Abstract
Objectives
To determine the extent to which thigh intermuscular fat (IMF) and quadriceps muscle (QM) volumes explained variance in knee extensor strength and physical performance in women with (ROA) and without (NROA) radiographic knee osteoarthritis.
Methods
Baseline data from 125 women in the Osteoarthritis Initiative, ≥50 years, with or at risk for ROA were included. Knee extensor strength was measured using a fixed force transducer, normalized to body mass (N/kg). Physical performance was the time required for five repeated chair stands (s). The IMF and QM volumes, normalized to height (cm3/m), were yielded from analyses of T1-weighted axial magnetic resonance images of the mid-thigh. Mean IMF and QM volumes, extensor strength and physical performance were compared between ROA and NROA, controlling for age. Hierarchical multiple regressions determined whether IMF and QM volumes were related to strength and performance after controlling for age, radiographic OA status (yes/no), alignment and pain.
Results
Compared to ROA, the NROA were stronger and performed chair stands faster (p<0.05). After adjusting for age, NROA had less IMF (61.1 ± 20.3 cm3/m) compared to ROA (72.0 ± 25.0 cm3/m, p<0.05). In the entire sample, 21.1% of variance in knee extensor strength was explained by alignment, pain and IMF. A model explaining 13.4% of variance in physical performance included OA status and IMF. QM volume was unrelated to strength and physical performance.
Conclusions
Intermuscular fat volume explained a small amount of variance in knee extensor strength and physical performance among women with, or at risk for ROA.
INTRODUCTION
Obesity is a substantive risk factor for incident knee osteoarthritis (OA) (1). A meta-analysis of 21 cohort studies showed that a five-unit increase in body mass index (BMI) was associated with a 35% increased risk of radiographic or pre-surgical knee OA (2). In OA, obesity fosters disability and inactivity by promoting pain, stiffness, instability, and muscle weakness (3). Body mass index correlated with disease severity in 300 community-recruited people with knee OA (r=Ȓ0.29, p<0.01) (4) and self-reported function scores in 69 people with knee OA (r=0.42, p<0.01) (5).
The complex mechanisms by which BMI promotes OA incidence and disability are still being revealed. At least three mechanisms have been described. First, obesity increases the overall load on the knee (6, 7), while reducing the capacity for dynamic shock absorption (8). Second, during activities of daily living, obesity is associated with slower self-selected speeds, shorter and wider steps, and altered kinematics (7, 9, 10) that are proposed to shift load-bearing to regions of articular cartilage that are not well-conditioned to withstand joint contact forces (11). Third, adipose tissue releases pro-inflammatory factors, such as cytokines and adipokines, which regulate chondrocyte anabolism and therefore play key roles in OA pathophysiology (12). These mechanisms reflect the impact of whole body obesity; however, less work has focused on the distribution of this fat tissue in the lower extremity and how this fat distribution affects mobility.
The local presence of the adipose tissue in the thigh influences mobility in healthy older adults. In 2,627 healthy old men and women, knee strength (extensor torque per cross-sectional area of quadriceps) was related to fatty infiltration of this muscle determined using a muscle tissue attenuation technique from cross-sectional computed tomography scans (r=0.26, p<0.01) (13). Fatty infiltration in the thigh musculature was associated with poorer performance in walking and repeated chair standing tasks in 3,075 well-functioning adults between 70 and 79 years of age, even after adjusting for total body fat, physical activity, and thigh muscle area (14). In fact, over 50 studies of aging show that fat accumulation in the whole body is more important than loss of lean muscle in the development of mobility limitations (15). This body of work provides a strong impetus to explore the role of fat tissue in osteoarthritic disease and physical functioning. However, to-date relatively little work has evaluated the impact of accumulated fat tissue around the knee on strength and mobility performance in OA.
Despite the strong evidence implicating fat in declining physical function with aging, skeletal muscle mass remains positively associated with strength and mobility (13, 15). In older adults, low lean muscle mass in the thigh predicted an increased risk of incident mobility limitations (odds ratio = 1.8, 95% CI 1.3−2.6); however this relationship was not significant once strength was used as a covariate (16). Thus lean tissue shares a small, and not always independent relationship, with mobility. Comparing the influence of fat and lean tissue mass on mobility in knee OA will provide some insight into the mechanisms leading to physical decline.
The purpose of this study was to (1) compare intermuscular fat and quadriceps volumes in the thigh, as well as knee extensor strength and physical performance, between women with radiographic knee OA and women at risk for radiographic knee OA; and (2) determine the extent to which intermuscular fat and quadriceps muscle volumes in the thigh explain variance in knee extensor strength and physical performance among women enrolled in the Osteoarthritis Initiative (OAI) who have, or are at risk for, radiographic knee OA. Measures of fat and muscle volume from magnetic resonance images of the thigh enable the robust quantification and tracking of these tissues in vivo (17). We hypothesized that women with radiographic knee OA will have greater intermuscular fat in the thigh, less quadriceps volume and poorer knee extensor strength and physical performance compared to women at risk for knee OA. In addition, we hypothesized that intermuscular thigh fat volume will explain a larger proportion of the variance in knee extensor strength and physical performance than quadriceps muscle volume in women at risk for, or with, prevalent radiographic knee OA.
PARTICIPANTS AND METHODS
Osteoarthritis Initiative Study Sample
The Osteoarthritis Initiative (OAI) is a multi-centre, longitudinal, observational cohort study designed to facilitate investigation of factors that influence the onset and progression of OA. The OAI study (n=4,796) includes individuals 45 to 79 years of age separated into control participants and individuals who are at risk for, or have OA at baseline (18). Controls (n=122) are an unexposed reference cohort with no radiographic evidence of OA and no risk factors, with the exception of age ≥ 70 years. The remaining participants (n=4,674) include (i) individuals with risk factors for the development of OA in the incidence subcohort and (ii) individuals with both symptomatic and radiographic evidence of OA in at least one knee at baseline in the progression subcohort.
Eligibility criteria for the incidence subcohort include no symptomatic knee OA in either knee at baseline, but various combinations of risk factors for developing the disease, including the following: Kellgren and Lawrence grades (KL) ≥ 2 on a fixed flexion radiograph with no frequent knee pain; frequent knee pain, but KL < 2; overweight; previous knee injury or knee surgery; family history or knee OA; Herbedens nodes; repetitive knee bending; and age 70–79 years. On the other hand, participants with symptomatic tibiofemoral knee OA at baseline are eligible for the progression subcohort if they have both of the following in at least one native knee: pain, aching or stiffness on most days for at least one month in the past year and tibiofemoral osteophytes equivalent to KL ≥ 2 on a fixed flexion radiograph. Exclusion criteria for the OAI included contraindications to magnetic resonance imaging (MRI) and bilateral end-stage knee OA, rheumatoid arthritis, pregnancy, or co-morbidities that may interfere with study participation (18). All participant data were obtained from the online OAI database (http://www.oai.ucsf.edu/), which is available for public access.
Sample for Current Study
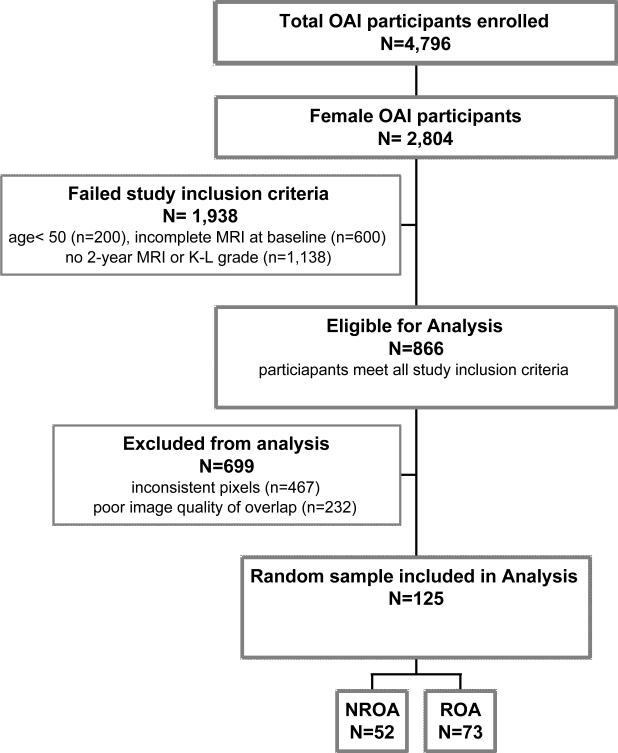
This was a cross-sectional study utilizing baseline data from the OAI sample. The OAI participant database was queried for women 50 years of age and older, in either the incidence or progression subcohort, with available baseline MRI scans of the thigh. For the purposes of a previous analysis (17), participants without 2 year follow-up MRIs of the thigh and those for whom Kellgren-Lawrence (KL) grades were unavailable at baseline were excluded. A random sample of participants meeting these inclusion and exclusion criteria, determined from a random number table, is included in these analyses (Figure 1).
Figure 1.
Consort diagram illustrating the inclusion and exclusion criteria applied to the Osteoarthritis Initiative database to create the sample of data form 125 participants analyzed in the current study.
Baseline KL grades determined from right knee radiographs were used to classify OA status (OAI variable: V00XRKL). Women with a KL grade of 0 or 1 were classified as having no radiographic OA in the right knee (NROA). Women with a KL grade of 2, 3 or 4 were classified as having radiographic OA in the right knee (ROA).
Dependent Variables
Knee extensor strength was the maximum isometric knee extensor force produced by the right leg against a fixed force transducer (OAI variable: V00REMAXF). As recommended by Bennell and colleagues, strength was normalized to body mass (N/kg) to account for differences in body mass (19). Maximum isometric knee extensor force was measured with the Good Strength chair (Metitur, Jyvaskyla, Finland), which uses a strain gauge to measure force. The strain gauge is in a pad positioned posteriorly, 2cm above the calcaneus, with a strap securely fastened around the lower leg. Standardized positioning set the knee angle at 60° from full extension. Two 50% effort warm-up trials preceded three 100% effort measurement trials. During the measurement trials, participants were instructed to push as hard and as fast as possible and were verbally encouraged throughout the test. Each trial was three seconds and participants were given a 30 second rest between trials. Knee extensor strength was the maximum force measured during one of the three measurement trials. Knee extensor strength was not expressed as torque because measurements of the distance between the strain gauge and knee axis of rotation, or lower leg length, were not available. Height was not used as a proxy because height is not consistently proportional to lower leg length (20). Isometric knee extensor efforts have demonstrated excellent reliability (r=0.92) among 203 well-functioning adults between 35 and 71 years of age (21).
Physical performance was represented by the amount of time each participant required to stand from sitting five times in succession (OAI variable: V00CSTREP1) (s). After viewing a demonstration and practising, participants were instructed to stand up five times as quickly as possible, with arms folded over the chest. They were instructed to come to a full standing and sitting position on each repetition. Gait aids were not permitted but verbal counting was provided. The time required to complete this task was recorded in seconds. The same repeated chair stand task, scored on a 4-point Likert scale based on ranges of performance times, produced data with acceptable test-retest reliability (ICC = 0.76–0.90) among 1,002 moderately to severely disabled older women (22). A similar repeated chair standing task, scored with time to completion, produced reliable data (r=0.80) among 203 adults between 35 and 71 years of age (21).
Independent Variables
Intermuscular thigh fat (IMF) and quadriceps muscle (QM) volumes (cm3) were determined using an image analysis protocol that has been described in detail elsewhere (17). Tissue volumes were normalized to height to account for differences in overall body size (cm3/m). Henceforth, references to IMF and QM volumes are those that are normalized to height.
Briefly, baseline MR images of the thigh were obtained from the OAI Coordinating Center (UCSF, San Francisco, CA) for the entire OAI study population. Bilateral MR images of the thighs, including 15 consecutive T1-weighted axial slices (5 mm slice thickness, spatial resolution of 0.977 mm × 0.977 mm), were acquired using a published protocol (18). Only the right thigh was segmented. The 7 cm region of interest scanned encompasses an area where the most distal slice of the thigh was acquired at a distance 10 cm proximal to the epiphyseal line of the distal right femur. A semi-automated software program featuring a watershed segmentation algorithm (SliceOmatic 4.3, TomoVision, Quebec, Canada) was applied to each thigh slice. Within SliceOmatic, the watershed algorithm was configured with thresholds set at 1 pixel surface and 0.01% mean difference. The morphological segmentation of the first analyzed slice was propagated forward to the next slice as previously described (17). The gold standard for measuring muscle volume is the segmentation of muscle from a series of contiguous MRI scans, which are acquired perpendicular to a muscle region of interest (23). On each slice, tissues that were identified and segmented included cortical bone, bone marrow, QM, all remaining muscles, subcutaneous fat, and IMF (17). Each tissue was tagged using a different colour. For this study, only the IMF volume and QM volume data were used. The IMF was considered as all tissue (including vessels) surrounding the muscles within the deep facial layer. Fat located within the muscle (intramuscular fat) was included in the segmentation of the muscle because of limitations in the resolution of the scans. Intra-rater and inter-rater reliability intraclass correlation coefficients for QM and IMF volumes were >0.95 and the root mean square coefficient of variation for QM and IMF volumes were <5.7% (17).
Covariates
In the regression analyses, we adjusted for age (17, 24), OA status (25), knee alignment in the frontal plane (24, 26) and pain intensity during the strength test (27, 28). The OA status was a dichotomous variable where participants were assigned NROA or ROA based on baseline right knee KL grade. Knee frontal plane alignment (OAI variable: V00RKALNMT) was measured while the participant stood facing the examiner with toes pointing straight ahead. A universal goniometer was placed on the center of the knee (Lafayette Instrument Co., Inc.) with the lower extendable arm aligned along the lower leg to the center of the ankle. The upper extendable arm was aligned and visually centered to the mid-thigh level. The goniometer angle was then recorded. Pain intensity during strength test (OAI variable: V00REXP1CV) was rated on a Likert scale by each participant between 0 and 4, with the anchors, “none”, “mild”, “moderate”, or “severe”; or “don't know” which was coded as missing data.
Statistical Analyses
Statistical analyses were performed using SPSS software (SPSS, Chicago, IL, V.18). Descriptive statistics of age, body mass, height, BMI, strength and performance tests were calculated. Because the NROA and ROA groups were of different sizes, Levene's test was used to test variance equality across groups prior to comparing means with independent t-tests. Means of the IMF and QM volumes in the NROA and ROA groups were compared using an analysis of covariance (ANCOVA), using age as a covariate given it is associated with increases in fat lean tissue and decreases in lean tissue (16, 29).
Pearson correlation coefficients were calculated between the covariate, dependent and independent variables. Bonferroni correction for 28 correlations at an alpha level of 0.05 in a two-tailed test requires a p-value <0.002 for significance. Finally, to address the primary study purpose, hierarchical multiple regression analysis was used to evaluate the extent to which variance in knee extensor strength could be explained by IMF and QM volumes normalized to height in women with NROA and ROA. Independent variables were entered in 3 blocks: (1) age; (2) OA status, right knee frontal plane alignment, pain intensity during strength test, and; (3) IMF, QM volumes. An analysis of multicollinearity was performed. Significance was set at p<0.05. This analysis was repeated using physical performance scores as the dependent variable.
RESULTS
Data from 125 participants were included in the analyses, with 52 in the NROA group and 73 in the ROA group. Descriptive data are shown in Table 1. Levene's test demonstrated variances in age, mass, height, BMI, knee extensor strength and physical performance were equal in the NROA and ROA groups. Compared to ROA, the NROA group was younger and demonstrated lower body mass, lower BMI, greater knee extensor strength and faster physical performance (p<0.05).
Table 1.
Descriptive statistics of the whole sample, NROA group and the ROA group.
| Whole Group | NROA | ROA Group | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Age (y) | 125 | 63.0 (7.2) | 52 | 60.7(7.1) | 73 | 64.6 (6.7)* |
| Body Mass (kg) | 124 | 73.1 (14.1) | 52 | 68.5 (13.2) | 72 | 76.5 (14.0)* |
| Height (m) | 122 | 1.62(0.64) | 51 | 1.61 (0.67) | 72 | 1.62(0.63) |
| BMI (kg/m2) | 123 | 28.0 (5.0) | 52 | 26.4 (5.0) | 71 | 29.1 (4.7)* |
| Knee Strength (N/kg) | 114 | 4.0(1.2) | 50 | 4.2(1.3) | 65 | 3.8(1.1)* |
| Physical Performance (s) | 123 | 12.5(4.1) | 52 | 11.4(3.4) | 71 | 13.3 (4.4)* |
BMI = Body mass index
(p < 0.05) between NROA and ROA groups
Variances in IMF and QM volumes were equal in the NROA and ROA groups. After adjusting for age, lower IMF volume was observed in the NROA compared with the ROA group (F=7.898; p=0.006) as summarized in Table 2. No differences were found in QM volume between the NROA and ROA despite adjusting for age (F=1.057; p=0.306).
Table 2.
Actual and age-adjusted intermuscular fat volume and quadriceps muscle volume (cm3/m) for the whole sample, NROA group, and the ROA group.
| Whole Group (n=125) | NROA (n=52) | ROA (n=73) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Intermuscular Fat Volume (cm3/m) | |||
| Actual | 67.4 (23.7) | 61.1 (20.3) | 72.0 (25.0)* |
| Age-adjusted | 60.3 (23.1) | 72.6 (23.7)* | |
| Quadriceps Muscle Volume (cm3/m) | |||
| Actual | 157.4(27.7) | 156.4(26.5) | 158.0(28.6) |
| Age-adjusted | 154.3 (23.7) | 160.0(30.1) |
(p < 0.05) between NROA and ROA groups
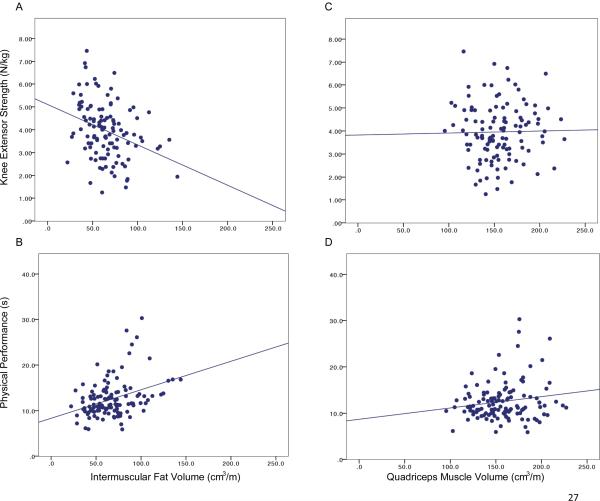
Table 3 shows that several relationships existed between tissue volumes and knee performance. In particular, the strongest correlations showed that BMI was related to IMF and QM volumes, as well as knee performance measures. The BMI and IMF shared 54% of variance. Figure 2 focuses on the relationships between thigh tissue volumes and knee function measures. While IMF volume related to knee extensor strength and physical performance, QM volume was not related to knee extensor strength or physical performance.
Table 3.
Correlation coefficients between covariate, independent and dependent variables.
| Age | Knee Alignment | Pain Intensity | Body Mass Index | Knee Extensor Strength | Physical Performance | IMF | QM | |
|---|---|---|---|---|---|---|---|---|
| Age (y) | 1 | |||||||
| Frontal Plane Knee Alignment (°) | −0.08 | 1 | ||||||
| Pain Intensity (0–4) | −0.03 | −0.16 | 1 | |||||
| Body Mass Index (kg/m2) | 0.46 | −0.16 | 0.08 | 1 | ||||
| Knee Extensor Strength (N/kg) | −0.14 | 0.36* | −0.26 | −0.45* | 1 | |||
| Physical Performance (s) | 0.09 | −0.03 | 0.14 | 0.47* | −0.37* | 1 | ||
| IMF (cm3/m) | −0.03 | −0.16 | 0.10 | 0.74* | −0.33* | 0.36* | 1 | |
| QM (cm3/m) | −0.21 | 0.08 | −0.21 | 0.53* | −0.02 | 0.17 | 0.39* | 1 |
Bonferroni corrected p-value (p < 0.002)
Figure 2.
Relationships between tissue volumes and knee function in women over age 50 years who have, or are at risk for, radiographic knee OA. [A] Relationship (r=−0.33, p<0.001) between intermuscular fat volume (cm3/m) and maximal isometric knee extensor strength (N/kg). [B] Relationship (r=0.36, p<0.001) between intermuscular fat volume (cm3/m) and timed performance of five repeated chair stands (s). [C] No relationship between quadriceps volume (cm3/m) and knee strength (N/kg). [D] No relationship between quadriceps volume (cm3/m) and physical performance (s).
Table 4 presents the hierarchical multiple regression analyses. A model explaining 21.1% of the variance in knee extensor strength included frontal plane knee alignment, pain intensity during the strength test and IMF volume. A model explaining 13.4% of the variance in physical performance included OA status and IMF volume. For both regressions, the tolerance statistics for multicollinearity were ≥ 0.81.
Table 4.
Hierarchical multiple regressions of knee extensor strength and physical performance. The addition of each subsequent variable significantly improved the model from the previous iteration.
| Model | Cumulative Adjusted R2 | Standardized β coefficient | p |
|---|---|---|---|
| Knee Extensor Strength | |||
| 1. Knee Alignment | 0.120 | 0.279 | 0.001 |
| 2. Knee Alignment + Pain Intensity | 0.155 | −0.191 | 0.001 |
| 3. Knee Alignment + Pain Intensity + IMF | 0.211 | −0.255 | 0.001 |
|
| |||
| Physical Performance | |||
| 1. OA Status | 0.065 | 0.215 | 0.004 |
| 2. OA Status + IMF | 0.134 | 0.282 | 0.001 |
DISCUSSION
Intermuscular fat volume in the thigh, a novel measure of local fat derived from MRI scans, was greater among women with radiographic knee than those at risk for knee OA. Furthermore, IMF explained a small amount of variance in knee function among women with radiographic knee OA, or at risk for knee OA. Among this subsample from the OAI, greater IMF volume at the mid-thigh corresponded somewhat with lower maximal knee extensor force production and slower performance of repeated chair stands. These relationships were relatively weak, however, with IMF adding only 5.6% and 6.9% to the explained variance in strength and physical performance over covariates, respectively. Meanwhile, QM volume was unrelated to these knee functions. While the magnitude of the relationships between IMF volume and strength or physical function was small, these findings have implications on the mechanisms underlying mobility limitations due to knee OA. First, obesity may impede knee function through an increased volume of fat surrounding muscle tissue above the knee, in addition to the biomechanical and systemic effects already acknowledged in the field (10). Second, lean tissue appears less influential than adipose tissue on declines in physical performance associated with knee OA.
While this study demonstrates a weak relationship between IMF and knee function in knee OA, similar phenomena are established in healthy aging. Whole-body fat mass relates to concurrent mobility limitations and predicts mobility decline among well-functioning older adults (15). In 30 different cohorts of adults over 65 years of age, large absolute amounts of fat mass in the whole body increased the odds of having incident disability (odds ratios = 1.08 to 3.04), with greater odds ratios for women than men in most studies (15). By comparison, low absolute amounts of lean tissue, typically measured using dual X-ray absorptiometry, also increased the odds of having incident disability, however to a lesser degree (odds ratios = 0.87 to 2.30) (15). Less work has focused on fat accumulation in the lower extremity. Among 3,075 adults between 70 and 79 years of age with no self-reported limitations in mobility at baseline, mid-thigh muscle area and mid-thigh adipose infiltration of muscle, measured using muscle attenuation from computed tomography scans, both related to decrements in mobility over 2.5 years (16). However, adipose infiltration remained a significant factor associated with incident mobility limitations when strength was entered as a covariate, while lean mass did not (16). It is important to note that this previous work used a measurement that reflected intramyocellular fat (16), rather than a measurement of fat volume within the fascial envelope of the thigh as in the current study. Together these studies suggest that fat accumulation around the thigh is implicated in a small way to declines in knee function in well-functioning older adults, women at risk for knee OA, and women with radiographic knee OA.
Modest evidence hints that exercise-induced reductions in fat accumulations in the thigh could have potential to improve knee function. In 32 adults over 55 years of age with a variety of medical histories, a supervised 12-week eccentric training program reduced fatty infiltration of thigh muscle by 11% (29). However, this pilot work did not report whether reductions in thigh fat were associated with improved knee function. Further work is warranted to explore whether exercise reduces IMF volume and whether the effect size is clinically important to knee strength and physical performance in aging populations, including those with knee OA. Exercise programs in knee OA likely have multiple mechanisms of effect, ranging from reduced joint loads through weight loss (6), improved skeletal muscle mass (19), enhanced muscle strength and power (30), pain reduction (30), cartilage conditioning (31), improved mental health (32) and, possibly, reduced accumulations of adipose tissue local to the knee. The novel local measures of IMF and QM volumes presented in the current study may have potential to examine the effect of exercise on tissue composition in the thigh and expand our understanding of mechanisms underlying declines in physical function. Intermuscular fat volume as measured in the current study was related with BMI, however, the magnitude of this relationship (R2 = 54%) indicates that these variables are not interchangeable. That is, IMF volume represents features distinct of an estimate of whole body obesity.
Quadriceps volume shared no relationship with physical performance or extensor muscle strength in this sample. This finding may be surprising given that quadriceps strengthening improves physical performance (30) and numerous cross-sectional studies document muscle weakness, activation failure and discoordination are associated with knee OA (19). The thigh region of interest reported in the current study may not be the best region to capture the strength-generating capacity of muscle. As well, the QM volume reported in this study includes intramuscular fat, which may confound the results. It is important to note, however, that strength has not consistently been related to knee OA incidence or progression. For example, women in the lowest tertile for concentric knee extensor strength had an elevated risk of incident joint space narrowing compared to women in the highest tertile (33). On the other hand, peak concentric knee extensor and flexor torque did not protect against incident knee symptoms after 30 months among 2,275 men and women between 50–79 years of age who were deemed at risk for knee OA due to obesity or previous injury/surgery (33). Peak concentric knee extensor torque did not influence progression of cartilage loss over 30 months in 265 people with symptomatic knee OA (34). These latter two studies suggest that quadriceps strength may not be profoundly influential on progression of knee OA, or its consequences. It is also possible that QM volume could share a stronger association with muscle functions other than peak force output as used in the current study, such as muscle power. Muscle power, which reflects the rate with which muscle can perform work, demonstrated stronger relationships (r=0.67–0.75, p<0.01) with physical performance scores (jumping and leg press) than muscle strength (r=0.48–0.61, p<0.01) among 32 healthy old women (35). It is possible that the volume of muscle will show stronger relationships with muscle power, compared to muscle strength, as well. Clearly more work is required to explore the complexity of muscle as it relates to physical performance of the osteoarthritic knee.
One limitation is that the sample did not demonstrate much variability in age. Despite including women with a range of radiographic signs, the homogeneity of the sample may have limited the ability of the regression to detect a relationship between QM volume and knee extensor strength or physical performance. In addition, the method utilized to assess muscle strength in this study, a force value, did not incorporate a measure of moment arm to reflect torque. Finally, generalizability from this sample may be limited because the OAI dataset is documented to favour milder disease (28). It is possible that different relationships between IMF and QM with physical performance and extensor strength would be found in women with severe disease. Future studies are required to confirm the importance of IMF volume on knee strength and physical performance in women with severe knee OA as well as men. Future work should evaluate associations between IMF volume and measures of fatty infiltration of muscle using a tissue attenuation technique.
Conclusions
Women with radiographic knee OA have more intermuscular thigh fat than women at risk for this disease. This study demonstrated that intermuscular thigh fat volume determined from magnetic resonance images is weakly related to knee function among women with, or at risk for, radiographic knee OA. Specifically, IMF volume explained a small amount of variance in isometric knee extensor strength and physical performance of a repeated chair standing task. In concordance with studies of healthy aging, QM volume related to knee extensor strength or performance in this sample. Further work examining these relationships in severe knee OA and men would enhance our understanding of the role of adipose tissue in knee function in knee OA.
Significance and Innovations
Thigh intermuscular fat and quadriceps muscle volumes are novel outcomes in knee osteoarthritis. Most previous research focuses on area of tissue derived from cross-sectional images as an outcome measure.
Thigh tissue volume was characterized in women with radiographic knee osteoarthritis and women at risk for knee osteoarthritis using images acquired in the Osteoarthritis Initiative Study.
Intermuscular fat volume from the thigh was greater among women with radiographic knee OA compared to women at risk for knee OA. Further, thigh intermuscular fat related weakly to knee extensor strength and physical performance among women over age 50 with, or at risk for, knee osteoarthritis.
Acknowledgement
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Financial Support: Monica Maly holds a New Investigator Award from the Canadian Institutes of Health Research. Karen Beattie holds a Network Scholar Award through The Arthritis Society/Canadian Arthritis Network. Kristina Calder holds a Canadian Institutes of Health Research-Joint Motion Program Post-Doctoral Fellowship. This study was funded in part by NSERC Discovery Grants (#353715 MRM and #311896 NJM).
REFERENCES
- 1.Blagojevic M, Jinks C, Jeffery A, Jordan K. Risk factors for the onset of osteoarthrtis of the knee in older adults: A systemati review and meta-analysis. Osteoarthritis Cart. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, et al. Body mass index and susceptibility to knee osteoarthritis: A systematic review and meta-analysis. Joint Bone Spine. doi: 10.1016/j.jbspin.2011.05.015. in press. [DOI] [PubMed] [Google Scholar]
- 3.Rosemann T, Grol R, Herman K, Wensing M, Szecsenyi J. Association between obesity, quality of life, physical activity and health service utilization in primary care patients with osteoarthritis. Int J Behav Nutrition Phys Activity. 2008;5:4. doi: 10.1186/1479-5868-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma L, Lou C, Cahue S, Dunlop D. The mechanism of the effect of obesity in knee osteoarthritis. Arthritis Rheum. 2000;43(3):568–75. doi: 10.1002/1529-0131(200003)43:3<568::AID-ANR13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Creamer P, Lethbridge-Cejku M, Hochberg M. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumtol. 2000;39:490–6. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 6.Messier S, Gutekunst D, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026–32. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 7.Browning R, Kram R. Effect of obesity on the biomechanics of walking at different speeds. Med Sci Sports Exerc. 2007;39:1632–41. doi: 10.1249/mss.0b013e318076b54b. [DOI] [PubMed] [Google Scholar]
- 8.Mikesky A, Meyer A, Tompson K. Relationship between quadriceps strength and rate of loading during gait in women. J Orthop Res. 2000;18:171–5. doi: 10.1002/jor.1100180202. [DOI] [PubMed] [Google Scholar]
- 9.Runhaar J, Koes B, Clockaerts S, Bierma-Zeinstra M. A systematic review on changed biomechanics of lower extremities in obese individuals: A possible role in development of osteoarthritis. Obesity Rev. 2011;12:1071–82. doi: 10.1111/j.1467-789X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 10.Messier S. Obesity and osteoarthritis: disease genesis and nonpharmacologic weight management. Rheum Dis Clin North Am. 2008;34:713–29. doi: 10.1016/j.rdc.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andriacchi T, Mundermann A, Smith R, Alexander E, Dyrby C, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–57. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 12.Conde J, Gomez R, Bianco G, Scotece M, Lear P, Diguez C, et al. Expanding the adipokine network in cartilage: Identification and regulation of novel factors in human and murine chondrocytes. Ann Rheum Dis. doi: 10.1136/ard.2010.132399. in press. [DOI] [PubMed] [Google Scholar]
- 13.Goodpaster B, Carlson C, Visser M, Kelley D, Scherzinger A, Harris T, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Kritchevsky S, Goodpaster B, Newman A, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: The Health, Aging and Body Composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 15.Kidde J, Marcus R, Dibble L, Smith S, LaStayo P. Regional muscle and whole-body composition factors related to mobility in older individuals: A review. Physiother Can. 2009;61:197–209. doi: 10.3138/physio.61.4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visser M, Goodpaster B, Kritchevsky S, Newman A, Nevitt M, Rubin S, et al. Muscle mass, muscle strength and muscle fat infiltration of predictors of incident mobility limitations in well-functioning older persons. J Gerontol Biol Med Sci. 2005;60A:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 17.Beattie K, MacIntyre N, Ramadan K, Inglis D, Maly M. Longitudinal changes in intermuscular fat volume and quadriceps muscle volume in the thighs of women with knee osteoarthritis. Arthritis Care Res. 2012;64:22–9. doi: 10.1002/acr.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevitt M, Felson D, Lester G. The Osteoarthritis Initiative. Protocol for the Cohort Study. 2006;v1.1 [Google Scholar]
- 19.Bennell K, Hunt M, Wrigley T, Lim B, Hinman R. Role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2008;34:731–54. doi: 10.1016/j.rdc.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Yeadon M, Morlock M. The appropriate use of regression equations for the estimation of segmental inertia parameters. J Biomech. 1989;22:683–9. doi: 10.1016/0021-9290(89)90018-3. [DOI] [PubMed] [Google Scholar]
- 21.Curb J, Ceria-Ulep C, Rodriguez B, Grove J, Guralnik J, Wilcox B, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54:737–42. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 22.Ostir G, Volpato S, Fried L, Chaves P, Guralnik J. Reliability and sensitivity to change assessed for a summary measure of lower body function. Results fromthe Women's Health and Aging study. J Clin Epi. 2002;55:916–21. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 23.Cotofana S, Hudelmaier M, Wirth W, Himmer M, Ring-Dimitriou S, Sanger A. Correlation between single-slice muscle anatomical cross-sectional area and muscle volume in thigh extensors, flexors and adductors of perimenopausal women. Eur J Appl Physiol. 2010;110:91–7. doi: 10.1007/s00421-010-1477-8. [DOI] [PubMed] [Google Scholar]
- 24.Sharma L, Cahue S, Song J, Hayes K, Pai Y, Dunlop D. Physical functioning over three years in knee osteoarthritis. Role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359–70. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- 25.Palmieri-Smith R, Thoma A. Karvonen-Gutierrez C, Sowers M. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoathritis. Am J Phys Med Rehabil. 2010;89:541–8. doi: 10.1097/PHM.0b013e3181ddd5c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma L, Dunlop D, Cahue S, Song J, Hayes K. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Internal Med. 2003;138:613–9. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 27.Stevens J, Mizner R, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21:775–9. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 28.Riddle D, Stratford P. Impact of pain reported during isometric quadriceps muscle strength testing in people with knee pain: Data from the Osteoarthritis Initiative. Phys Ther. 2011;91:1478–89. doi: 10.2522/ptj.20110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus R, Addison O, Kidde J, Dibble L, Lastayo P. Skeletal muscle fat infiltration: Impact of age, inactivity and exercise. J Nutrition Health Aging. 2010;14:362–6. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fransen M, McConnell S. Exercise for osteoarthritis of the knee.: Cochrane Database of Systematic Reviews. 2008. p. Art. No. CD004376. [DOI] [PubMed] [Google Scholar]
- 31.Roos E, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage. Arthritis Rheum. 2005;52:3507–14. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 32.Lorig K, Gonzalez V, Laurent D, Morgan L, Laris B. Arthritis self-management program variations: Three studies. Arthritis Care Res. 1998;11:448–54. doi: 10.1002/art.1790110604. [DOI] [PubMed] [Google Scholar]
- 33.Segal N, Glass N, Torner J, Yang M, Felson D, Sharma L, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cart. 2010;18:769–75. doi: 10.1016/j.joca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, et al. Quadriceps strength and the risk fo cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60(189–198) doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forte R, Macaluso A. Relationship between performance-based and laboratory tests for lower-limb muscle strength and power assessment in healthy older women. J Sports Sci. 2008;26:1431–6. doi: 10.1080/02640410802208994. [DOI] [PubMed] [Google Scholar]