Abstract
Membrane-type 2 matrix metalloproteinase (MT2-MMP; also called MMP15) is a membrane-bound protease that degrades extracellular matrix and activates proMMPs such as proMMP-2. MMP-2 expression in avian embryos is well documented, but it is not clear how proMMP-2 is activated during avian embryogenesis. Here, we report that MT2-MMP mRNA is expressed in several tissues including the neural folds and epidermal ectoderm, intermediate mesoderm, pharyngeal arches, limb buds, and dermis. Several, but not all, of these tissues are known to express MMP-2. These observations suggest MT2-MMP may play a role during embryonic development not only through its own proteolytic activity, but also by activating proMMP-2.
Keywords: MT-MMP, MMP15, chicken embryo
Introduction
Matrix metalloproteinases (MMPs) are extracellular proteases that facilitate cell migration, regulate embryonic development and tissue remodeling events, and mediate epithelial–mesenchymal transitions (see reviews by Sternlicht and Werb, 2001; Mott and Werb, 2004; Bourboulia and Stetler-Stevenson, 2010; Klein and Bischoff, 2011). Soluble MMPs, such as MMP-2, are secreted as latent proenzymes (proMMPs) with an inhibitory N-terminal pro-domain that must be proteolytically removed, often by another MMP, in order to be activated. Membrane-type MMPS (MT-MMPs) are MMPs that are anchored to the plasma membrane either through a single transmembrane domain (MT1-MMP, MT2-MMP, MT3-MMP, and MT5-MMP; Imai et al., 1996; Fillmore et al., 2001; Lang et al., 2004) or a GPI linkage (MT4-MMP and MT6-MMP; Itoh et al., 1999; Kojima et al., 2000).
The activation of proMMPs often involves MT-MMPs and tissue inhibitors of matrix metalloproteinases (TIMPs; reviewed in Nagase et al., 2006). Although TIMPs inhibit the proteolytic activity of MMPs, they participate in proMMP activation by recruiting proMMPs to the cell surface where they can be cleaved and activated by a MT-MMP. The best-studied example of this is the activation of proMMP-2 by TIMP-2 and MT1-MMP (Butler et al., 1998; English et al., 2006). In fact, the primary mode of proMMP-2 activation in mice involves MT1-MMP and TIMP-2, as evidenced by numerous in vitro studies (reviewed in Woessner and Nagase, 2000; Murphy et al., 2003) and the fact that TIMP-2-null mice show greatly reduced levels of activated MMP-2 (Wang et al., 2000).
During avian embryogenesis, however, this appears not to be the case. Although MMP-2 is fairly widely expressed (Cai et al. 2000; Duong and Erickson, 2004), TIMP-2 and MT1-MMP expression are much more restricted (Brauer and Cai, 2002; Cantemir et al., 2004; our unpublished observations). In addition, chicken MT1-MMP has a 5-amino-acid deletion in a structure called the “MT loop” that in mammals is critical for proMMP-2 activation (English et al., 2001; Yang et al. 2008). Thus, chicken MT1-MMP likely could not activate proMMP-2 even if the two were co-expressed.
How else could proMMP-2 be activated during avian embryogenesis? MT2-MMP (also called MMP15) can activate proMMP-2 in a TIMP-independent manner (Kolkenbrock et al., 1997; Morrison et al., 2001) and is an important mediator of basement membrane turnover and cell invasiveness in its own right (e.g. Hotary et al., 2000; Reimar et al., 2005; Hotary et al., 2006). MT2-MMP is expressed in the ectoderm of Xenopus embryos (Harrison et al., 2004) and has been implicated in endocardial cushion formation (Tao et al., 2011) and submandibular gland morphogenesis (Rebustini et al., 2009) in mouse development. However, nothing is known about the role or expression of MT2-MMP during avian development. Thus, the purpose of this study was to determine where and when MT2-MMP is expressed during early avian embryogenesis.
Materials and Methods
Characterization of a chicken MT2-MMP EST
A chicken EST corresponding to MT2-MMP (EST number 88a20) was identified by database searching and acquired from Geneservice Ltd. (Cambridge, UK). A 526 bp fragment from this EST was subcloned into pBluescript II and sequenced. The sequence was compared to published and predicted sequences of other MT-MMPs using BLAST and ClustalW2. A maximum likelihood phylogenetic comparison of this sequence to that of other MT-MMPs was performed using MEGA5 software (www.megasoftware.net).
In situ hybridization
15–25 embryos per stage were analyzed. Hamilton-Hamburger (HH) stage 8–22 chicken embryos were fixed in 4% paraformaldehyde overnight, rinsed in phosphate-buffered saline (PBS) and treated with proteinase K (1 μg/ml for 10 min for HH stages 8–13, 10 μg/ml for 10 min for HH stages 14–18, and 10 μg/ml for 15 min for HH stages 19–22) before being subjected to whole mount in situ hybridization as described previously (Kos et al., 2001). The 526 bp subclone described above was used to make sense and antisense digoxigenin-labeled riboprobes via in vitro transcription (Roche Applied Sciences). Very stringent hybridization conditions (70°C, 5X SSC, 50% formamide) and post-hybridization washes (65–68°C, 0.5X SSC, 50% formamide) were used. Hybridized probes were detected using an alkaline phosphatase-conjugated anti-digoxigenin antibody and NBT/BCIP phosphatase substrates (Roche Applied Sciences). Embryos were post-fixed in 4% paraformaldehyde, photographed as whole mounts, and then embedded and sectioned at 10–15 μm on a cryostat.
Immunostaining
Immunostaining for chicken MMP-2 was performed as described in Cai et al. (2000). Briefly, embryos were fixed for 30 min in 4% paraformaldehyde while on ice and frozen sections were immunostained for one hour with rabbit anti-chicken MMP-2 IgG (Hahn-Dantona et al., 2000) and binding visualized using goat anti-rabbit IgG-FITC (Jackson ImmunoResearch, West Grove, PA). Sections were washed and coverslipped with Vectashield mounting medium (Vector Laboratories, Burlingame, CA), examined on an epifluorescent microscope, and images captured using a SPOT CCD camera (Diagnostic Instruments, Sterling Heights, MI).
Results
Characterization of a MT2-MMP EST
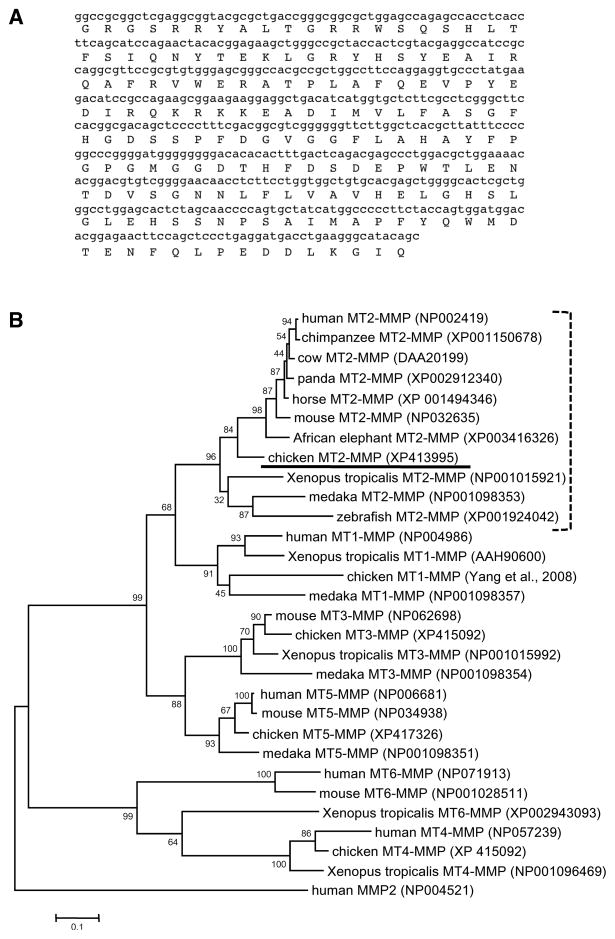
The sequence of the 526 bp fragment from EST 88a20 used in this study is shown in Figure 1A. Domain analysis and BLAST searching revealed that it corresponded to the catalytic and TIMP-binding region of MT2-MMP. Although this EST was not full-length and the catalytic domains of different MT-MMPs are similar at the amino acid level, we were confident it represented MT2-MMP and not another MT-MMP for two reasons. First, BLASTing the nucleotide sequence against the chicken genome database revealed it mapped to the predicted MT2-MMP locus on chicken chromosome 11 (NCBI accession number XM413995). This region of chromosome 11 is not fully sequenced, and the first exon of MT2-MMP lies within an unsequenced region. In addition, maximum likelihood phylogenetic analysis showed this sequence clusters with MT2-MMPs from other species, with a bootstrap support value (confidence) of 84% (Fig. 1B).
Figure 1.
A). Nucleotide and predicted amino acid sequence of the 526 bp subclone from EST 88a20 used in this study. B). Maximum-likelihood phylogenetic tree of MT-MMPs using human MMP2 as the outgroup (255 informative sites, 1000 repetitions). The chicken MT2-MMP sequence (underlined) clusters with 84% confidence with MT2-MMP sequences from other species (bracket). The accession numbers of each sequence used are listed after the sequence name. Bootstrap support values for each branch are shown. Scale bar = substitutions per site.
Ectodermal Expression of MT2-MMP
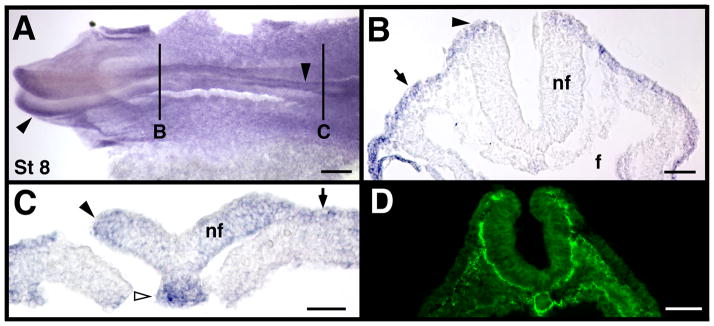
Consistent with reports from Xenopus (Harrison et al., 2004), at HH stage 8 MT2-MMP was expressed in low levels throughout the epidermal ectoderm (black arrows in Fig. 2B, C). This widespread expression in the early epidermal ectoderm is interesting, as MMP-2 protein is widespread in the underlying mesoderm at stage 8 (Fig. 2D).
Figure 2.
MT2-MMP mRNA expression at stage 8. A). Representative whole mount, with solid black lines indicating the approximate cross-section level shown in panels B and C. D). MMP-2 antibody labeling in a stage 8 embryo for comparison. Black arrowheads indicate neural folds, black arrows indicate epidermal ectoderm, and white arrowhead indicate the caudal notochord. Scale bar = 200 μm in panel A, 50 μm in panels B, and C, and 100 μm in D.
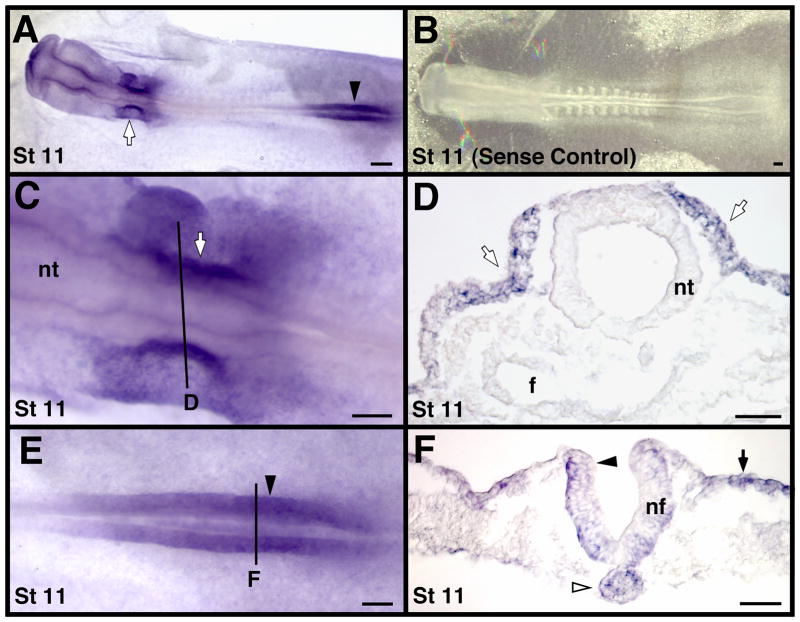
At stage 8, MT2-MMP transcripts were also detected in the cranial and caudal neuropores (black arrowheads in Fig. 2A, C). Expression in the caudal neuropore continued at later stages (black arrowheads in Fig. 3A, E, F and in 4A, C). MT2-MMP mRNA was not detected in the closed neural tube (e.g. Figs. 2B, 3D and 4B, D, G), suggesting MT2-MMP expression was transient and related to the neurulation process itself. MT2-MMP was also expressed in the developing otic placode and otic vesicle (white arrows in Fig. 3A, C, D). Once the otic vesicle fully formed, however, it did not express MT2-MMP transcripts (not shown), similar to how MT2-MMP expression was downregulated in the neural tube following the completion of neurulation.
Figure 3.
MT2-MMP mRNA expression in stage 11 chicken embryos. A). Representative whole-mount. B). Embryo hybridized with a sense probe as a negative control. This embryo was photographed with substage rather than oblique lighting to provide enough contrast to see detail in the otherwise clear embryo. C and E). Higher magnification view of the embryo in A showing the otic placode (in C) and caudal neuropore (in E) regions. Solid black lines indicate the approximate cross-section level shown in corresponding labeled images D and F. Black arrowheads indicate neural folds, black arrows indicate epidermal ectoderm, white arrowheads indicate the caudal notochord, and white arrows indicate the otic placode. Scale bar = 100 μm in A and B, 50 μm in panels C–F. Abbreviations: nf, neural fold; and nt, neural tube.
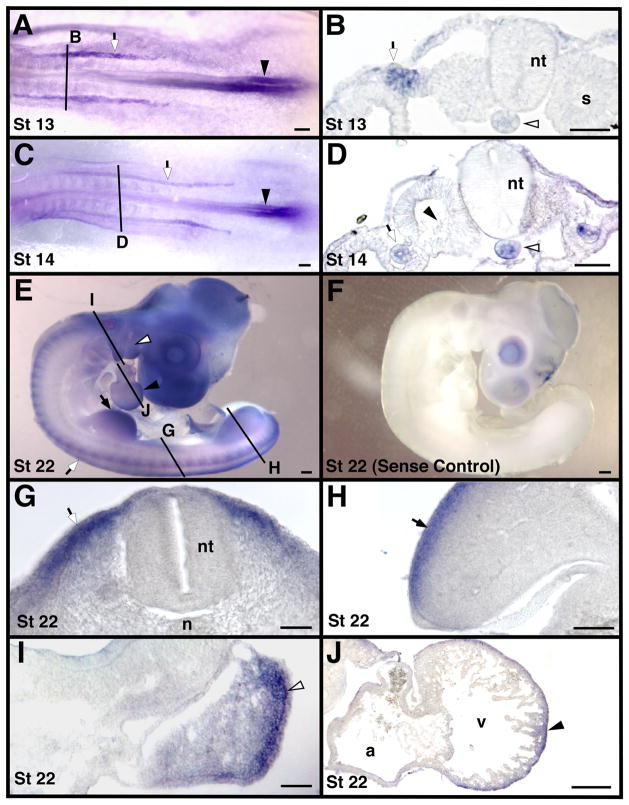
Figure 4.
Localization of MT2-MMP mRNA in whole mount and transverse sections of stage 13 – 22 chicken embryos. Solid black lines indicate approximate cross-section level shown in corresponding labeled images. Panel F is an embryo hybridized with a sense probe, all other panels are embryos hybridized with an antisense probe for MT2-MMP. Shown are stages 13 in panels A and B, 14 in panels C and D, and 22 in panels E–J. Caudal notochord expression continues until approximately stage 14 (white arrowheads in B and D) as does neural fold expression (black arrowheads in A and C). The intermediate mesoderm (white arrows in A–D) also expressed MT2-MMP. MT2-MMP was expressed in the dermamyotome (white arrows in E and G) and in the limb (black arrows in E and H). The pharyngeal arches (white arrowheads in E and I) and the heart (black arrowheads in E and J) also expressed MT2-MMP. Note the lack of expression in the sclerotome (black arrowhead in D). Scale bar = 50 μm in B, D, G, H, I and J and 100 μm in A, C and E. Abbreviations: a, atrium; n, notochord; nt, neural tube; s, somite; and v, ventricle.
Mesodermal Expression of MT2-MMP
MT2-MMP transcripts were detected in axial, paraxial, intermediate, and lateral mesoderm-derived structures. MT2-MMP was expressed in the caudal notochord as early as stage 8 and was maintained there until approximately stage 14 (white arrowheads in Fig. 2C, 3F and 4B, D). We did not detect MT2-MMP expression in the cranial notochord at any stage (e.g., Fig. 2B, 3D). MT2-MMP was also strongly expressed in the pronephrogenic cord of the developing intermediate mesoderm between stages 12–14 (white arrows in Fig. 4A–D). We did not detect MT2-MMP transcripts in the developing mesonephros at later stages.
From stages 19–22, strong expression of MT2-MMP was observed in the dermamyotome and later in the subectodermal mesenchyme that likely becomes dermis (white arrow in Fig. 4G). MT2-MMP was also strongly expressed in the dorsal subectodermal mesenchyme of the developing limbs (black arrows in Fig. 4E, H). This limb bud expression began around stage 19/20 and persisted until at least stage 22, which is the latest stage examined. Morphologically, the MT2-MMP-expressing cells in the limb did not appear to be hypaxial muscle progenitors, but were likely either dermis or lateral plate mesoderm-derived. Also in older embryos (stage 21/22), MT2-MMP expression was found in both the pharyngeal arches (white arrowheads in Fig. 4E, I) and in the compact layer of the myocardium (black arrowheads in Fig. 4E, J).
Discussion
MT2-MMP in an important mediator of basement membrane turnover and cell invasion, and can also activate pro-MMP2. Here we report that MT2-MMP is expressed in the early chicken embryo in several morphogenetically active tissues. MT2-MMP expression was seen in the cranial and caudal neuropores and in the developing otic placodes, areas of considerable basement membrane remodeling (e.g. Martins-Green, 1988; Hemond and Morest, 1991; Fernandez et al., 1992; Hilfer and Randolph, 1993; Visconti and Hilfer, 2002). In particular, laminin turnover has been implicated in both otic vesicle formation (Visconti and Hilfer, 2002) and primary neurulation (Zagris et al., 2004), and MT2-MMP is particularly efficient at degrading laminin (d’Ortho et al., 1997). At these same stages MMP-2 protein is localized to basement membranes underlying the epidermal ectoderm and the neural folds (Cai et al., 2000), so MT2-MMP may also help to activate proMMP-2 at these sites.
MT2-MMP was also expressed in the developing pronephros. At stages 12–13, the rostral limit of MT2-MMP expression in the intermediate mesoderm was adjacent to somites 7–8, and expression extended caudally to the level of the segmental plate mesoderm. This correlates both temporally and spatially with the extension of the pronephric cord and the separation of the pronephric duct and nephric tubule progenitor tissues (Hiruma and Nakamura, 2003), suggesting that MT2-MMP may play a role in these processes.
Previous studies have reported that during the same stages of avian development examined here, MMP-2 is expressed by or localized within head mesenchyme, cardiogenic splanchnic mesoderm and the early heart tube, sclerotome, dermamyotome/dermis, trunk neural crest cells, and limb dermis (Cai et al. 2000; Duong and Erickson, 2004). Our findings suggest MT2-MMP could play a role in proMMP-2 activation in some, but not all, of these regions. We did not detect MT2-MMP mRNA in several areas of strong MMP-2 expression, including epithelial somites, sclerotome, early cardiac mesoderm, or migrating neural crest cells. However, MT2-MMP was expressed in the dermamyotome and subectodermal mesenchyme, pharyngeal arches, neural folds, and epidermal ectoderm, tissues where MMP-2 expression has been reported previously. Our observations suggest MT2-MMP may play a role during embryonic development not only through its own proteolytic activity, but also by activating proMMP-2 in some tissues.
Acknowledgments
We are grateful to Dr. Soochin Cho for assistance with the phylogenetic analysis. This work was made possible by NIH grant numbers P20 RR016469 (NCCR), 5P20RR016469 (NCCR) and 8P20GM103427 (NIGMS). Its contents are the sole responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH. This project was also supported by grant number 0555683Z from the American Heart Association—Heartland Affiliate.
Literature Cited
- Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Sem Cancer Biol. 2010;20:161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer PR, Cai DH. Expression of tissue inhibitor of metalloproteinases (TIMPs) during early cardiac development. Mech Dev. 2002;113:175–179. doi: 10.1016/s0925-4773(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, Crabbe T, Clements J, d’Ortho MP, Murphy G. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- Cai DH, Vollberg TM, Sr, Hahn-Dantona E, Quigley JP, Brauer PR. MMP-2 expression during early avian cardiac and neural crest morphogenesis. Anat Rec. 2000;259:168–179. doi: 10.1002/(SICI)1097-0185(20000601)259:2<168::AID-AR7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Cantemir V, Cai DH, Reedy MV, Brauer PR. Tissue inhibitor of metalloproteinase-2 (TIMP-2) expression during cardiac neural crest cell migration and its role in proMMP-2 activation. Dev Dyn. 2004;231:709–719. doi: 10.1002/dvdy.20171. [DOI] [PubMed] [Google Scholar]
- d’Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- Duong TD, Erickson CA. MMP-2 plays an essential role in producing epithelial-mesenchymal transformations in the avian embryo. Dev Dyn. 2004;229(1):42–53. doi: 10.1002/dvdy.10465. [DOI] [PubMed] [Google Scholar]
- English WR, Holtz B, Vogt G, Knauper V, Murphy G. Characterization of the role of the “MT-loop”: an eight-amino acid insertion specific to progelatinase A (MMP2) activating membrane-type matrix metalloproteinases. J Biol Chem. 2001;276:42018–42026. doi: 10.1074/jbc.M107783200. [DOI] [PubMed] [Google Scholar]
- English JL, Kassiri Z, Koskivirta I, Atkinson SJ, Di Grappa M, Soloway PD, Nagase H, Vuorio E, Murphy G, Khokha R. Individual Timp deficiencies differentially impact pro-MMP-2 activation. J Biol Chem. 2006;281:10337–10346. doi: 10.1074/jbc.M512009200. [DOI] [PubMed] [Google Scholar]
- Fernandez Caso M, De Paz P, Fernandez Alvarez JG, Chamorro C, Villar JM. Delamination of neuroepithelium and nonneural ectoderm and its relation to the convergence step in chick neurulation. J Anat. 1992;180(Pt 1):143–153. [PMC free article] [PubMed] [Google Scholar]
- Fillmore HL, VanMeter TE, Broaddus WC. Membrane-type matrix metalloproteinases (MT-MMPs): expression and function during glioma invasion. J Neurooncol. 2001;53:187–202. doi: 10.1023/a:1012213604731. [DOI] [PubMed] [Google Scholar]
- Hahn-Dantona EA, Aimes RT, Quigley JP. The isolation, characterization, and molecular cloning of a 75-kDa gelatinase B-like enzyme, a member of the matrix metalloproteinase (MMP) family. An avian enzyme that is MMP-9-like in its cell expresssion pattern but diverges from mammalian gelatinase B in sequence and biochemical properties. J Biol Chem. 2000;275:40827–40838. doi: 10.1074/jbc.M006234200. [DOI] [PubMed] [Google Scholar]
- Harrison M, Abu-Elmagd M, Grocott T, Yates C, Gavrilovic J, Wheeler GN. Matrix metalloproteinase genes in Xenopus development. Dev Dyn. 2004;231:214–220. doi: 10.1002/dvdy.20113. [DOI] [PubMed] [Google Scholar]
- Hemond SG, Morest DK. Ganglion formation from the otic placode and the otic crest in the chick embryo: mitosis, migration, and the basal lamina. Anat Embryol (Berl) 1991;184:1–13. doi: 10.1007/BF01744256. [DOI] [PubMed] [Google Scholar]
- Hilfer SR, Randolph GJ. Immunolocalization of basal lamina components during development of chick otic and optic primordia. Anat Rec. 1993;235:443–452. doi: 10.1002/ar.1092350313. [DOI] [PubMed] [Google Scholar]
- Hiruma T, Nakamura H. Origin and development of the pronephros in the chick embryo. J Anat. 2003;203:539–552. doi: 10.1046/j.1469-7580.2003.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K, Allen E, Punturieri A, Yana I, Weiss S. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 2006;20:2673–2686. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Ochuchi E, Aoki T, Nomura H, Fujii Y, Sata H, Seiki M, Okada Y. Membrane-type matrix metalloproteinase 1 is a gelatinolytic enzyme and is secreted in a complex with tissue inhibitor of metalloproteinase 1. Canc Res. 1996;56:2707–2710. [PubMed] [Google Scholar]
- Itoh Y, Kajita M, Kinoh H, Mori H, Okada A, Seiki M. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J Biol Chem. 1999;274:34260–34266. doi: 10.1074/jbc.274.48.34260. [DOI] [PubMed] [Google Scholar]
- Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Itoh Y, Matsumoto S, Masuho Y, Seiki M. Membrane-type 6 matrix metalloproteinase (MT6-MMP, MMP-25) is the second glycosyl-phosphatidyl inositol (GPI)-anchored MMP. FEBS Lett. 2000;480:142–146. doi: 10.1016/s0014-5793(00)01919-0. [DOI] [PubMed] [Google Scholar]
- Kolkenbrock H, Hecker-Kia A, Orgel D, Ulbrich N, Will H. Activation of progelatinase A and progelatinase A/TIMP-2 complex by membrane type 2-matrix metalloproteinase. Biol Chem. 1997;378:71–76. doi: 10.1515/bchm.1997.378.2.71. [DOI] [PubMed] [Google Scholar]
- Kos R, Reedy MV, Johnson RL, Erickson CA. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128:1467–1479. doi: 10.1242/dev.128.8.1467. [DOI] [PubMed] [Google Scholar]
- Lang R, Braun M, Sounni NE, Noel A, Frankenne F, Foidart JM, Bode W, Maskos K. Crystal structure of the catalytic domain of MMP-16/MT3-MMP: characterization of MT-MMP specific features. J Mol Biol. 2004;336:213–225. doi: 10.1016/j.jmb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Martins-Green M. Origin of the dorsal surface of the neural tube by progressive delamination of epidermal ectoderm and neuroepithelium: implications for neurulation and neural tube defects. Development. 1988;103:687–706. doi: 10.1242/dev.103.4.687. [DOI] [PubMed] [Google Scholar]
- Morrison CJ, Butler GS, Bigg HF, Roberts CR, Soloway PD, Overall CM. Cellular activation of MMP-2 (gelatinase A) by MT2-MMP occurs via a TIMP-2-independent pathway. J Biol Chem. 2001;276:47402–47410. doi: 10.1074/jbc.M108643200. [DOI] [PubMed] [Google Scholar]
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Knauper V, Lee MH, Amour A, Worley JR, Hutton M, Atkinson S, Rapti M, Williamson R. Role of TIMPs (tissue inhibitors of metalloproteinases) in pericellular proteolysis: the specificity is in the detail. Biochem Soc Symp. 2003;70:65–80. doi: 10.1042/bss0700065. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG, Hoffman MP. MT2-MMP-dependent release of collagen IV NCI domains regulates sunmandibular gland branching morphogenesis. Dev Cel. 17:482–493. doi: 10.1016/j.devcel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimar A, Schafer J, Rothe M, Bange J, Knyazev P, Ullrich A. Identification of MMP-15 as an anti-apoptotic factor in cancer cells. J Biol Chem. 2005;280:34123–34132. doi: 10.1074/jbc.M508155200. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Ann Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao G, Levay AK, Gridley T, Lincoln J. Mmp15 is a direct target of Snai1 during endothelial to mesenchymal transformation and endocardial cushion development. Dev Biol. 2011;359:209–221. doi: 10.1016/j.ydbio.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti RP, Hilfer SR. Perturbation of extracellular matrix prevents association of the otic primordium with the posterior rhombencephalon and inhibits subsequent invagination. Dev Dyn. 2002;223:48–58. doi: 10.1002/dvdy.1237. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jutterman R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner JF, Nagase H. Activation of the zymogen forms of MMPs. In: Woessner JF Jr, Nagase H, editors. Matrix metalloproteinases and TIMPs. Oxford University Press; New York: 2000. pp. 72–86. [Google Scholar]
- Yang M, Zhang B, Zhang L, Gibson G. Contrasting expression of membrane metalloproteinases, MT1-MMP and MT3-MMP, suggest distinct functions in skeletal development. Cell Tissue Res. 2008;333:81–90. doi: 10.1007/s00441-008-0619-3. [DOI] [PubMed] [Google Scholar]
- Zagris N, Christopoulos M, Giakoumaki A. Developmentally regulated expression and functional role of alpha 7 integrin in the chick embryo. Dev Growth Differ. 2004;4:299–307. doi: 10.1111/j.1440-169X.2004.00747.x. [DOI] [PubMed] [Google Scholar]