Abstract
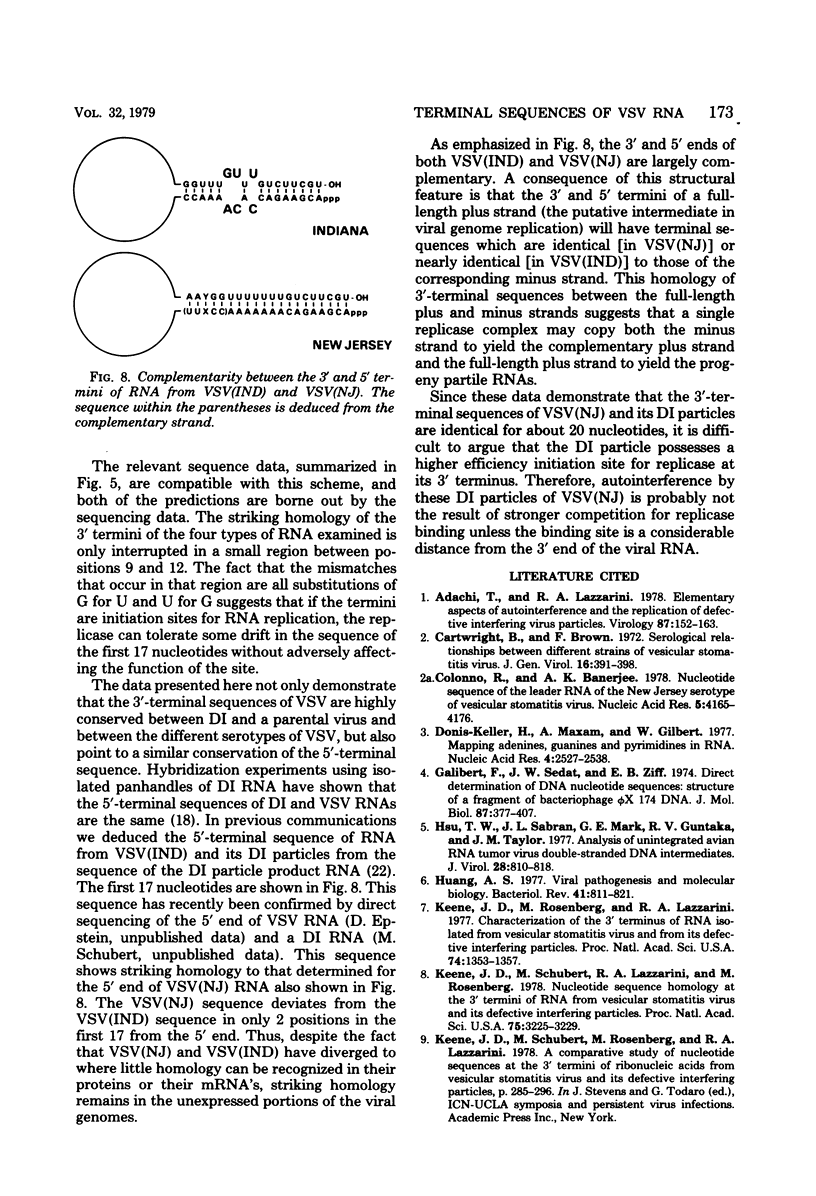
The nucleotide sequences at the 5' and 3' termini of RNA isolated from the New Jersey serotype of vesicular stomatitis virus [vsV(NJ)] and two of its defective interfering (DI) particles have been determined. The sequence differs from that previously demonstrated for the RNA from the Indiana serotype of VSV at only 1 of the first 17 positions from the 3' terminus and at only 2 of the first 17 positions from the 5' terminus. The 5'-terminal sequence of VSV(NJ) RNA is the complement of the 3'-terminal sequence, and duplexes which are 20 bases long and contain the 3' and 5' termini have been isolated from this RNA. The RNAs isolated from DI particles of VSV(NJ) have the same base sequences as do the RNAs from the parental virus. These results are in sharp contrast to those obtained with the Indiana serotype of VSV and its DI particles, in which the 3'-terminal sequences differ in 3 positions within the first 17. However, with both serotypes, the 3'-terminal sequence of the DI RNA is the complement of the 5'-terminal sequence of the RNA from the infectious virus. These findings suggest that the 3' and 5' RNA termini are highly conserved in both serotypes and that the 3' terminus of DI RNA is ultimately derived by copying the 5' end of the VSV genome, as recently proposed (D. Kolakofsky, M. Leppert, and L. Kort, in B. W. J. Mahy and R. D. Barry, ed., Negative-Strand Virus and the Host Cell, 1977; M. Leppert, L. Kort, and D. Kolakofsky, Cell 12:539-552, 1977; A. S. Huang, Bacteriol. Rev. 41:811-8218 1977).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Lazzarini R. A. Elementary aspects of autointerference and the replication of defective interfering virus particles. Virology. 1978 Jun 1;87(1):152–163. doi: 10.1016/0042-6822(78)90167-8. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Brown F. Serological relationships between different strains of vesicular stomatis virus. J Gen Virol. 1972 Sep;16(3):391–398. doi: 10.1099/0022-1317-16-3-391. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Nucleotide sequence of the leader RNA of the New Jersey serotype of vesicular stomatitis virus. Nucleic Acids Res. 1978 Nov;5(11):4165–4176. doi: 10.1093/nar/5.11.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Sedat J., Ziff E. Direct determination of DNA nucleotide sequences: structure of a fragment of bacteriophage phiX172 DNA. J Mol Biol. 1974 Aug 15;87(3):377–407. doi: 10.1016/0022-2836(74)90093-x. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Rosenberg M., Lazzarini R. A. Characterization of the 3' terminus of RNA isolated from vesicular stomatitis virus and from its defective interfering particles. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1353–1357. doi: 10.1073/pnas.74.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A., Rosenberg M. Nucleotide sequence homology at the 3' termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3225–3229. doi: 10.1073/pnas.75.7.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. R., Lazzarini R. A. The relationship between autointerference and the replication of defective interfering particle. Virology. 1977 Mar;77(1):189–201. doi: 10.1016/0042-6822(77)90417-2. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Weber G. H., Johnson L. D., Stamminger G. M. Covalently linked message and anti-message (genomic) RNA from a defective vesicular stomatitis virus particle. J Mol Biol. 1975 Sep 25;97(3):289–307. doi: 10.1016/s0022-2836(75)80042-8. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Perrault J. Cross-linked double-stranded RNA from a defective vesicular stomatitis virus particle. Virology. 1976 Apr;70(2):360–371. doi: 10.1016/0042-6822(76)90278-6. [DOI] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J Gen Virol. 1978 Jan;38(1):35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Reichmann M. E., Schnitzlein W. M., Bishop D. H., Lazzerini R. A., Beatrice S. T., Wagner R. R. Classification of the New Jersey serotype of vesicular stomatitis virus into two subtypes. J Virol. 1978 Jan;25(1):446–449. doi: 10.1128/jvi.25.1.446-449.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repik P., Flamand A., Clark H. F., Obijeski J. F., Roy P., Bishop D. H. Detection of homologous RNA sequences among six rhabdovirus genomes. J Virol. 1974 Jan;13(1):250–252. doi: 10.1128/jvi.13.1.250-252.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzlein W. M., Reichmann M. E. A possible effect of viral proteins on the specificity of interference by defective vesicular stomatitis virus particles. Virology. 1977 Jul 15;80(2):275–288. doi: 10.1016/s0042-6822(77)80004-4. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Soria M., Little S. P., Huang A. S. Characterization of vesicular stomatitis virus nucleocapsids. I. Complementary 40 S RNA molecules in nucleocapsids. Virology. 1974 Sep;61(1):270–280. doi: 10.1016/0042-6822(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]