Abstract
Background
Thymidine analog 5-ethynyl-2-deoxyuridine (EdU) has recently been employed for tracking mesenchymal stem cells (MSCs). In the present study we tested whether EdU was cytotoxic and whether it interfered with MSC’s differentiation, cytokine secretion, and migration.
Methods
EdU labeling was performed by incubating adipose-derived stem cells (ADSCs) with 10 μM of EdU for 48 hours. Incorporation of EdU was detected by reaction with azide-conjugated Alexa594. The labeled and unlabeled ADSCs were compared for proliferation and apoptosis as determined by CellTiter and comet assays, respectively. They were also compared for neuron-like and endothelial differentiation as determined by morphology, marker expression, and function. Comparison of their secreted cytokine profile was performed by cytokine antibody array. Comparison of their response to homing factor SDF-1 was performed by migration assay.
Results
EdU was incorporated into the nucleus in approximately 70% of ADSCs. No significant differences in proliferation and apoptosis rates were observed between EdU-labeled and unlabeled ADSCs. Isobutylmethylxanthine (IBMX) induced both EdU-labeled and unlabeled ADSCs to assume a neuron-like morphology and to express β-III tubulin. Endothelial growth medium-2 (EGM2) induced endothelial differentiation in both EdU-labeled and unlabeled ADSCs, including the ability to uptake low-density lipoprotein (LDL) and to form capillary-like structures as well as the expression of vWF, eNOS, and CD31. EdU-labeled and unlabeled ADSCs exhibited identical secreted cytokine profile and identical migratory response to SDF-1.
Discussion
At the recommended dosage of 10 μM EdU is non-toxic to ADSCs. EdU label did not interfere with ADSC’s differentiation, cytokine secretion, or migratory response to SDF-1.
Keywords: EdU, mesenchymal stem cells, adipose-derived stem cells, cell labeling, differentiation, cytokine expression, migration
Introduction
Thymidine analogs have been used extensively for the analysis of cellular DNA synthesis, tracking of transplanted cells, and identification of tissue-resident stem cells [1-4]. The importance of these thymidine analogs in biomedical research is best exemplified by the utilization of 5-bromo-2-deoxyuridine (BrdU) in more than 20,000 peer-reviewed studies [1]. However, the histological detection of BrdU requires harsh conditions that affect cellular structure and protein antigenicity. In addition, the resulting histological images can be difficult to assess due to low signal versus noise ratio. As such, in 2008 Salic and Mitchison introduced a new thymidine analog, 5-ethynyl-2′-deoxyuridine (EdU), which can be easily detected without affecting cellular structure or protein antigenicity [5]. In 2009 we reported for the first time the use of EdU for the labeling and tracking of mesenchymal stem cells (MSCs), specifically, adipose-derived stem cells (ADSCs) [2]. We have since used this method to track transplanted ADSCs in several preclinical studies, for example, in a rat model of stress urinary incontinence [3]. We have also used it for the detection of potential tissue-resident stem cells via the “label-retaining cell” strategy [4]. In studies that examined possible cellular differentiation of transplanted ADSCs, we occasionally observed smooth muscle or endothelial differentiation based on the colocalization of the EdU label with specific cell lineage markers [3,6,7]. But such colocalization occurred only rarely and therefore, we proposed that ADSC’s therapeutic effects might derive from its paracrine action rather than cellular differentiation [3,8,9]. However, it has been shown that BrdU was cytotoxic and inhibited cell differentiation and migration [10,11]. Thus, it is possible that the infrequent cellular differentiation of transplanted ADSCs was a consequence of EdU labeling. On the other hand, it has also been shown that BrdU labeling did not have adverse effects on ADSCs [12]. Therefore, amid these conflicting reports on BrdU’s adverse effects or lack of, the present study aimed at testing whether EdU labeling affects cell proliferation, apoptosis, cytokine secretion, differentiation, and migration.
Material and Methods
EdU labeling of ADSC
Isolation and culture of rat ADSCs have been described previously [3]. Cells at 4th passage were used in the present study. For EdU labeling, 1 ×105 cells were seeded in each well of a 6-well plate in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Twenty-four hours later, EdU (Invitrogen, Carlsbad, CA) was added to the medium at 10 μM. Another 48 hours later, cells were fixed for EdU staining or for further tests. For EdU staining, the cells were fixed with methanol, washed twice with phosphate-buffered saline (PBS), incubated in 3% bovine serum albumin (BSA) in PBS, and then incubated in 0.5% Triton® X-100 in PBS for 20 min at room temperature. The cells were then incubated with freshly made Click-iT reaction cocktail, which contained azide-conjugated Alexa594 (Invitrogen), for 30 min at room temperature in the dark. The cells were further stained with 4′,6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 μg/ml, Sigma-Aldrich, St. Louis, MO) and then mounted in standard mounting media. The stained cells were examined with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera. For determination of the percentage of EdU-positive cells, the number of red-fluorescent (Alexa594-stained) cells was divided by the number of blue-fluorescent (DAPI-stained) cells. The experiment was performed in triplicate and data presented in Results are the average of three independent experiments.
Proliferation assay
EdU-labeled or unlabeled ADSCs were seeded into a 96-well plate at 2,000 cells/well in DMEM with 10% FBS and incubated at 37°C. At 0, 24, 48, 72, and 96 hours, 10 μl of CellTiter-96 reagent (Promega Inc., Madison, WI) was added to each well. After 1 hour of further incubation at 37°C, the cells were scanned in a plate reader (Molecular Devices Corp., Sunnyvale, CA) at 490-nm absorbance. All assays were performed in triplicate and all data presented in Results are the average of three independent experiments.
Apoptosis assay
Cellular apoptosis was analyzed by the CometAssay Electrophoresis Systems (Trevigen, Inc., Gaithersburg, MD). Briefly, 50 μl (5,000 cells) of EdU-labeled or unlabeled ADSCs in PBS were mixed with 500 μl of molten low-melting agarose at 37°C. An aliquote of 75 μl of the mixture was immediately pipeted and spread evenly onto a CometSlide (Trevigen). The slide was then placed in a refrigerator for 10-30 min and immersed in pre-chilled lysis solution for another 30-60 min. Afterward, the slide was immersed in freshly prepared alkaline solution for 20-60 min at room temperature in the dark. The slide was then washed with Tris-borate-EDTA (TBE) buffer twice for 5 min each and electrophoresed in TBE at 1 v/cm for 10 min. Afterward, the slide was dipped in 70% ethanol for 5 min and air-dried (until the agarose became dry). An aliquote of 50 μl of diluted SYBR Green I (Trevigen) was then added onto each well of the dried agarose. The slide was then observed with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera. For determination of the percentage of apoptotic cells, the number of cells with comet tail (indication of apoptosis) was divided by the number of all cells. The experiment was performed in triplicate and data presented in Results are the average of three independent experiments.
In vitro neuronal differentiation
ADSC neuron-like differentiation was performed as previously described [13]. Briefly, EdU-labeled or unlabeled ADSCs were seeded into a 6-well plate at 5 × 105 cells per well in DMEM with 10% FBS. The next day, the cells were washed 3 times with PBS and the medium changed to DMEM supplemented with 500 μM IBMX (Sigma-Aldrich). One hour later the cells were stained for the presence of EdU and for neuron-specific marker β-III tubulin [14] (using anti-β-III tubulin antibody from Abcam, Inc., Cambridge, MA), followed by nuclear stain with DAPI.
In vitro endothelial differentiation
ADSC endothelial differentiation was performed as previously described [7]. Briefly, EdU-labeled or unlabeled ADSCs were seeded into a 6-well plate at 5 × 105 cells per well in DMEM with 10% FBS. The next day, the culture medium was replaced with EGM2 (Lonza Biologics Inc., Portsmouth, NH) and the cells further incubated for 7 days with the medium replenished every three days. The cells were then analyzed for endothelial marker expression and for LDL uptake and tube formation. For endothelial marker expression the cells were stained for the presence of EdU and by antibodies against endothelial specific markers CD31 (Abcam), vWF (Abcam), and eNOS [15] (BD Biosciences, San Jose, CA), followed by nuclear stain with DAPI.
Immunofluorescence staining
Cells were fixed in cold methanol for 5 min at 4°C, permeabilized with 0.05% Triton X-100 for 5 min, and blocked with 5% normal equine serum in PBS for 1 hour at room temperature. The cells were then incubated with primary antibody for 1 hour at room temperature. After washing with PBS three times, the cells were incubated with Texas red- or fluorescein isothiocyanate-conjugated secondary antibody for another hour at room temperature. After three washes with PBS, the cells were further stained with DAPI.
Low-density lipoprotein (LDL) uptake
LDL uptake was performed as previously described [7]. Briefly, cells were seeded into 6-well plates at of 5×104 cells per well in 3 ml of EGM2 and incubated at 37°C. The next day, 10 mg/ml of DiI-conjugated acetylated low-density lipoprotein complex (DiI-AcLDL, Invitrogen) was added to the culture medium. The next day, after the medium was removed, the cells were washed 3 times with PBS, examined by phase-contrast and fluorescence microscopy, and photographed. Quantification of LDL-uptake (in pixel number) in each well was performed on photographic images of 9 randomly chosen fields at 200x magnification using Image-Pro Plus 5.1 (Media Cybernetics, Silver Spring, MD). The experiment was performed in triplicate and data presented in Results are the average of three independent experiments.
Matrigel-based capillary-like tube formation assay
This assay was performed as previously described [7], and was initiated by coating a 12-well culture plate with 300 μl of growth factor-reduced Matrigel (BD Biosciences) per well. Approximately 1.5 × 105 cells in 1 ml of EGM2 were then seeded into each well and incubated at 37°C. Sixteen hours later, develop ment of capillary-like networks was examined by phase-contrast microscopy and photographed. Endotubes were quantified by counting their numbers in 9 random fields/sample under the microscope (x50). The experiment was performed in triplicate and data presented in Results are the average of three independent experiments.
Cytokine Antibody Array
An antibody-based cytokine array system (RayBio Rat Cytokine Antibody Array 1, RayBiotech, Norcross, GA) was used to detect cytokines in the culture medium of EdU-labeled or unlabeled ADSCs as previously described [16]. Briefly, the cells were cultured in 10% FBS-supplemented DMEM in 6-well dish to 80% confluence. The culture medium was then changed to serum-free DMEM. Twenty-four hours later, the medium was collected and centrifuged at 13,000×g for 10 s. The supernatant was recovered and used in the array experiment as follows. The array membrane was placed in an eight-well tray and incubated in 4 ml of 1X blocking buffer for 45 min at room temperature. Each test sample (2 ml of cell culture medium) was added to the membrane, which was then shaken for 2 hours at room temperature. After washing the membrane three times, a cocktail of biotinated anti-cytokine antibodies was added and the reaction was allowed to proceed for 1 hour at room temperature on shaker. After washing the membrane three times, horseradish-conjugated streptavidin was added and the reaction was allowed to proceed for 1 hour at room temperature on shaker. Finally, the membrane was washed as above, incubated in enhanced chemiluminescence detection regents (Amersham Life Sciences Inc., Arlington Heights, IL) for 5 min, and exposed to Kodak autoradiography film for 1–15 min.
Cell migration assay
SDF-1α-induced migration of EdU-labeled or unlabeled ADSCs was assessed by using 24-well BioCoat with an 8-μm pore size (BD Biosciences) as previously described [17]. Briefly, 500 μl of DMEM containing 1% FBS and 0 or 100 ng/ml of SDF-1α (R&D Systems, Inc., Minneapolis, MN) was added to the lower chamber, and 300 μl of DMEM containing 1 × 104 ADSCs was added to the upper chamber. After incubation for 24 hours at 37°C in a humidified atmosphere with 5% CO 2, migrated cells, which adhered to the lower surface of a glass cover slide, were stained with calcein (Invitrogen) for 10 min and manually counted in 8 random microscopic fields. The experiment was performed in triplicate and data presented in Results are the average of three independent experiments.
Statistical analysis
Data was analyzed with Prism 5 (GraphPad Software, Inc., San Diego, CA) and expressed as mean ± standard error of the mean for continuous variables. The continuous data was compared among the groups using one-way analysis of variance. The Tukey-Kramer test was used for post-hoc comparisons. Statistical significance was set at p < 0.05.
Results
EdU labeling did not inhibit cell proliferation or increase cell death
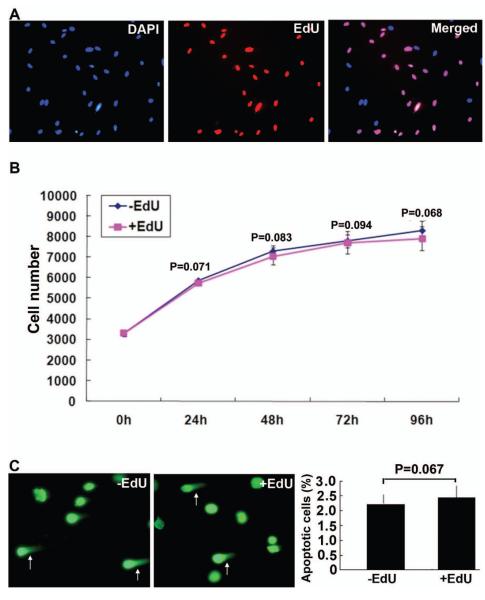
Incubation of ADSCs with 10μM EdU for 48h resulted in the incorporation of EdU in the nucleus of approximately 70% of cells (Fig. 1A). When labeled cells were compared to unlabeled cells for proliferation rate, no statistical difference was noted (Fig. 1B). Likewise, no statistical difference in apoptosis rate was observed between labeled and unlabeled cells (Fig. 1C).
Figure 1.
EdU labeling efficiency and effects on proliferation and apoptosis of ADSCs. (A) ADSCs were labeled with EdU at 10 μM for 48 hours and stained with azide-conjugated Alexa594 (red fluorescence) and DAPI (blue fluorescence). Original magnification was 200x. (B) Proliferation of EdU-labeled and unlabeled ADSCs was assessed by CellTiter at the indicated time points. (C) Apoptosis of EdU-labeled and unlabeled ADSCs was assessed by CometAssay. Representative images are shown on the left with arrows pointing at apoptotic cells. The number of apoptotic cells as a percentage of total cells is shown in the bar chart on the right.
EdU labeling did not affect the ability of ADSCs to differentiate
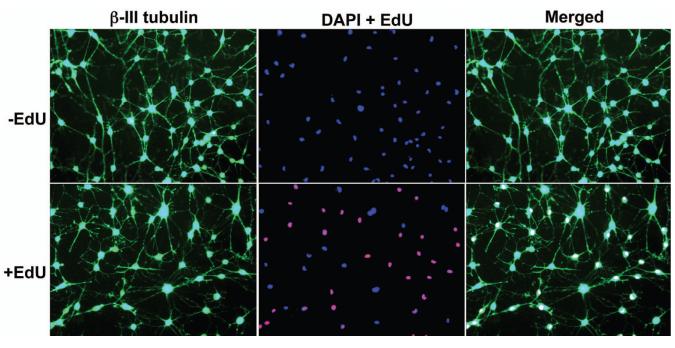
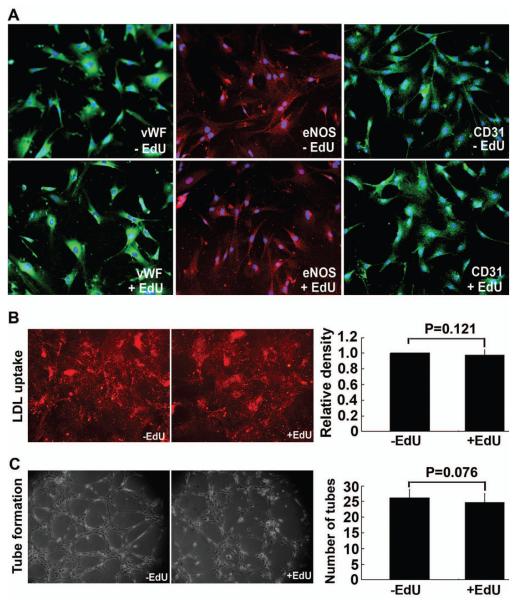
When ADSCs were treated with 500 μM IBMX, they assumed the morphology of neuron-like cells with extensive intercellular networks and stained positive for neuronal marker β-III tubulin. These phenotypical changes occurred in both EdU-labeled and unlabeled ADSCs (Fig. 2). When ADSCs were grown in EGM2, they expressed endothelial markers vWF, eNOS, and CD31 (Fig. 3A). They also acquired the ability to uptake LDL and to form capillary-like tubular structures (Fig. 3B & C). These endothelial phenotypes were displayed by both EdU-labeled and unlabeled ADSCs (Fig. 3).
Figure 2.
Effects of EdU labeling on neuron-like differentiation of ADSCs. EdU-labeled and unlabeled ADSCs were induced to differentiate into neuron-like cells by IBMX and stained for the presence of EdU (red fluorescence) and for β-III tubulin (green fluorescence), followed by nuclear stain with DAPI (blue fluorescence). Original magnification was 200x.
Figure 3.
Effects of EdU labeling on endothelial differentiation of ADSCs. EdU-labeled and unlabeled ADSCs were induced to differentiate into endothelial cells as verified by (A) the expression of endothelial markers CD31, vWF (green fluorescence), and eNOS (red fluorescence); (B) LDL uptake (red fluorescence); and (C) tube formation. Blue fluorescence in panel A indicates cell nuclei. Quantitative data of LDL uptake and tube formation are shown in the bar charts on the rightmost column of panels B and C, respectively.
EdU labeling did not affect the secreted cytokine profile of ADSCs
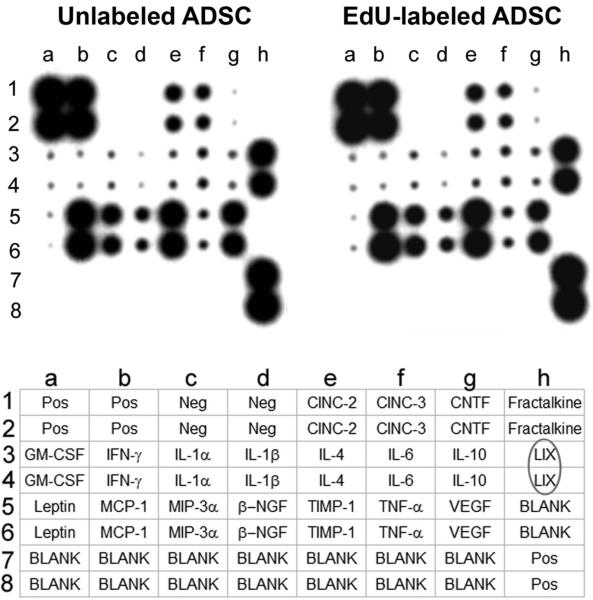
Secretion of a panel of 19 different cytokines was analyzed by an antibody-based array, and the results show little or no difference between EdU-labeled and unlabeled ADSCs (Fig. 4).
Figure 4.
Effects of EdU labeling on cytokine secretion of ADSCs. EdU-labeled and unlabeled ADSCs were analyzed for cytokine secretion by the RayBio Rat Cytokine Antibody Array, whose key is shown at the bottom. Cytokine LIX (CXCL5) is circled in the key to highlight the characteristic high-level secretion of this cytokine by ADSCs.
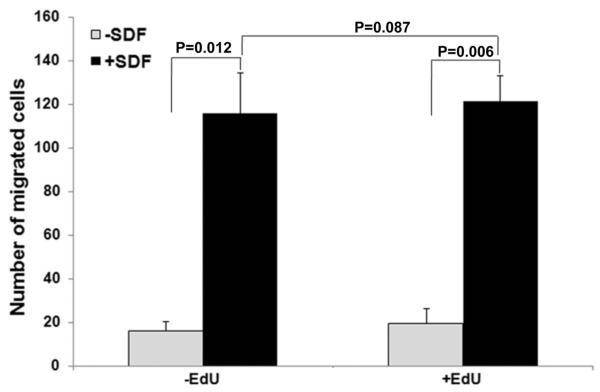
EdU labeling did not affect the migration of ADSCs
EdU-labeled and unlabeled ADSCs were analyzed for their migratory response to homing factor SDF-1α; the results show no statistical difference (Fig. 5).
Figure 5.
Effects of EdU labeling on migratory response of ADSCs toward SDF-1α. EdU-labeled and unlabeled ADSCs were analyzed for migratory response to SDF-1α by using 24-well BioCoat with an 8-μm pore size. The migrated cells were visualized by calcein stain and counted. The number of migrated cells was averaged from 3 independent experiments.
Discussion
Several different labels have been used for tracking transplanted cells; however, most of them are problematic due to “leakage”, cytotoxicity, and/or detection difficulties [18,19]. EdU is a recently developed label that is easy to use and can be detected without ambiguity [5]. However, due to its nascency, whether EdU labeling has adverse effects remains unexplored. In our earlier studies with EdU labeling we chose a labeling time of 24 hours or overnight and this resulted in a labeling rate of approximately 50% [2,6]. In our more recent studies we changed the labeling time to 48 hours [8,20] and this resulted in a labeling rate of approximately 70%. While this change of labeling time has been mentioned in some of our published studies [8,20], the actual data is presented for the first time in the present study. More importantly, we found that EdU-labeled ADSCs were able to proliferate at the same rate as unlabeled ADSCs, and they also underwent apoptosis at the same rate as unlabeled ADSCs. These findings suggest that, when used at the recommended dosage of 10 μM, EdU is non-cytotoxic.
One of the purposes of using labeled stem cells (SCs) is to determine whether SCs can differentiate into certain cell types after their transplantation. However, many studies, including ours, have not been able to convincingly demonstrate such differentiations [9]. This raised the possibility that the labels may impede SC differentiation, and as such, in the present study we investigated whether EdU had this adverse effect. For induction of neuronal differentiation in vitro we repeated our previously published protocol in which IBMX was the inducing agent [13,21]. Like most other neuronal induction methods, our procedure resulted in the morphological transformation of SCs into neuron-like cells and the expression of neuronal markers such as β-III tubulin. Importantly, we found that both EdU-labeled and unlabeled ADSCs were able to undergo such neuron-like differentiation. For induction of endothelial differentiation in vitro we repeated our previously published protocol in which EGM2 medium was the inducing agent [7]. The results were reproducible, including the expression of endothelial markers (vWF, eNOS, and CD31) and acquirement of endothelial functions (LDL uptake and tube formation) in the EGM2-treated ADSCs. More importantly, we observed no discernible difference between EdU-labeled and unlabeled ADSCs in any of these tests.
The scarcity of evidence for SC’s in vivo differentiation has prompted the consideration that SC’s therapeutic efficacy might derive from their paracrine actions [9]. In the case with ADSCs we have previously shown that these cells’ neuroprotective effects might derive from their abundant secretion of the CXCL5 cytokine [16]. In the present study we repeated the protocol of using the cytokine antibody array for the analysis of secreted cytokines in both EdU-labeled and unlabeled ADSCs. The results were reproducible in terms of the levels of all 19 tested cytokines between our present and previous studies, and this of course included the characteristic high-level CXCL5 secretion. More importantly, we observed no discernible difference in the level of any of these cytokines between EdU-labeled and unlabeled ADSCs.
One of the proposed mechanisms for SC’s therapeutic effects is their ability to home in to injury sites, and this homing mechanism is believed to be primarily mediated by SDF-1 [22]. In the case with ADSCs we have previously demonstrated the expression of SDF-1 in injured tissues and the appearance of ADSCs in the vicinity of SDF-1 expression after their systemic administration [3,20]. In the present study we compared EdU-labeled and unlabeled ADSCs for their ability to migrate toward SDF-1. The results showed that both cell preparations were responsive to SDF-1 and there was no discernible difference between them.
From the above discussion it can be concluded that EdU was non-toxic to ADSCs and did not interfere with ADSC’s differentiation, cytokine secretion, or migratory response to SDF-1. EdU therefore appears to be a reliable label for tracking transplanted cells. However, although we have not observed EdU leakage (i.e., absorption by unlabeled cells) in our routine experiments, this issue needs to be addressed in a specifically designed experiment in the future. Additionally, it would be of value to investigate the in vivo differentiation potential and migratory behavior of EdU-labeled ADSCs in other experiments as well.
Acknowledgements
This work was supported by grants from the National Institutes of Health (DK64538, DK045370, and DK069655).
Abbreviations
- BrdU
5-bromo-2-deoxyuridine
- EdU
5-ethynyl-2′-deoxyuridine
- ADSCs
adipose-derived stem cells
- MSCs
mesenchymal stem cells
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
- TBE
Tris-borate-EDTA
- IBMX
Isobutylmethylxanthine
- EGM2
Endothelial growth medium-2
- SCs
stem cells
- LDL
Low-density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of interest: None.
References
- 1.Cavanagh BL, Walker T, Norazit A, Meedeniya AC. Thymidine analogues for tracking DNA synthesis. Molecules. 2011;16:7980–93. doi: 10.3390/molecules16097980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin G, Huang YC, Shindel AW, Banie L, Wang G, Lue TF, et al. Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy. 2009;11:864–73. doi: 10.3109/14653240903180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin G, Xin Z, Zhang H, Banie L, Wang G, Qiu X, et al. Identification of active and quiescent adipose vascular stromal cells. Cytotherapy. 2011 doi: 10.3109/14653249.2011.627918. [DOI] [PubMed] [Google Scholar]
- 5.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–20. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7:1391–400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ning H, Liu G, Lin G, Yang R, Lue TF, Lin CS. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967–79. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–40. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CS, Lue TF. Adipose-Derived Stem Cells: Therapy through Paracrine Actions. In: Hayat MA, editor. Stem Cells and Cancer Stem Cells. Vol. 4. Springer; New York, NY: 2012. pp. 203–16. [Google Scholar]
- 10.Duque A, Rakic P. Different effects of bromodeoxyuridine and [3H]thymidine incorporation into DNA on cell proliferation, position, and fate. J Neurosci. 2011;31:15205–17. doi: 10.1523/JNEUROSCI.3092-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehner B, Sandner B, Marschallinger J, Lehner C, Furtner T, Couillard-Despres S, et al. The dark side of BrdU in neural stem cell biology: detrimental effects on cell cycle, differentiation and survival. Cell Tissue Res. 2011;345:313–28. doi: 10.1007/s00441-011-1213-7. [DOI] [PubMed] [Google Scholar]
- 12.Lequeux C, Oni G, Mojallal A, Damour O, Brown SA. Adipose derived stem cells: efficiency, toxicity, stability of BrdU labeling and effects on self-renewal and adipose differentiation. Mol Cell Biochem. 2011;351:65–75. doi: 10.1007/s11010-011-0712-x. [DOI] [PubMed] [Google Scholar]
- 13.Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–8. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan KF, Cleveland DW. Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. Proc Natl Acad Sci U S A. 1986;83:4327–31. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Liu X, Jiang Y, Chu L, Hao H, Liua Z, et al. MAPK/ERK signalling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med. 2008;12:2395–406. doi: 10.1111/j.1582-4934.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin CS. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J Sex Med. 2011;8:437–46. doi: 10.1111/j.1743-6109.2010.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Ning H, Banie L, Wang G, Lin G, Lue TF, et al. Adipose tissue-derived stem cells secrete CXCL5 cytokine with chemoattractant and angiogenic properties. Biochem Biophys Res Commun. 2010;402:560–4. doi: 10.1016/j.bbrc.2010.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CS, Lue TF. Stem cell therapy for stress urinary incontinence: a critical review. Stem Cells Dev. 2012;21:834–43. doi: 10.1089/scd.2011.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CS, Xin ZC, Wang Z, Deng C, Huang YC, Lin G, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012;21:343–51. doi: 10.1089/scd.2011.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, et al. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61:201–10. doi: 10.1016/j.eururo.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning H, Lin G, Fandel T, Banie L, Lue TF, Lin CS. Insulin growth factor signaling mediates neuron-like differentiation of adipose-tissue-derived stem cells. Differentiation. 2008;76:488–94. doi: 10.1111/j.1432-0436.2007.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–96. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]