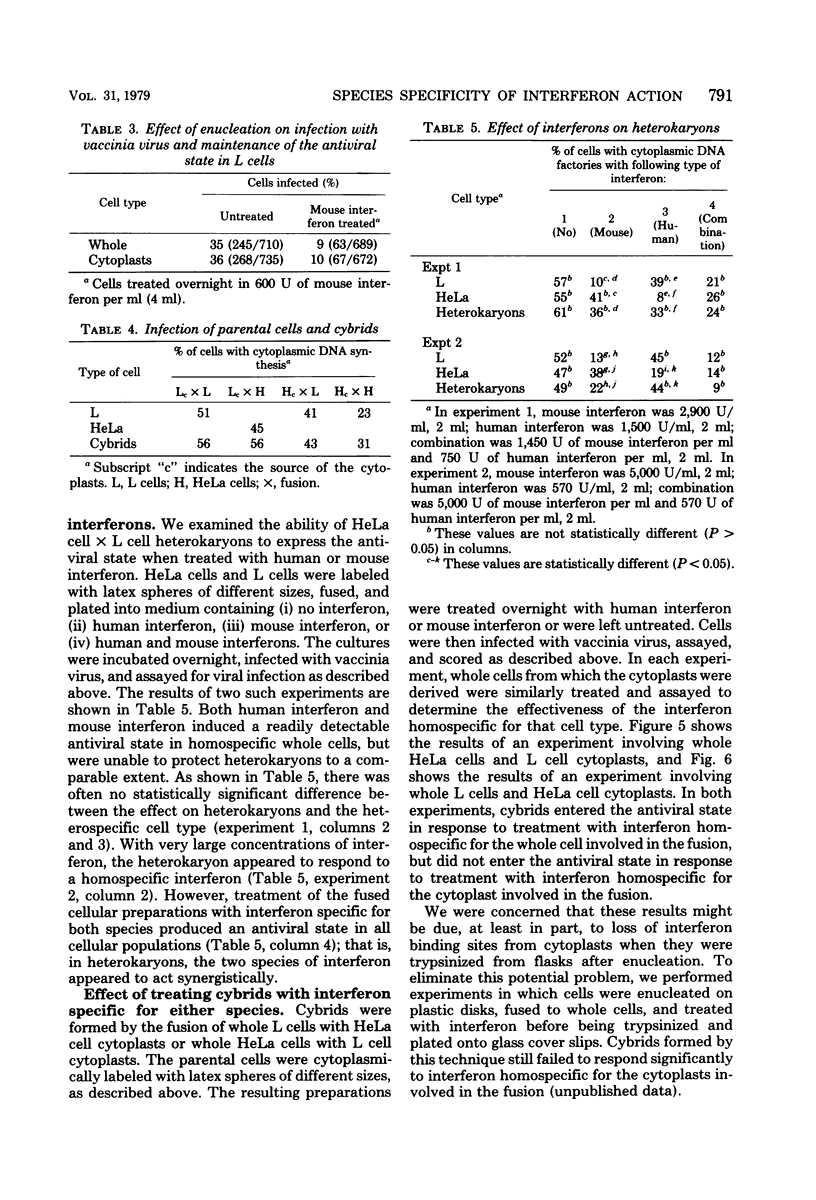
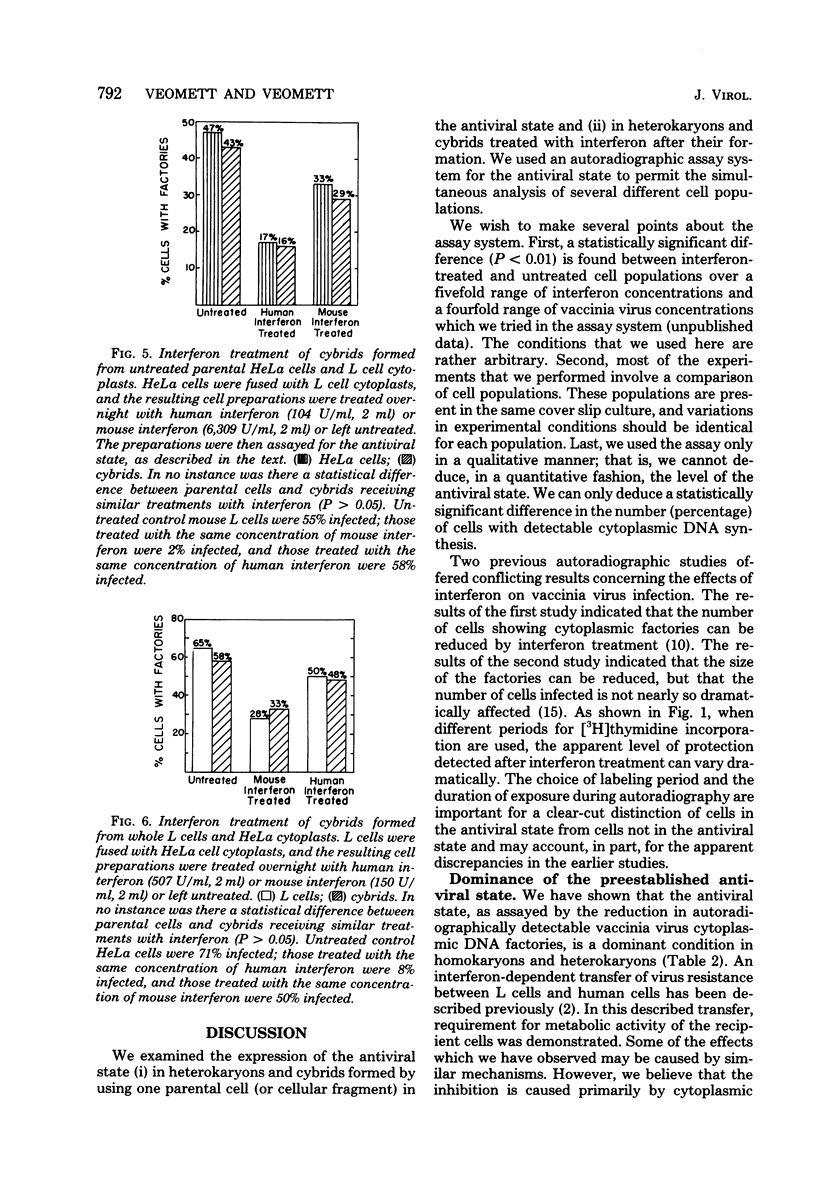
The expression of the interferon-induced antiviral state was studied in heterokaryons and cytoplasmic hybrids (cybrids). An autoradiographic assay for the antiviral state, in which the percentage of cells containing vaccinia viral DNA factories was determined, was used. The expression of the antiviral state was dominant in homokaryons and heterokaryons formed by fusion of interferon-treated cells with untreated cells. Cytoplasts derived from treated cells conferred resistance to virus growth on cybrids formed by fusing such cytoplasts with untreated cells. Treatment of L cell x HeLa cell heterokaryons with human interferon or mouse interferon was much less effective in inducing a detectable antiviral state than was similar treatment of parental cells with homospecific interferon. The antiviral state was fully induced when heterokaryons were treated simultaneously with both types of interferon. Cybrids formed by fusing L cell cytoplasts with HeLa cells or HeLa cytoplasts with L cells did not enter a detectable antiviral state after treatment with interferon specific for the cell type of the enucleated parent. However, treatment of cybrids with interferon specific for the cell type of the nucleated parent was effective in inducing a detectable antiviral state.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A., White C. N. Oligonucleotide inhibitor of protein synthesis made in extracts of interferon-treated chick embryo cells: comparison with the mouse low molecular weight inhibitor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1167–1171. doi: 10.1073/pnas.75.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock J. E., Baron S. Mechanisms of interferon induced transfer of viral resistance between animal cells. J Gen Virol. 1979 Feb;42(2):363–372. doi: 10.1099/0022-1317-42-2-363. [DOI] [PubMed] [Google Scholar]
- Bourgeade M. F. Interferon cell species specificity: role of cell membrane receptors. Proc Soc Exp Biol Med. 1974 Jul;146(3):820–824. doi: 10.3181/00379727-146-38198. [DOI] [PubMed] [Google Scholar]
- Burke D. C., Veomett G. Enucleation and reconstruction of interferon-producing cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3391–3395. doi: 10.1073/pnas.74.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassingena R., Chany C., Vignal M., Suarez H., Estrade S., Lazar P. Use of monkey-mouse hybrid cells for the study of the cellular regualtion of interferon production and action. Proc Natl Acad Sci U S A. 1971 Mar;68(3):580–584. doi: 10.1073/pnas.68.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chany C. Membrane-bound interferon specific cell receptor system: role in the establishment and amplification of the antiviral state. Biomedicine. 1976 Jun;24(3):148–157. [PubMed] [Google Scholar]
- De Clercq E., Edy V. G., Cassiman J. J. Chromosome 21 does not code for an interferon receptor. Nature. 1976 Nov 18;264(5583):249–251. doi: 10.1038/264249a0. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN R. M., SONNABEND J. A. INHIBITION OF INTERFERON ACTION BY P-FLUOROPHENYLALANINE. Nature. 1964 Jul 25;203:366–367. doi: 10.1038/203366a0. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN R. M., SONNABEND J. A., MCDEVITT H. INTERFERON INHIBITION OF CYTOPLASMIC DNA ACCUMULATION IN VACCINIA VIRUS INFECTION. A RADIOAUTOGRAPHIC STUDY. Proc Soc Exp Biol Med. 1965 Jun;119:551–553. doi: 10.3181/00379727-119-30235. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Brown R. E., Kerr I. M. Synthesis of low molecular weight inhibitor of protein synthesis with enzyme from interferon-treated cells. Nature. 1977 Aug 11;268(5620):537–540. doi: 10.1038/268537a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L. D., Friedman R. M., Holmes J. M., Lee G. Use of thyrotropin and cholera toxin to probe the mechanism by which interferon initiates its antiviral activity. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3695–3699. doi: 10.1073/pnas.73.10.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Magee W. E., Hamilton R. D., Miller O. V. Effect of interferon on early enzyme and viral DNA synthesis in vaccinia virus infections. Virology. 1967 May;32(1):33–40. doi: 10.1016/0042-6822(67)90249-8. [DOI] [PubMed] [Google Scholar]
- Meldolesi M. F., Friedman R. M., Kohn L. D. An interferon-induced increase in cyclic AMP levels precedes the establishment of the antiviral state. Biochem Biophys Res Commun. 1977 Nov 7;79(1):239–246. doi: 10.1016/0006-291x(77)90086-9. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Kates J., Kirkpatrick J. B. Replication of vaccinia virus DNA in enucleated L-cells. J Mol Biol. 1971 Aug 14;59(3):505–508. doi: 10.1016/0022-2836(71)90313-5. [DOI] [PubMed] [Google Scholar]
- Radke K. L., Colby C., Kates J. R., Krider H. M., Prescott D. M. Establishment and maintenance of the interferon-induced antiviral state: studies in enucleated cells. J Virol. 1974 Mar;13(3):623–630. doi: 10.1128/jvi.13.3.623-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Bash D., Ruddle F. H. Antibodies to a cell-surface component coded by human chromosome 21 inhibit action of interferon. Nature. 1976 Mar 11;260(5547):139–141. doi: 10.1038/260139a0. [DOI] [PubMed] [Google Scholar]
- Slate D. L., Shulman L., Lawrence J. B., Revel M., Ruddle F. H. Presence of human chromosome 21 alone is sufficient for hybrid cell sensitivity to human interferon. J Virol. 1978 Jan;25(1):319–325. doi: 10.1128/jvi.25.1.319-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. H. T98G: an anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in vitro. J Cell Physiol. 1979 Apr;99(1):43–54. doi: 10.1002/jcp.1040990107. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. STUDIES ON THE MECHANISM OF ACTION OF INTERFERON. I. INTERFERON ACTION AND RNA SYNTHESIS IN CHICK EMBRYO FIBROBLASTS INFECTED WITH SEMLIKI FOREST VIRUS. Virology. 1965 Mar;25:340–349. doi: 10.1016/0042-6822(65)90053-x. [DOI] [PubMed] [Google Scholar]
- TYRRELL D. A. Interferon produced by cultures of calf kidney cells. Nature. 1959 Aug 8;184(Suppl 7):452–453. doi: 10.1038/184452a0. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veomett G., Shay J., Hough P. V., Prescott D. M. Large-scale enucleation of mammalian cells. Methods Cell Biol. 1976;13:1–6. doi: 10.1016/s0091-679x(08)61794-x. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]