Abstract
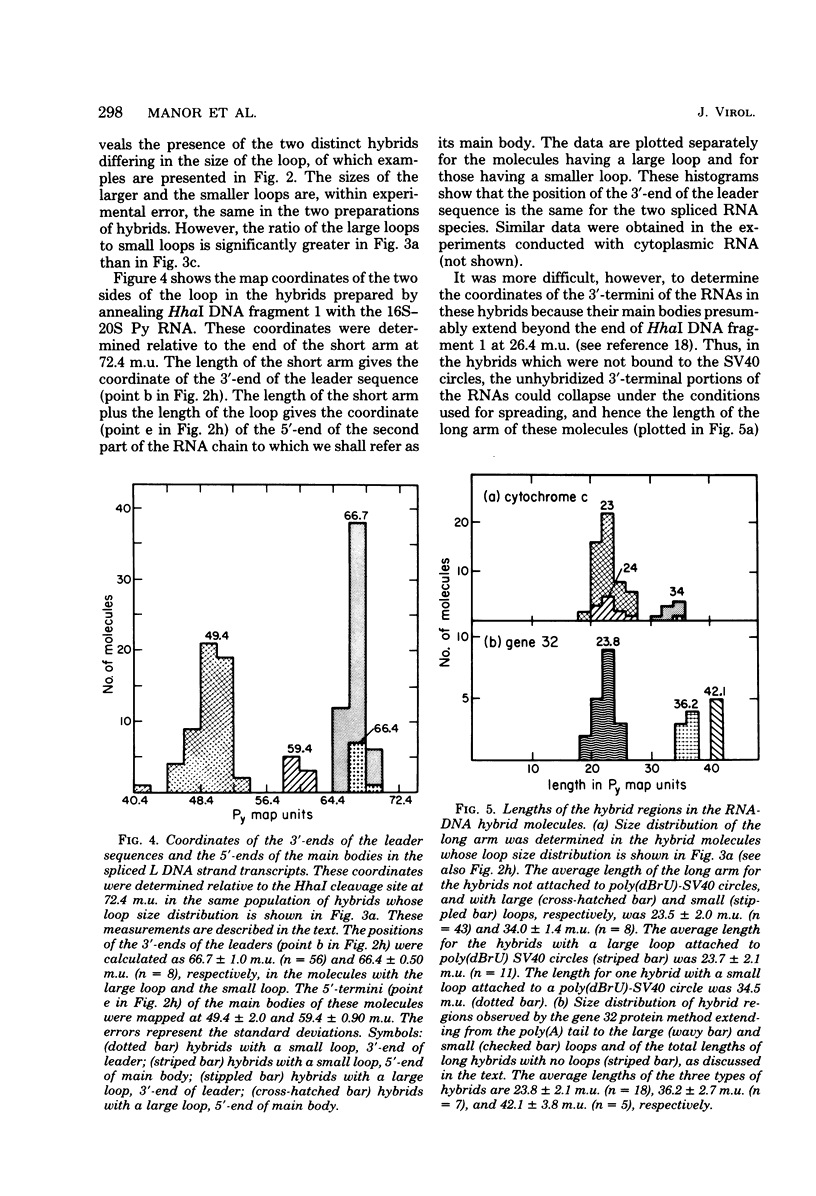
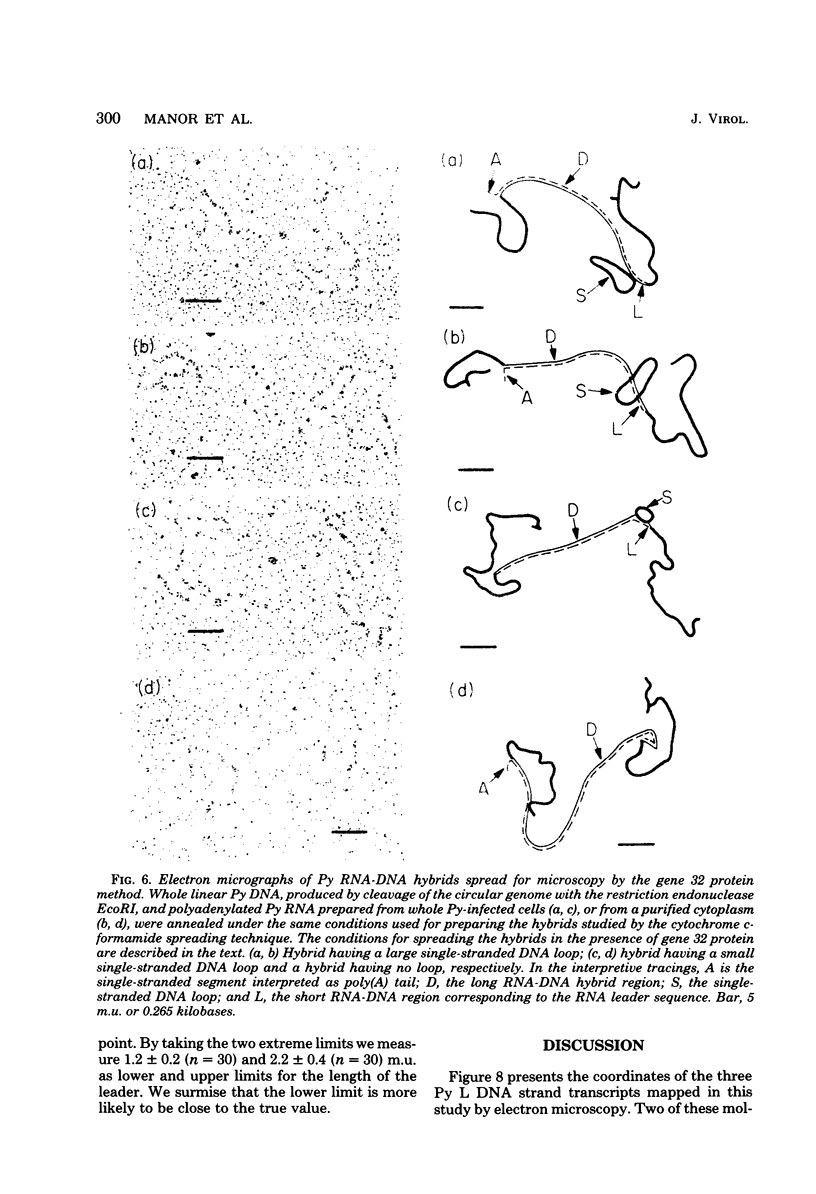
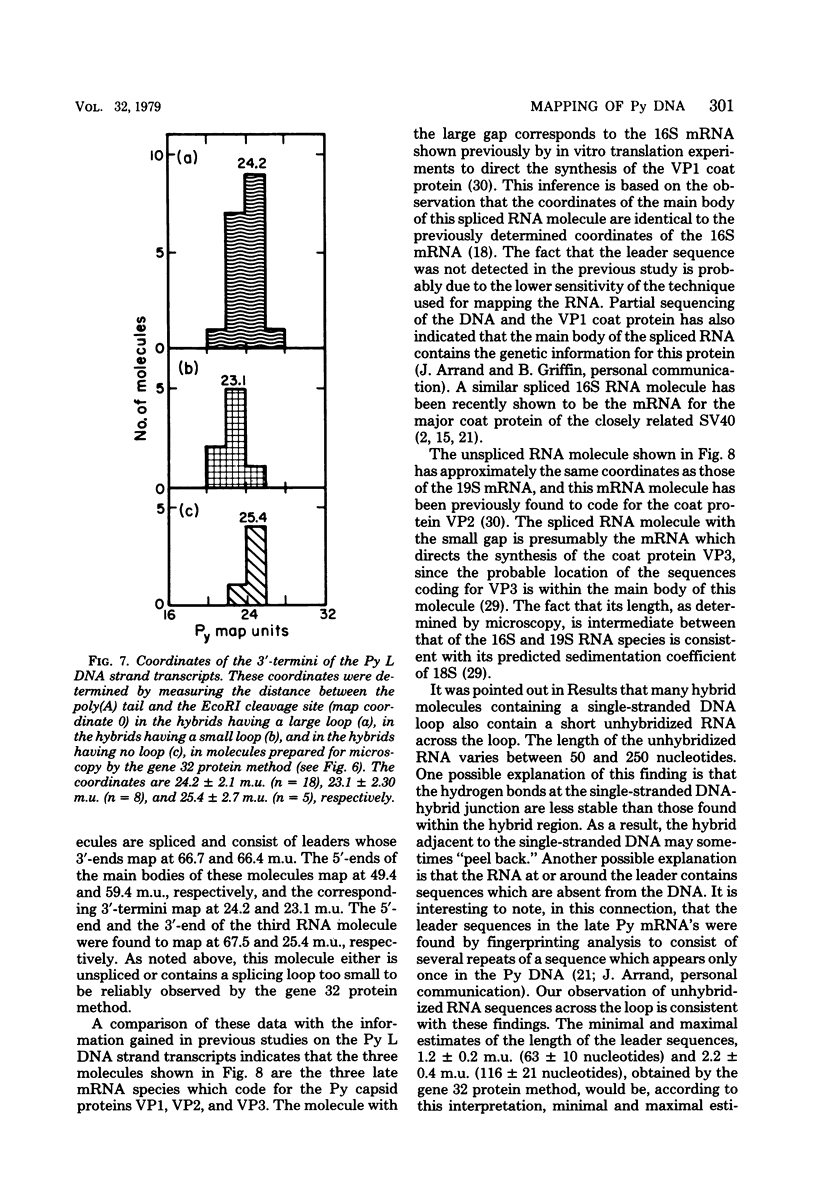
The polyoma virus (Py) RNA species transcribed from the L DNA strand of the "late" region of the Py genome in Py-infected mouse cells have been mapped by hybridization with specific fragments of Py DNA followed by electron microscopic visualization of the hybrids. Total cellular polyadenylated Py-specific RNA molecules having an S value in the range of 16S to 20S were purified by oligodeoxythymidylic acidcellulose column chromatography, preparative hybridization with Py DNA, and sucrose gradient centrifugation. Cytoplasmic Py-specific RNA was similarily purified, except that it was not fractionated by sucrose gradient centrifugation. Hybrids of these RNA molecules and Py DNA fragments were spread for electron microscopy by either the cytochrome c technique or the bacteriophage T4 gene 32 protein method. The polyadenylic acid at the 3'-end of the RNA in the hybrids was identified by labeling with simian virus 40 DNA circles to which polybromodeoxyuridylic acid tails had been covalently attached. These experiments revealed the presence of three L DNA strand transcripts in both RNA preparations. Two of these RNA molecules were found to be spliced from chains transcribed from two noncontiguous parts of the late region. The third molecule either is a continuous transcript of the entire late region or contains a splicing feature which is too small to be reliably observed by the electron microscope methods used. The 5'-ends of the three RNA species map within a region extending from 68 to 70 map units on the Py restriction endonuclease map. Each of the two spliced molecules contains a 5'-terminal leader sequence transcribed from a DNA segment with an estimated length of 60 to 110 nuvleotides. The 3'-ends of the leaders map at 66.7 +/- 1.0 and 66.4 +/- 0.50 map units. In these molecules the 5'-ends of the other part (the main body) map at 59.4 +/- 0.90 and 49.4 +/- 2.0 map units, respectively. The 3'-termini of all three RNA species map at 24 to 25 map units.
Full text
PDF


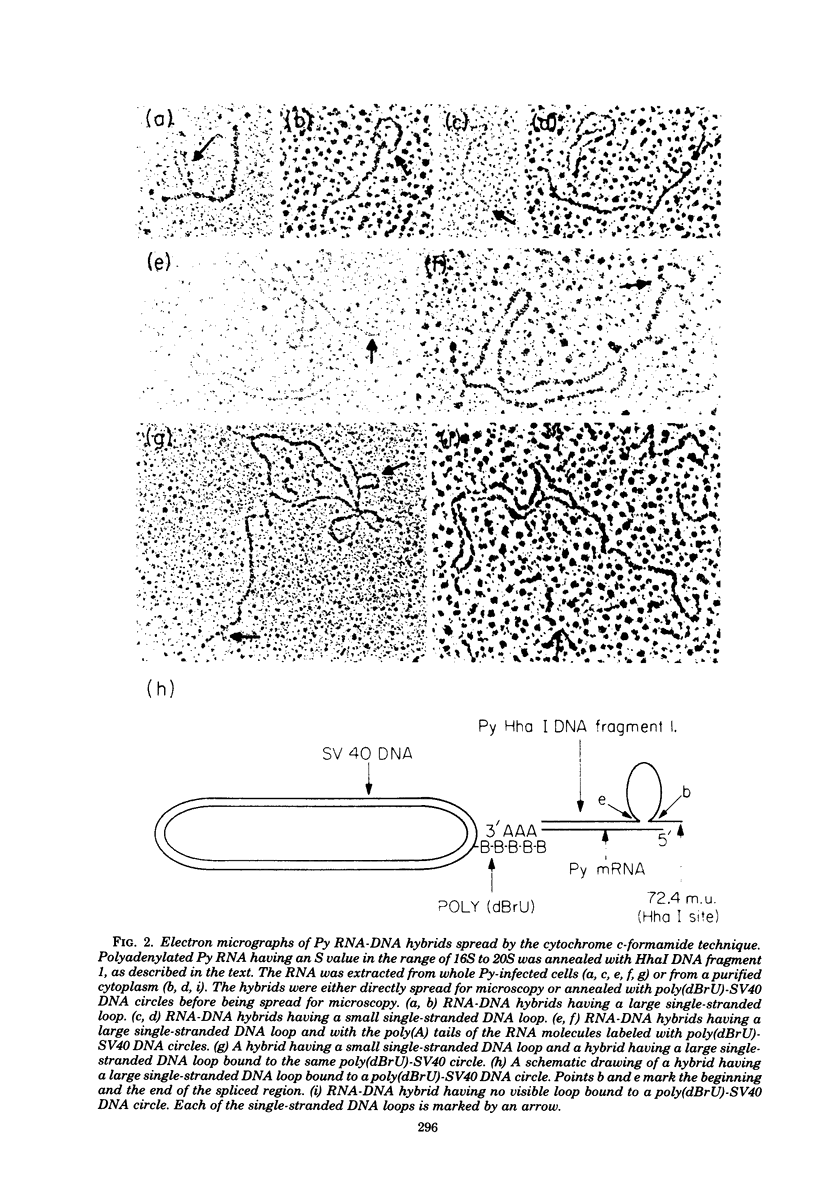
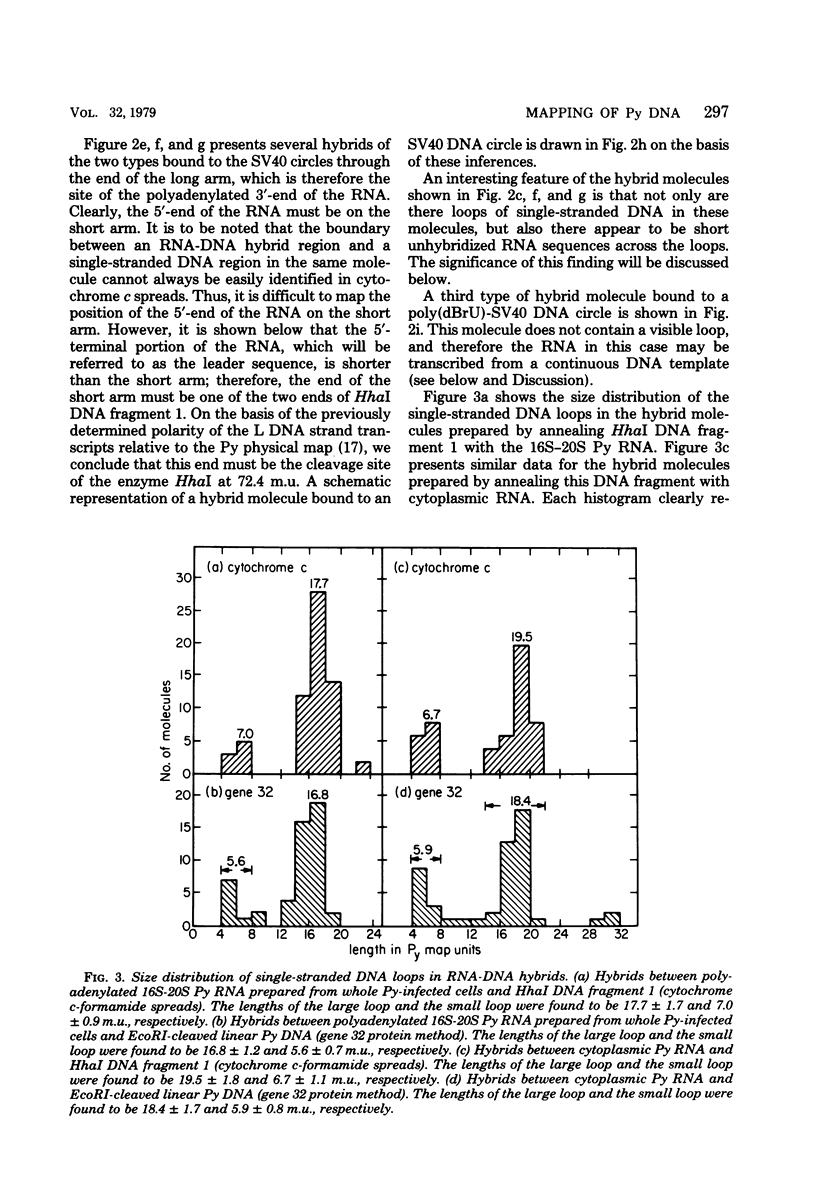



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Buetti E., Scherrer K., Weil R. Transcription of the polyoma virus genome: synthesis and cleavage of giant late polyoma-specific RNA. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2231–2235. doi: 10.1073/pnas.68.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Dhar R., Laub O., Horowitz M., Khoury G. Novel mechanism for RNA maturation: the leader sequences of simian virus 40 mRNA are not transcribed adjacent to the coding sequences. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3686–3690. doi: 10.1073/pnas.74.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Davidson N., Kindle K. L., Taylor W. C., Silverman M., Firtel R. A. The structure of M6, a recombinant plasmid containing Dictyostelium DNA homologous to actin messenger RNA. Cell. 1978 Nov;15(3):779–788. doi: 10.1016/0092-8674(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birg F., Favaloro J., Kamen R. Analysis of polyoma virus nuclear RNA by mini-blot hybridization. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3138–3142. doi: 10.1073/pnas.74.8.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Dunn A. R., Hassell J. A. A novel method to map transcripts: evidence for homology between an adenovirus mRNA and discrete multiple regions of the viral genome. Cell. 1977 Sep;12(1):23–36. doi: 10.1016/0092-8674(77)90182-9. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Kamen R. Strand-specific transcription of polyoma virus DNA late during productive infection. J Mol Biol. 1977 Sep 15;115(2):237–242. doi: 10.1016/0022-2836(77)90099-7. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M. Amplification of a specific region of the polyoma virus genome. Nature. 1975 Jul 17;256(5514):175–179. doi: 10.1038/256175a0. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M., Bratosin S., Aloni Y. Polyoma infected cells contain at least three spliced late RNAs. Nucleic Acids Res. 1978 Dec;5(12):4663–4675. doi: 10.1093/nar/5.12.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. T., Ford J. Sequence arrangement of the 5' ends of simian virus 40 16S and 19S mRNAs. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4982–4985. doi: 10.1073/pnas.74.11.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- Kamen R., Shure H. Topography of polyoma virus messenger RNA molecules. Cell. 1976 Mar;7(3):361–371. doi: 10.1016/0092-8674(76)90165-3. [DOI] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Lavi S., Groner Y. 5'-Terminal sequences and coding region of late simian virus 40 mRNAs are derived from noncontiguous segments of the viral genome. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5323–5327. doi: 10.1073/pnas.74.12.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S., Flavell A. J., Cowie A., Kamen R. Amplification in the leader sequence of late polyoma virus mRNAs. Cell. 1979 Feb;16(2):373–388. doi: 10.1016/0092-8674(79)90013-8. [DOI] [PubMed] [Google Scholar]
- Lev Z., Kamen R., Manor H. Topography of polyoma virus-specific giant nuclear RNA molecules containing poly(A) sequences. Virology. 1979 Mar;93(2):445–457. doi: 10.1016/0042-6822(79)90248-4. [DOI] [PubMed] [Google Scholar]
- Lev Z., Manor H. Amount and distribution of virus-specific sequences in giant RNA molecules isolated from polyoma-infected mouse kidney cells. J Virol. 1977 Mar;21(3):831–842. doi: 10.1128/jvi.21.3.831-842.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Darnell J. E. SV40-specific RNA in the nucleus and polyribosomes of transformed cells. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1089–1096. doi: 10.1073/pnas.65.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor H., Kamen R. Polyoma virus-specific RNA synthesis in an inducible line of polyoma virus-transformed rat cells. J Virol. 1978 Mar;25(3):719–729. doi: 10.1128/jvi.25.3.719-729.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor H., Neer A. Effects of cycloheximide on virus RNA replication in an inducible line of polyoma-transformed rat cells. Cell. 1975 Jul;5(3):311–318. doi: 10.1016/0092-8674(75)90106-3. [DOI] [PubMed] [Google Scholar]
- Parish J. H., Kirby K. S. Reagents which reduce interactions between ribosomal RNA and rapidly labelled RNA from rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal L. J., Salomon C., Weil R. Isolation and characterization of poly(A)-containing intranuclear polyoma-specific "giant" RNA'S. Nucleic Acids Res. 1976 May;3(5):1167–1183. doi: 10.1093/nar/3.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Smith A. E. Polyoma virus has three late mRNA's: one for each virion protein. J Virol. 1978 Aug;27(2):427–431. doi: 10.1128/jvi.27.2.427-431.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Kamen R., Mangel W. F., Shure H., Wheeler T. Location of the sequences coding for capsid proteins VP1 and VP2 on polyoma virus DNA. Cell. 1976 Nov;9(3):481–487. doi: 10.1016/0092-8674(76)90093-3. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N. Use of gene 32 protein staining of single-strand polynucleotides for gene mapping by electron microscopy: application to the phi80d3ilvsu+7 system. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4506–4510. doi: 10.1073/pnas.72.11.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Roberts R. J., Davidson N. Structure of the inverted terminal repetition of adenovirus type 2 DNA. J Virol. 1977 Feb;21(2):766–777. doi: 10.1128/jvi.21.2.766-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]