Abstract
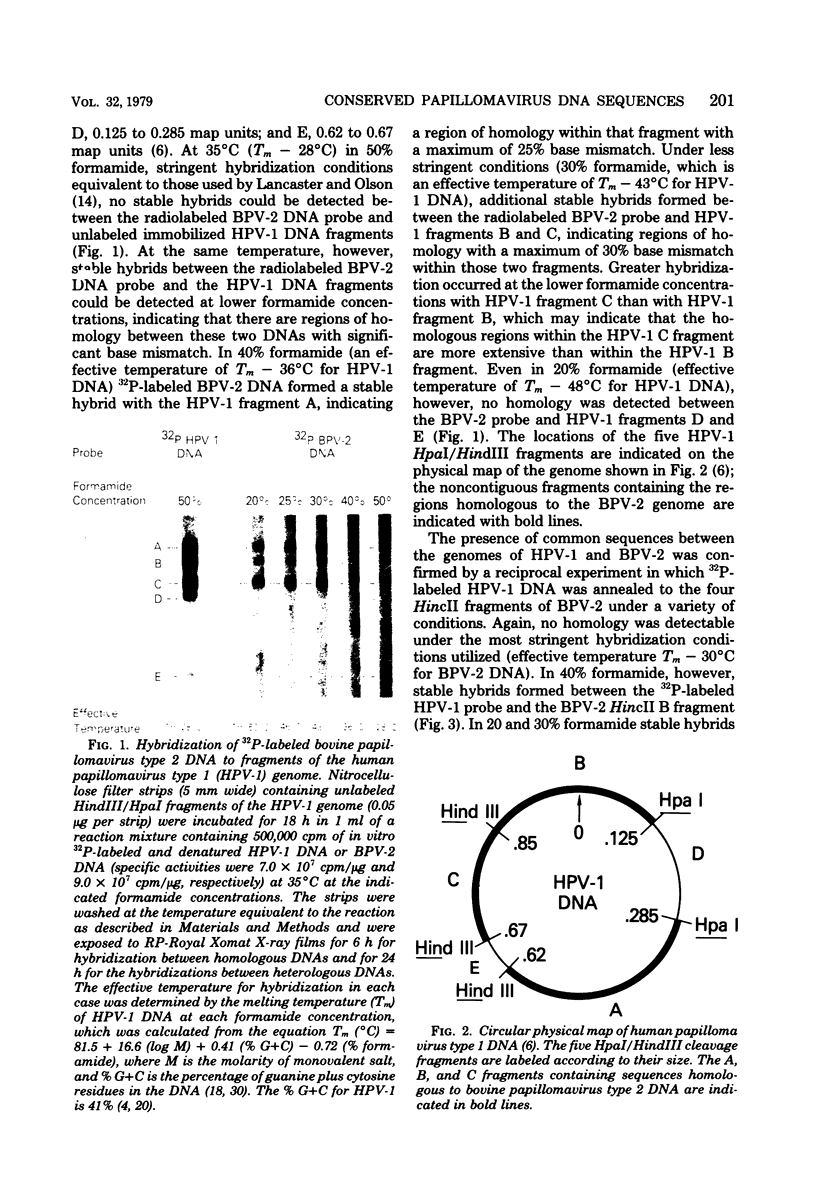
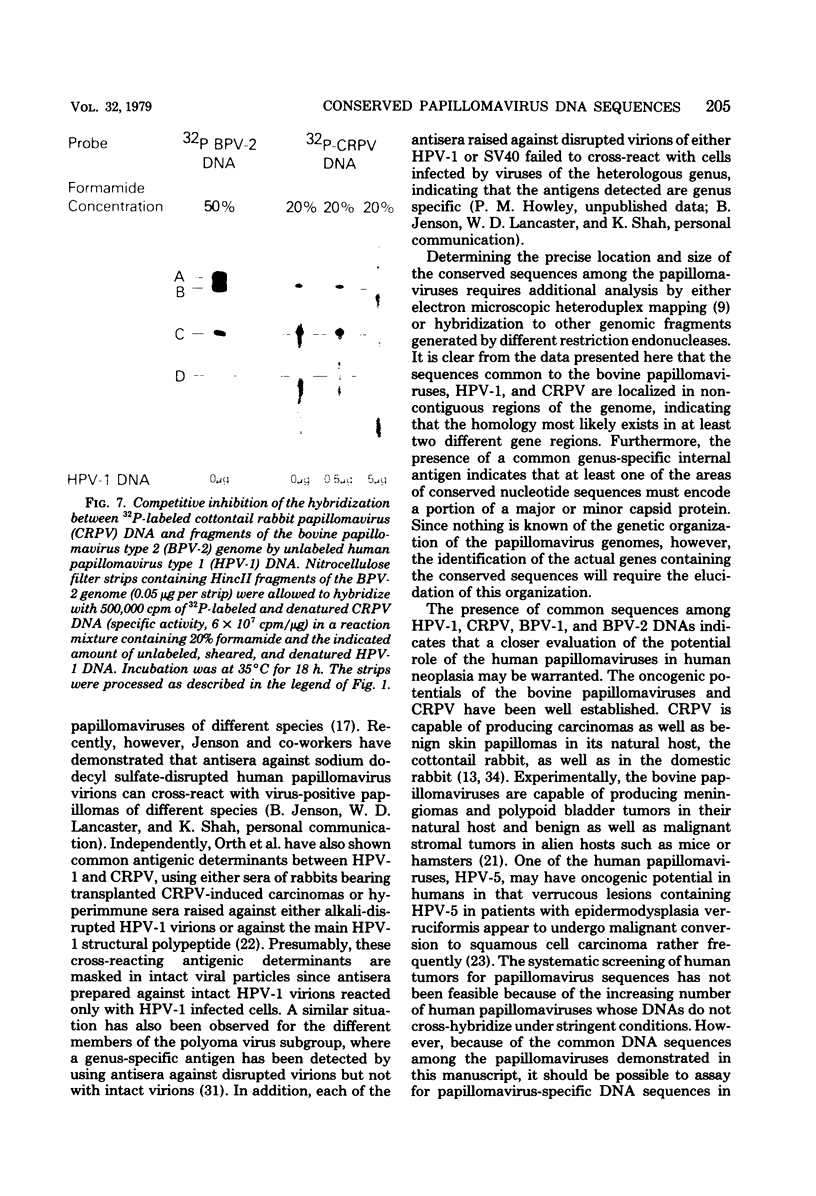
The DNAs of different members of the Papillomavirus genus of papovaviruses were analyzed for nucleotide sequence homology. Under standard hybridization conditions (Tm - 28 degrees C), no homology was detectable among the genomes of human papillomavirus type 1 (HPV-1), bovine papillomavirus type 2 (BPV-2), or cottontail rabbit (Shope) papillomavirus (CRPV). However, under less stringent conditions (i.e., Tm - 43 degrees C), stable hybrids were formed between radiolabeled DNAs of CRPV, BPV-1, or BPV-2 and the HindIII-HpaI A, B, and C fragments of HPV-1. Under these same conditions, radiolabeled CRPV and HPV-1 DNAs formed stable hybrids with HincII B and C fragments of BPV-2 DNA. These results indicate that there are regions of homology with as much as 70% base match among all these papillomavirus genomes. Furthermore, unlabeled HPV-1 DNA competitively inhibited the specific hybridization of radiolabeled CRPV DNA to bpv-2 DNA fragments, indicating that the homologous DNA segments are common among these remotely related papillomavirus genomes. These conserved sequences are specific for the Papillomavirus genus of papovaviruses as evidenced by the lack of hybridization between HPV-1 DNA and either simian virus 40 or human papovavirus BK DNA under identical conditions. These results indicate a close evolutionary relationship among the papillomaviruses and further establish the papillomaviruses and polyoma viruses as distinct genera.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butel J. S. Studies with human papilloma virus modeled after known papovavirus systems. J Natl Cancer Inst. 1972 Feb;48(2):285–299. [PubMed] [Google Scholar]
- CRAWFORD L. V., CRAWFORD E. M. A COMPARATIVE STUDY OF POLYOMA AND PAPILLOMA VIRUSES. Virology. 1963 Oct;21:258–263. doi: 10.1016/0042-6822(63)90265-4. [DOI] [PubMed] [Google Scholar]
- Crawford L. V. Nucleic acids of tumor viruses. Adv Virus Res. 1969;14:89–152. doi: 10.1016/s0065-3527(08)60558-8. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Favre M., Orth G., Croissant O., Yaniv M. Human papillomavirus DNA: physical map. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4810–4814. doi: 10.1073/pnas.72.12.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M., Orth G., Croissant O., Yaniv M. Human papillomavirus DNA: physical mapping of the cleavage sites of Bacillus amyloliquefaciens (BamI) and Haemophilus parainfluenzae (HpaII) endonucleases and evidence for partial heterogeneity. J Virol. 1977 Mar;21(3):1210–1214. doi: 10.1128/jvi.21.3.1210-1214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. Classification and nomenclature of viruses. Second report of the International Committee on Taxonomy of Viruses. Intervirology. 1976;7(1-2):1–115. doi: 10.1159/000149938. [DOI] [PubMed] [Google Scholar]
- Ferguson J., Davis R. W. An electron microscopic method for studying and mapping the region of weak sequence homology between simian virus 40 and polyoma DNAs. J Mol Biol. 1975 May 15;94(2):135–149. doi: 10.1016/0022-2836(75)90073-x. [DOI] [PubMed] [Google Scholar]
- Gissmann L., Pfister H., Zur Hausen H. Human papilloma viruses (HPV): characterization of four different isolates. Virology. 1977 Feb;76(2):569–580. doi: 10.1016/0042-6822(77)90239-2. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Demonstration of two distinct classes of bovine papilloma virus. Virology. 1978 Sep;89(2):372–379. doi: 10.1016/0042-6822(78)90179-4. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C., Meinke W. Quantitation of bovine papilloma viral DNA in viral-induced tumors. J Virol. 1976 Mar;17(3):824–831. doi: 10.1128/jvi.17.3.824-831.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Takemoto K. K., Howley P. M. Characterization of the genome of the murine papovavirus K. J Virol. 1979 Apr;30(1):90–97. doi: 10.1128/jvi.30.1.90-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Allison A. C., Butel J. S., Eckhart W., Eddy B. E., Kit S., Levine A. J., Miles J. A., Pagano J. S., Sachs L. Papovaviridae. Intervirology. 1974;3(1-2):106–120. doi: 10.1159/000149746. [DOI] [PubMed] [Google Scholar]
- Morrison J. M., Keir H. M., Subak-Sharpe H., Crawford L. V. Nearest neighbour base sequence analysis of the deoxyribonucleic acids of a further three mammalian viruses: Simian virus 40, human papilloma virus and adenovirus type 2. J Gen Virol. 1967 Jan;1(1):101–108. doi: 10.1099/0022-1317-1-1-101. [DOI] [PubMed] [Google Scholar]
- Olson C., Gordon D. E., Robl M. G., Lee K. P. Oncogenicity of bovine papilloma virus. Arch Environ Health. 1969 Dec;19(6):827–837. doi: 10.1080/00039896.1969.10666938. [DOI] [PubMed] [Google Scholar]
- Orth G., Breitburd F., Favre M. Evidence for antigenic determinants shared by the structural polypeptides of (Shope) rabbit papillomavirus and human papillomavirus type 1. Virology. 1978 Dec;91(2):243–255. doi: 10.1016/0042-6822(78)90373-2. [DOI] [PubMed] [Google Scholar]
- Orth G., Favre M., Croissant O. Characterization of a new type of human papillomavirus that causes skin warts. J Virol. 1977 Oct;24(1):108–120. doi: 10.1128/jvi.24.1.108-120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G., Jablonska S., Breitburd F., Favre M., Croissant O. The human papillomaviruses. Bull Cancer. 1978;65(2):151–164. [PubMed] [Google Scholar]
- Orth G., Jablonska S., Favre M., Croissant O., Jarzabek-Chorzelska M., Rzesa G. Characterization of two types of human papillomaviruses in lesions of epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1537–1541. doi: 10.1073/pnas.75.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SYVERTON J. T. The pathogenesis of the rabbit papilloma-to-carcinoma sequence. Ann N Y Acad Sci. 1952 Jul 10;54(6):1126–1140. doi: 10.1111/j.1749-6632.1952.tb39983.x. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Ozer H. L., Ghazey H. N., Kelly T. J., Jr Common structural antigen of papovaviruses of the simian virus 40-polyoma subgroup. J Virol. 1977 Jan;21(1):179–186. doi: 10.1128/jvi.21.1.179-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]








