Abstract
The treatment of diseased vasculature remains challenging, in part because of the difficulty in implanting drug-eluting devices without subjecting vessels to damaging mechanical forces. Implanting materials using adhesive forces could overcome this challenge, but materials have previously not been shown to durably adhere to intact endothelium under blood flow. Marine mussels secrete strong underwater adhesives that have been mimicked in synthetic systems. Here we develop a drug-eluting bioadhesive gel that can be locally and durably glued onto the inside surface of blood vessels. In a mouse model of atherosclerosis, inflamed plaques treated with steroid-eluting adhesive gels had reduced macrophage content and developed protective fibrous caps covering the plaque core. Treatment also lowered plasma cytokine levels and biomarkers of inflammation in the plaque. The drug-eluting devices developed here provide a general strategy for implanting therapeutics in the vasculature using adhesive forces and could potentially be used to stabilize rupture-prone plaques.
Keywords: biomaterials, catechol, delivery, endoluminal paving
Diseases of the vasculature are numerous and can be deadly. Local delivery of drugs is an important challenge in experimental biology and medicine for understanding and controlling diseases of the vasculature, such as atherosclerosis, ischemia, inflammation, edema, oxidative stress, thrombosis, hemorrhage, metabolic and oncological diseases, and others (1). One prominent example involves the rupture of the endothelium or endothelialized fibrous cap on atherosclerotic plaques, a trigger of heart attacks and strokes (2). Although many technologies are being developed to identify plaques at risk of rupturing (3–6), effective local treatments for these vulnerable plaques do not currently exist (7, 8). Substantial effort has been devoted to creating devices that locally deliver therapeutics to diseased vasculature (7, 9–11). The ideal device would shield the diseased vasculature from the bulk blood stream and stabilize the diseased tissue without causing substantial damage to it, but this has not yet been achieved (12, 13). Drug-eluting stents are an example of devices used for local delivery. Drug-eluting materials are incorporated into or onto stiff expandable struts that apply a strong mechanical force against the blood vessel wall to remain in place. The stents that are used clinically are valuable for rapidly widening vessels with large plaques to increase blood flow, but their primary function in the clinic is not to preventively stabilize and heal plaques that are at risk of rupture. In fact, stents are known to cause substantial tissue injury when they are deployed, which over time can lead to impaired reendothelialization, restenosis, and thrombosis, and these challenges will need to be overcome if stents are considered as a preventive therapy for plaque rupture (12–16). Locally depositing therapeutics in blood vessels via catheters is a promising approach for local delivery but is limited in some applications by the rapid removal of the agents due to blood flow (7). Endoluminal paving, another local therapy, is a catheter-based technique that deposits a thin layer of degradable hydrogels temporarily on the inside of blood vessels (11, 17–20). This technique has been intensively explored for almost 20 y, and many methods have been developed, including interfacial photo-polymerization. Although it reduced restenosis following balloon angioplasty in animals, it has not been used to treat inflamed atherosclerotic plaques (17). Endoluminal paving holds substantial promise for clinical applications, but durable adherence has thus far been limited to vasculature mechanically denuded of its endothelium using balloon angioplasty (20). The development of a coating that could be directly applied on the vasculature, remains chronically adhered, induces minimal hyperplasia, and stabilizes the diseased area would overcome a major barrier to local delivery of drugs to inflamed plaques and other regions of the vasculature.
This paper describes the development of an adhesive hydrogel capable of durably attaching to the inside of blood vessels and over atherosclerotic plaques, with spatial resolution on the scale of millimeters. The adhesion of the gel emerges from catechol moieties in the gel. This gel mimics aspects of the adhesion of marine mussels, which adhere to a variety of surfaces in the ocean. Mussels secrete adhesive proteins from their feet that contain 3,4-dihydroxy-L-phenylalanine (DOPA), an amino acid–containing catechol (21, 22). This biomimetic chemistry has been used in synthetic systems and gels for a variety of adhesive applications (23–26). We hypothesized that by mimicking the mussel’s underwater adhesion, materials could be designed that adhere to blood vessels under blood flow. The motivation to test this hypothesis was that the material and technique would be useful for studying and promoting healing of diseased vasculature in general, and atherosclerotic plaques in particular, due to the ability to deliver the material locally and release therapeutics on a controllable time scale. Additionally, an appropriate material may promote characteristics of plaque healing by remodeling and strengthening the fibrous cap on atherosclerotic plaques. A thrombus-induced acute event such as myocardial infarction or stroke occurs when the endothelium or fibrous cap on a plaque erodes, allowing blood to contact nonendothelial thrombogenic tissue of the plaque core and coagulate (2). Properties of so-called vulnerable plaques at risk of rupture include a thin cap of fibrous tissue and cells, high content of inflammatory cells, metalloproteinases, cholesterol and tissue factor, and decreased extracellular matrices (2, 27). Both strengthening the fibrous cap that covers the inflamed core and mechanically shielding the lesion have been proposed as ways to stabilize plaques and prevent them from rupturing (16).
Results and Discussion
Catechol-Based Gel Adheres to Vascular Cells and Tissue Under Physiological Shear Stress.
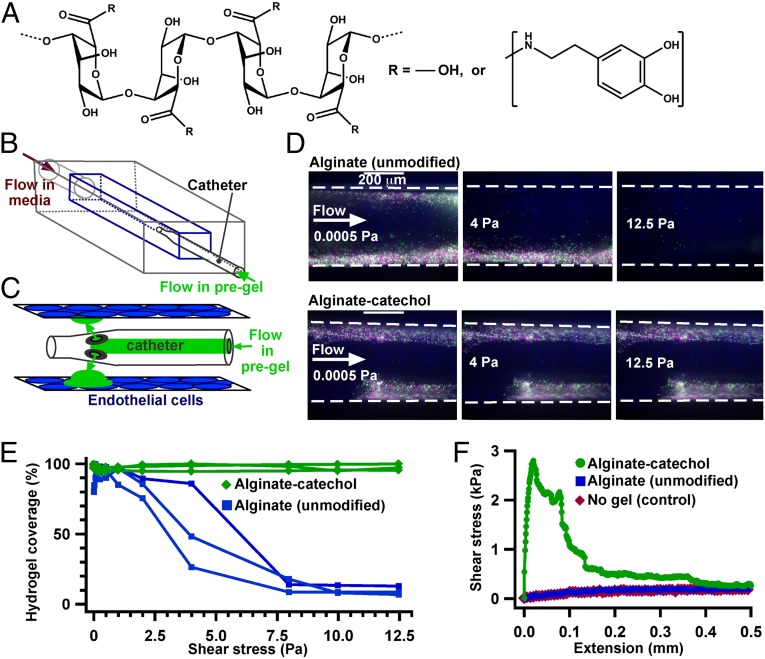
The adhesive gel was synthesized based on alginate, a polysaccharide that forms a biostable and generally biocompatible hydrogel (28), and catechol, which provides sites for cross-linking and adhesive properties (Fig. 1A). The mechanism by which catechols adhere to cellular surfaces is a combination of hydrogen bonding through phenolic protons and covalent bonding through the reaction of nucleophiles on the surface of cells and matrices with the unstable quinone group formed by oxidation of catechols (23). The gel covalently cross-linked in 10–20 min following the addition of periodate, an oxidant that has been used to cross-link catechol-containing hydrogels in vivo for long-term implantation of islets (25). The shear strength of the gel adhesion to endothelial cells was determined to predict whether the gel would be able to withstand the shear forces present inside blood vessels under flow. This shear strength was measured both in a microfluidic device that mimicked aspects of the vasculature (Fig. 1 B–E) and in a lap-shear tensile strain test (Fig. 1F). A microfluidic device was used as a simplified model of the vasculature due to the ease of controlling channel size, flow, and shear stress, and as a tool to develop a catheter-based approach for delivering the gel. The gel adhered to endothelial cells growing on the walls of the microfluidic channel when deposited locally in a 1- to 3-mm-long region of the device via a catheter. The adhesive shear strength of the gel was 12 Pa, a shear stress several times higher than that generated by physiological blood flow (29). In a lap-shear tensile strain test, the gel was applied to the inside of a bovine carotid artery that had been excised for 3 d and had denuded endothelium. The shear strength of the gel to the artery was 2 ± 1.3 kPa, which is two to three orders of magnitude higher than physiological shear stress. Moderate concentrations of the gel did not cause significant toxicity to cultured cells in vitro. The gel and the oxidative cross-linking process did not cause measurable toxicity in vivo, assessed in several types of assays (Figs. S1–S3). These assays included measuring the body mass of mice following i.p. injections, assessing inflammation surrounding s.c.-injected deposits, and measuring liver enzymes and cytokines in serum and analyzing histology of organs following intravascular deposition. Degradation of the gel was not observed within 28 d in vitro and in vivo, which is consistent with alginate gels that are irreversibly cross-linked. Alginate that is reversibly cross-linked with a solution containing Ca2+ is typically stable for weeks or longer in a cellular environment, and stronger cross-linking, such as with Ba2+, is known to stabilize gels further (30, 31).
Fig. 1.
An adhesive gel, alginate-catechol, remains adhered to endothelial cells under flow above physiological shear stress. (A) Chemical structure of alginate-catechol, synthesized from alginate. (B) Schematic of a microfluidic system used to compare the adhesion of alginate and alginate-catechol gels. Alginate (unmodified) was cross-linked with a solution containing Ca2+ and used as a control due to its low adhesive strength. (C) Schematic of the gel (green) being deposited on endothelial cells (blue) grown inside the device. A catheter was temporarily inserted into the device to deliver the gel to the annular region between the catheter and cells while the flow of media was stopped. (D) Fluorescence images of the gels containing fluorescent particles (green and purple) coated on endothelial cells in microfluidic channels (dashed lines). The unmodified alginate does not remain adhered at physiological shear stress, whereas the alginate-catechol remains adhered above physiological shear stress. (E) Graph quantifying the percent of gel that remained covered on the microfluidic channels at various shear stresses. (F) Quantifying the shear strength of the gels using a lap-shear tensile strain test.
Adhesive Gel Can Be Locally and Durably Implanted Inside Arteries.
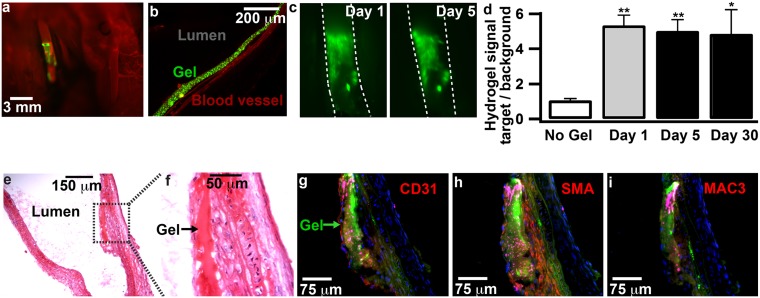
Using the techniques developed in microfluidic devices, the adhesive gel was coated locally onto the carotid arteries of WT mice (Fig. 2 A and B). The carotid artery was temporarily ligated to stop blood flow, a catheter containing a solution of alginate-catechol and fluorescent particles was inserted through an incision in the artery and positioned away from the incision, and 0.3–0.6 μL of the solution was applied through the catheter to place the viscous solution in contact with the vessel wall (see SI Text and Fig. S4 for further description of the catheter system). Deposition and cross-linking of alginate-catechol was monitored by imaging fluorescent particles immersed in the gel using intravital fluorescence microscopy. The gel cross-linked via oxidation of the catechol moieties, and then the catheter was removed, leaving a film of gel 10–100 μm thick adhered to the vessel wall. The incision in the artery was closed, the ligations were removed, and blood flow resumed. Intravital microscopy was used to monitor the deployment of the gel. Several different types of arteries were successfully coated, including the common carotid, the bifurcation of the carotid, the external carotid artery, and the abdominal aorta. Various lengths along blood vessels could also be coated, from 10 μm to several millimeters (Fig. S4). The gel had remarkable adherence to the blood vessel wall. When mice were imaged 5 d after treatment, the gel maintained its coverage on the wall and its overall shape, indicating that the gel did not break or detach in that time (Fig. 2C). The amount of gel in the vessels did not significantly decrease within 5 or 30 d (P > 0.7; n = 10; Fig. 2D). The thickness of the gel was 53 ± 24 μm in three mice at day 5 and 88 ± 50 μm in three other mice at day 30 after gel application. All WT mice survived in this experiment, and the vessels coated with the gel were not occluded by the gel or clotted blood, as evidenced by blood flow through the vessel on intravital microscopy, further supporting the notion that the gel coating is stable. The absence of neurological deficits indicated that no major strokes occurred. The gel was still present in vessels 4 mo after treatment in the two mice that were tested at that time (Fig. S4). These results demonstrate that an adhesive coating can have long-term durability in vivo under blood flow. To investigate the biological response to the gel, we analyzed sections of the vessel by histology in three mice at day 5 and three other mice at day 30. The gel elicited the growth of new endothelial cells and smooth muscle cells within 30 d in all coated arteries (Fig. 2 E–H). The layer of smooth muscle cells was 10 ± 1 μm thick at day 5 and present in the tunica media, similar to untreated mice. The layer of smooth muscle cells increased to 74 ± 24 μm at day 30 after application. This increased growth was not seen in regions without gel. An acute, low-level inflammatory response, typical of implanted biomaterials, was seen at day 5, which was likely the stimulus for the growth of the fibrous layer of smooth muscle cells on both sides of the gel (Figs. S1 and S5). Macrophages were not observed in the vessel near the gel at 30 d, indicating that the gel did not elicit a prolonged chronic inflammatory response (Fig. 2I). The gel thickened the tissue on the inner surface of the blood vessel and shielded the vessel surface that was previously in contact with the bulk blood stream. Controlled growth of an endothelialized fibrous cap is likely a major advantage for this technology, which focuses, in part, on remodeling the tissue and isolating the thrombogenic plaque core from clotting factors in the plasma. Severe luminal narrowing and stenosis was not observed. The deposition process itself did not cause substantial damage at the site of gel application. Four days after treatment, the elastin and smooth muscle cells in the vessel wall were viable, no thrombosis was observed, and there were cells expressing CD31, a marker of endothelial cells on the gel and underneath the gel (Figs. S5 and S6).
Fig. 2.
The adhesive gel can be durably coated on the inside of blood vessels in vivo. (A) Intravital fluorescence microscopy image of a carotid artery in a living mouse coated with the adhesive gel containing 200-nm particles (green). A fluorescent dye (red) is seen in the artery after it was injected intravenously, indicating that blood flowed through it. (B) Histological section of one wall of a carotid artery (red, elastin) coated for 28 d with the adhesive gel (green fluorescent particles). (C) Intravital microscopy images of the gel adhered to a carotid artery in a living mouse at days 1 and 5 after deposition. (D) Chart quantifying the amount of gel in vessels over time. The average fluorescence intensity in the coated region of each vessel was measured, and data are displayed as mean ± SEM for 10 mice that were imaged on day 1 and then imaged again either on day 5 (5 mice) or day 30 (5 mice). *P < 0.05, **P < 0.01 vs. control without gel. (E and F) Lengthwise histological section stained with H&E at day 30. (G–I) Immunofluorescence sections show gel (green autofluorescence and pink particles), cell nuclei (blue), and red staining for either CD31, a marker for endothelial cells (G), smooth muscle actin (SMA), a marker for smooth muscle cells (H), or CD107b (MAC-3), a marker for macrophages (I) (see Fig. 4B for an example of positive MAC-3 staining).
Small Molecules Can Be Locally Delivered into Vasculature.
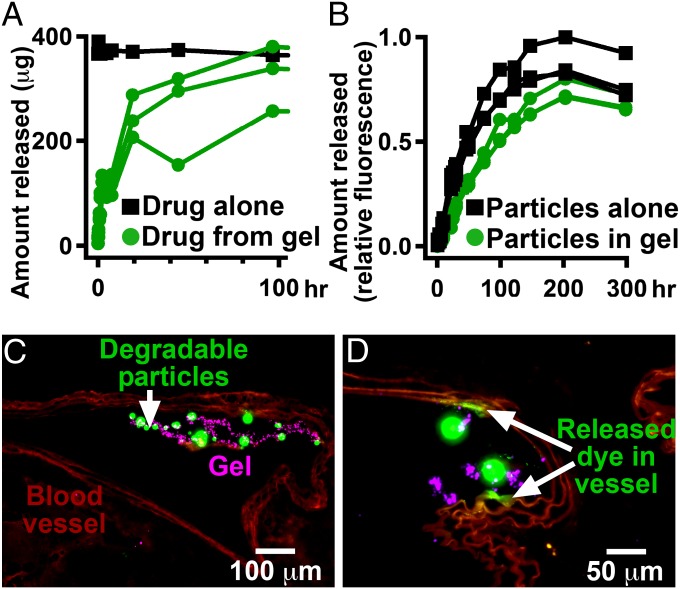
We hypothesized that additional benefit could be derived from the local delivery of anti-inflammatory agents. We developed a gel formulation capable of releasing a corticosteroid over the course of days. In vitro, >50% of the steroid, dexamethasone 21-phosphate disodium salt (dexamethasone), was released within a day, and the entire amount of steroid was released within 5 d (Fig. 3A). This fast release would be useful for delivering a local burst of therapeutics, but in some cases a more sustained release would be desired. To slow the release of small molecules, we incorporated them into degradable microparticles (9, 10). When a small-molecule fluorescent dye, 4,4-Difluoro-1,3,5,7,8-Pentamethyl-4-Bora-3a,4a-Diaza-s-Indacene (BODIPY), was incorporated into degradable poly(lactic-coglycolic acid) particles and blended into the gel, only 50% of dye was released after 5 d (Fig. 3B). These degradable particles could be affixed to the inside of blood vessels using the adhesive gel (Fig. 3C). The small-molecule fluorescent dye was taken up into the vessel wall from the gel and microparticles within 5 d (Fig. 3D). This technique was used to deliver small-molecule therapeutics to the bifurcation of the internal and external carotid arteries, which in our mouse model measured ∼1 mm in diameter.
Fig. 3.
Small molecules can be controllably released from the adhesive gel into blood vessel walls. (A) Release of a steroid, dexamethasone, occurs on the time scale of days, in vitro, when directly blended into the gel. (B) Release of a fluorescent dye, BODIPY, occurs on the time scale of weeks, in vitro, when incorporated in degradable poly(lactic-coglycolic acid) microparticles that are blended into the gel. (C) Histological section of the degradable particles containing BODIPY (green) and nondegradable 200-nm particles (purple) inside of the adhesive gel and coated on a vessel (red, elastin) at a bifurcation for 5 d. (D) Section of the blood vessel wall stained with BODIPY that was released into the vessel wall from nearby particles.
Painted Atherosclerotic Plaques Have Reduced Inflammation and Thicker Fibrous Caps.
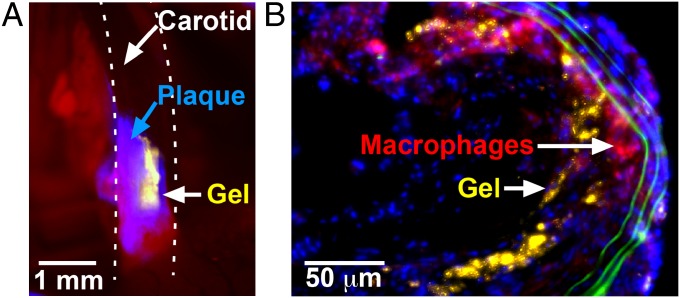
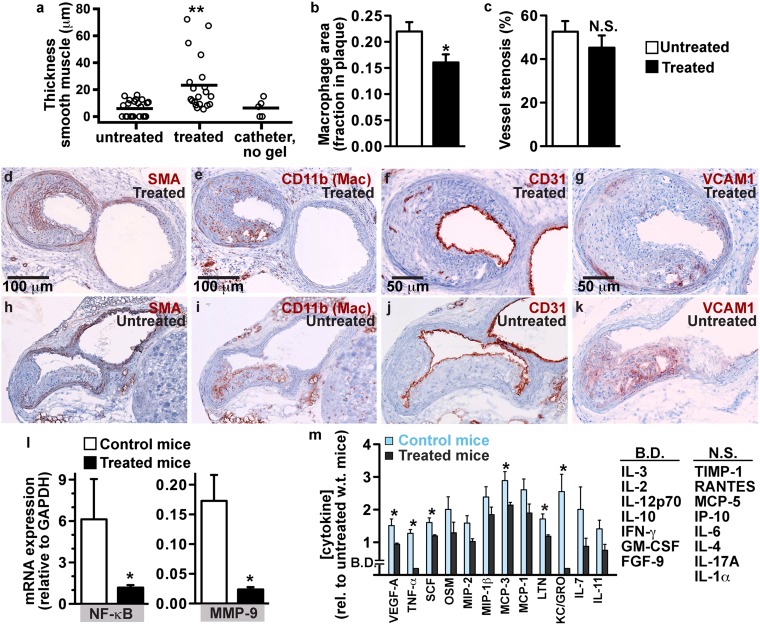
To test whether this vascular paint could be used to treat diseased areas of the vasculature, we tested whether the adhesive gel could be coated onto atherosclerotic plaques. A mouse model of atherosclerosis was used [hypercholesterolemic apolipoprotein E–deficient (apoE−/−) mice] (32), rather than a pig or rabbit model, to take advantage of in vivo molecular imaging tools developed for mice (4, 7, 32, 33). There is no widely accepted animal model for human vulnerable plaques, but histological analysis of plaque morphology can provide valuable insights into the inflammatory activity of a plaque (2, 33). Both in vivo intravital microscopy and histological analysis of excised vessels confirmed that the gel could be selectively delivered to cover inflamed plaques (Fig. 4). Initial experiments treating plaques with the gel alone, without steroid, did not show significant decreases in the number of macrophages compared with untreated mice (Fig. S7). To further test whether this technique could be used to reduce inflammation in the plaque, plaques at the bifurcations of the carotid arteries were treated with the gel containing dexamethasone (Fig. 5). We tested dexamethasone because it is a well-characterized anti-inflammatory agent, but other drugs may be more suitable for pacifying inflamed plaques (12). Histological sections of treated vessels were compared with contralateral untreated carotid arteries from the same mice (Fig. 5 A–K). All treated plaques had a fibrous cap containing smooth muscle cells, with a mean thickness of 23 ± 4.5 μm. In untreated plaques, the mean thickness of the layer of smooth muscle cells was 6 ± 1.2 μm, three times less than the treated plaques (P < 0.001), and only 12 of 22 untreated plaques had smooth muscle cells covering them (Fig. 5A). In an additional control experiment using the same catheter-based delivery method but without deployment of the gel, only three of five plaques had smooth muscle cell growth (mean of 6 ± 2.9 μm, P < 0.01 compared with gel treated). This indicates that the gel treatment was able to thicken the fibrous cap covering the plaque core. Histological sections were also analyzed to determine the amount of staining for CD11b, a marker of inflammatory cells, particularly macrophages. The average CD11b (macrophage) content of the treated plaques was ∼25% reduced compared with untreated plaques in the contralateral carotid (P = 0.01; Fig. 5B), indicating that the treatment locally reduced inflammation. The treatment did not induce higher-grade stenosis (P = 0.7) measured as the total area of tunica intima, including the plaque, fibrous cap, and hydrogel (Fig. 5C). The endothelium in the treated region was viable in 10 sections from 10 mice (Fig. 5 F and J). Staining for vascular adhesion molecule 1 (VCAM-1), an adhesion molecule that recruits leukocytes, showed the activated endothelium that lined untreated plaques robustly expressed VCAM-1, whereas treated plaques expressed much less VCAM-1 (Fig. 5 G and K). In a separate cohort of mice, plaque mRNA levels of NF-κB and matrix metallopeptidase-9 (MMP-9) were lower in treated mice compared with mice that received saline, measured using qPCR (Fig. 5L). Furthermore, the gel treatment reduced serum cytokines, measured using an immunoassay with multianalyte profiling (Fig. 5M). Reduced levels of these biomarkers further indicate that the treatment attenuated inflammation.
Fig. 4.
The adhesive gel can be coated on atherosclerotic plaques in live apoE−/− mice. (A) Intravital fluorescence microscopy of the adhesive gel (yellow particles), without degradable particles or steroid, immediately after it was selectively coated on an atherosclerotic plaque expressing cathepsin (blue fluorescent substrate) in a carotid artery (dashed line). (B) Cross-section of a vessel showing gel (yellow) coated on an atherosclerotic plaque containing macrophages (red) after 30 d. Cell nuclei (blue) and elastin (green) are also shown.
Fig. 5.
Treatment with the gel and soluble dexamethasone induced growth of fibrous caps over atherosclerotic plaques and reduced inflammation. (A–K) Histochemical analysis of plaques treated for 30 d. (A) Thickness of the layer of smooth muscle cells (fibrous cap) grown over plaques (n = 20–22 sections from 10 treated and untreated vessels, n = 5 sections from 3 control vessels treated with dexamethasone via catheter without gel). (B) Fraction of tissue expressing CD11b, a marker of macrophages and other inflammatory cells (n = 36–40 sections from 10 mice). (C) Degree of stenosis of vessel lumens by plaque, fibrous cap, and gel, indicating that the treatment does not result in further stenosis (n = 20–22 sections from 10 mice). (D–G) Histochemical-stained cross-sections of a gel-treated plaque at the bifurcation of the external and internal carotid; red indicates staining for smooth muscle cells (D) (72 μm thick on plaque), macrophages (E) (25% of the plaque), endothelium (F), and VCAM-1 (G). (H–K) An untreated plaque; smooth muscle cells (H) (0 μm thick on plaque), macrophages (I) (41% of the plaque), endothelium (J), and VCAM-1 (K). (L) Plaque expression of NK-κB and MMP-9 in treated and untreated vessels, determined using qPCR relative to GAPDH (a housekeeping gene) (n = 5). (M) Serum cytokines 1 wk after treatment with either the gel or saline (control). Values indicate cytokine levels in treated and control apoE−/− mice (n = 5) relative to the average levels in four untreated WT mice. B.D., below detection limit; N.S., additional cytokines that were not significantly different. *P < 0.05, **P < 0.001 vs. untreated plaques or control mice. Data are displayed as mean ± SEM.
Conclusion
The developed synthetic materials were directly coated inside blood vessels with high spatial control, remained adherent for over a month, and reduced inflammation in atherosclerotic plaques. The synthetic materials used here were inspired by the underwater adhesives of marine mussels (21, 22), and by mimicking their functional chemistry (23–26), adhesion under blood flow was achieved. These results show that materials can be durably implanted into the vasculature for long periods of time with adhesive forces and provide an alternative to using mechanical forces for intravascular implants. Testing in large animals, such as pigs, by delivering materials that reduce inflammation and increase the thickness of the fibrous cap is an important next step and necessary before this material is advanced toward clinical applications (27, 33). Microporous balloon or double-balloon catheters, which are already used clinically for local delivery of solutions, may facilitate consistent gel deployment in larger-sized vessels. Catheters compatible with a double barrel injection system would likely reduce the time necessary to deliver the adhesive and may be required for delivery to coronary arteries. Here we used biostable hydrogels to conclusively test whether the gel would remain adhered to the vessel. Degradation of alginate occurs more rapidly when it is oxidized, and the rate of degradation of alginate-catechol at atherosclerotic plaques over months to years will need to be determined in the future (34). For other applications, rapidly degradable adhesive gels may be desired. We found that a degradable adhesive gel, hyaluronan-catechol (26), could be coated on blood vessels and degraded on the time scale of days (Fig. S8).
The developed materials provide a tool to deliver therapeutics to any area of the vasculature that is accessible by a catheter. We foresee numerous applications for this technology, from healing damaged vessels to deploying intravascular glues. Potential applications include treating the injury caused by balloon angioplasty or stent implantation, strengthening the vessel walls of aneurisms, and delivering anticancer drugs to vessels feeding tumors. Future work will need to explore the type of therapeutics that can be delivered, including small molecules, proteins, nucleic acids, and stem cells.
Materials and Methods
See SI Materials and Methods for detailed materials and methods.
Synthesis and Gelation of Adhesive Hydrogels.
Alginate-catechol was synthesized by coupling alginate to dopamine hydrochloride (see SI Materials and Methods for details). To form the gel, alginate-catechol was dissolved in PBS (5% wt/vol) and mixed 3:1 with the gelation solution. The gelation solution was prepared fresh by combining sodium periodate (NaIO4, 8.33 mg/mL in PBS) with a solution of NaOH (0.4 M in H2O) in a 10:1 ratio. The solution turned light brown and gelled in 10–20 min. Unmodified alginate (no catechol) did not gel under these conditions but was gelled by adding a solution of CaCl2 (200 mM in H2O) in a ratio of 3:1. In specified experiments, near-infrared fluorescent particles (100–200 nm) were added to the gelation solution (0.3% solids). In experiments where other agents were incorporated into the gel, these agents were added 1 min after the alginate-catechol solution and NaIO4 gelation solution were mixed. When degradable poly lactic-coglycolic acid) (PLGA) particles were incorporated into the gel, a solution of particles (10 mg in 100 μL PBS) was added in a ratio of 1:10. In experiments treating atherosclerotic plaques with steroid, dexamethasone 21-phosphate was added (5 μg/μL), but degradable or fluorescent micro/nanoparticles were not.
Measuring the Adhesive Strength of Alginate-Catechol In Vitro.
Microfluidic devices were fabricated by rapid prototyping in polydimethylsiloxane. The channels were coated with fibronectin overnight, and then the device was filled with 100 μL of human umbilical vein endothelial cells (HUVECs; 500,000 cells/mL) in growth media and incubated for 3 h. Growth medium was then flowed through the device overnight at 0.5 μL/min. To deposit the gel, a catheter was inserted into the device, and 0.8–1 μL of the gel solution was injected, coated onto the channel, and allowed to gel for 20 min. To measure the adhesive strength of the coatings, growth medium was flowed through the device at rates between 0 and 50,000 μL/min. The gel coverage was quantified from fluorescence images using a microscope. The ex vivo adhesive strength of the alginate-catechol to bovine carotid arteries was measured in a lap-shear tensile strain test using an Instron 5542 single column testing system. The gel solutions (100 μL) were injected between two parallel plates, one presenting the inside surface of the bovine artery. Force was measured as the plates were slid away from each other at 3.3 μm/s, and adhesive failure occurred between the gel and bovine carotid.
Gelation In Situ in the Arteries of Living Mice.
Experimental protocols were approved by the Massachusetts Institute of Technology Committee on Animal Care, and the Massachusetts General Hospital Institutional Animal Care and Use Committee. Female apoE−/− mice and C57BL/6J mice were purchased from the Jackson Laboratory at 8–10 wk of age. apoE−/− mice were 21–24 wk old at the time of surgery and had been kept on a high-cholesterol diet (Harlan Laboratories) since 8–10 wk of age. The mice had substantial plaque growth at the time they were initially treated. A fluorescent substrate for proteases in atherosclerotic plaques, Prosense 680 or Prosense 750 (2 nmol; Visen), was added 18–24 h before surgery to visualize the plaque. In each mouse, the common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) were isolated and temporarily ligated with sutures. The gel was deposited in either the CCA (data in Fig. 2 and Figs. S2–S6 and S8) or the ICA/ECA bifurcation (data in Figs. 3–5). See SI Materials and Methods for a further description of surgical procedures. For deposition of gel on plaques in the ICA/ECA bifurcation, a small hole was made at the anterior wall of the CCA using a 31-gauge needle, allowing the catheter to be inserted into the CCA, moved to the plaque, and secured. The gel was injected and allowed to cross-link while the catheter was in place for 30 min. The hole at the CCA anterior wall was closed with suture, and all of the ligations were released to restore blood flow. The presence of the gel was confirmed using an Olympus OV110 Small Animal Fluorescence Imaging System, and then the surgical wound was closed. After a specified time, the gel was reimaged, and serum and carotid arteries were collected for histological, cytokine, and qRT-PCR analysis.
Releasing Small Molecules from Alginate-Catechol.
Dexamethasone (0.5 mg) was dissolved in 50 μL of gel and submerged in 5 mL of PBS. The amount of dexamethasone released from the gel was measured using HPLC and ELISA (Fig. S9). To test whether small molecules could be released from degradable particles blended into the gel, a fluorescent dye, BODIPY, was incorporated into PLGA particles (2–25 μm) and blended with alginate-catechol before gelation (see SI Materials and Methods for details). In vitro, the amount of BODIPY released from the particles and gel into PBS was measured using a fluorometer. In vivo, the release of BODIPY was detected by analyzing fluorescence in histological sections at the vessel wall near particles in the gel (Fig. S9).
Supplementary Material
Acknowledgments
We thank E. Aikawa, J. Donners, E. Edelman, A. Jaklenec, S. Lyu, S. Lyle, A. Nathan, S. Oesterle, R. Sparer, P. Wojciechowski, and H. Yin for helpful suggestions; and Z. Mueller and J. Sullivan for help with in vivo imaging. C.K. is a Johnson & Johnson Fellow of the Life Sciences Research Foundation. This work was funded by National Institutes of Health Grants DE016516 (to D.G.A. and R.L.), R01HL096576 (to M.N.), and U24-CA92782 (to R.W.); Medtronic, Inc.; Canadian Institutes of Health Research Grant MOP119426; the Pioneer Research Program Grants R31-10071 and WCU-2z011-0001696 (to H.L.); and the Juvenile Diabetes Research Foundation (D.G.A. and R.L.). This work was carried out in part through the use of Massachusetts Institute of Technology’s Microsystems Technology Laboratories, Swanson Biotechnology Center, and the Mouse Imaging Program in the Center for Systems Biology at Massachusetts General Hospital.
Footnotes
Conflict of interest statement: A patent application has been filed with the US Patent and Trade Office on the work reported in this article.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217972110/-/DCSupplemental.
References
- 1.Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009;335(1):283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Fayad ZA, et al. dal-PLAQUE Investigators Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): A randomised clinical trial. Lancet. 2011;378(9802):1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;116(9):1052–1061. doi: 10.1161/CIRCULATIONAHA.106.647164. [DOI] [PubMed] [Google Scholar]
- 5.Leuschner F, Nahrendorf M. Molecular imaging of coronary atherosclerosis and myocardial infarction: Considerations for the bench and perspectives for the clinic. Circ Res. 2011;108(5):593–606. doi: 10.1161/CIRCRESAHA.110.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451(7181):953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 7.Spratt JC, Camenzind E. Plaque stabilisation by systemic and local drug administration. Heart. 2004;90(12):1392–1394. doi: 10.1136/hrt.2004.034975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobatto ME, Fuster V, Fayad ZA, Mulder WJM. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat Rev Drug Discov. 2011;10(11):835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nat Biotechnol. 2003;21(10):1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 10.Chan JM, et al. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc Natl Acad Sci USA. 2010;107(5):2213–2218. doi: 10.1073/pnas.0914585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slepian MJ, Hubbell JA. Polymeric endoluminal gel paving: Hydrogel systems for local barrier creation and site-specific drug delivery. Adv Drug Deliv Rev. 1997;24(1):11–30. [Google Scholar]
- 12.Waxman S, Ishibashi F, Muller JE. Detection and treatment of vulnerable plaques and vulnerable patients: Novel approaches to prevention of coronary events. Circulation. 2006;114(22):2390–2411. doi: 10.1161/CIRCULATIONAHA.105.540013. [DOI] [PubMed] [Google Scholar]
- 13.Parikh SA, Costa MA. Secondary prevention, the interventional way: Prophylactic drug-eluting stents for nonobstructive saphenous vein graft disease. Circulation. 2009;120(20):1940–1942. doi: 10.1161/CIRCULATIONAHA.109.903146. [DOI] [PubMed] [Google Scholar]
- 14.Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation. 2003;108(14):1701–1706. doi: 10.1161/01.CIR.0000091115.05480.B0. [DOI] [PubMed] [Google Scholar]
- 15.Kuchulakanti PK, et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation. 2006;113(8):1108–1113. doi: 10.1161/CIRCULATIONAHA.105.600155. [DOI] [PubMed] [Google Scholar]
- 16.Ramcharitar S, et al. First case of stenting of a vulnerable plaque in the SECRITT I trial-the dawn of a new era? Nat Rev Cardiol. 2009;6(5):374–378. doi: 10.1038/nrcardio.2009.34. [DOI] [PubMed] [Google Scholar]
- 17.Hill-West JL, Chowdhury SM, Slepian MJ, Hubbell JA. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proc Natl Acad Sci USA. 1994;91(13):5967–5971. doi: 10.1073/pnas.91.13.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thierry B, Winnik FM, Merhi Y, Tabrizian M. Nanocoatings onto arteries via layer-by-layer deposition: Toward the in vivo repair of damaged blood vessels. J Am Chem Soc. 2003;125(25):7494–7495. doi: 10.1021/ja034321x. [DOI] [PubMed] [Google Scholar]
- 19.Livnat M, Beyar R, Seliktar D. Endoluminal hydrogel films made of alginate and polyethylene glycol: Physical characteristics and drug-eluting properties. J Biomed Mater Res A. 2005;75(3):710–722. doi: 10.1002/jbm.a.30474. [DOI] [PubMed] [Google Scholar]
- 20.West JL, Hubbell JA. Separation of the arterial wall from blood contact using hydrogel barriers reduces intimal thickening after balloon injury in the rat: The roles of medial and luminal factors in arterial healing. Proc Natl Acad Sci USA. 1996;93(23):13188–13193. doi: 10.1073/pnas.93.23.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyne KJ, Qin XX, Waite JH. Extensible collagen in mussel byssus: A natural block copolymer. Science. 1997;277(5333):1830–1832. doi: 10.1126/science.277.5333.1830. [DOI] [PubMed] [Google Scholar]
- 22.Harrington MJ, Masic A, Holten-Andersen N, Waite JH, Fratzl P. Iron-clad fibers: a metal-based biological strategy for hard flexible coatings. Science. 2010;328(5975):216–220. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BP, Dalsin JL, Messersmith PB. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules. 2002;3(5):1038–1047. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- 24.Ryu JH, et al. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules. 2011;12(7):2653–2659. doi: 10.1021/bm200464x. [DOI] [PubMed] [Google Scholar]
- 25.Brubaker CE, Kissler H, Wang L-J, Kaufman DB, Messersmith PB. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials. 2010;31(3):420–427. doi: 10.1016/j.biomaterials.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, et al. Substrate-independent layer-by-layer assembly by using mussel-adhesive-inspired polymers. Adv Mater. 2008;20(9):1619–1623. doi: 10.1002/adma.200702378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libby P. The molecular mechanisms of the thrombotic complications of atherosclerosis. J Intern Med. 2008;263(5):517–527. doi: 10.1111/j.1365-2796.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6(8):623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 29.Gnasso A, et al. Association between intima-media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation. 1996;94(12):3257–3262. doi: 10.1161/01.cir.94.12.3257. [DOI] [PubMed] [Google Scholar]
- 30.Bajpai SK, Sharma S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React Funct Polym. 2004;59(2):129–140. [Google Scholar]
- 31.Hunt NC, Smith AM, Gbureck U, Shelton RM, Grover LM. Encapsulation of fibroblasts causes accelerated alginate hydrogel degradation. Acta Biomater. 2010;6(9):3649–3656. doi: 10.1016/j.actbio.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Aikawa E, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115(3):377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 33.Granada JF, et al. Porcine models of coronary atherosclerosis and vulnerable plaque for imaging and interventional research. EuroIntervention. 2009;5(1):140–148. doi: 10.4244/eijv5i1a22. [DOI] [PubMed] [Google Scholar]
- 34.Bouhadir KH, et al. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17(5):945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.