Abstract
Plasmodium falciparum causes the deadliest form of human malaria. Its virulence is attributed to its ability to modify the infected RBC and to evade human immune attack through antigenic variation. Antigenic variation is achieved through tight regulation of antigenic switches between variable surface antigens named “P. falciparum erythrocyte membrane protein-1” encoded by the var multicopy gene family. Individual parasites express only a single var gene at a time, maintaining the remaining var genes in a transcriptionally silent state. Strict pairing between var gene promoters and a second promoter within an intron found in each var gene is required for silencing and counting of var genes by the mechanism that controls mutually exclusive expression. We have identified and characterized insulator-like DNA elements that are required for pairing var promoters and introns and thus are essential for regulating silencing and mutually exclusive expression. These elements, found in the regulatory regions of each var gene, are bound by distinct nuclear protein complexes. Any alteration in the specific, paired structure of these elements by either deletion or insertion of additional elements results in an unregulated var gene. We propose a model by which silencing and mutually exclusive expression of var genes is regulated by the precise arrangement of insulator-like DNA pairing elements.
Keywords: gene expression, allelic exclusion, PfEMP1
The deadliest form of human malaria, affecting millions worldwide every year, is caused by the protozoan parasite Plasmodium falciparum (1). Its virulence is attributed to its ability to evade the human immune system by modifying the host red blood cells to adhere to the vascular endothelium and to undergo antigenic variation. The main antigenic ligands responsible for both cytoadherence and antigenic variation are members of the P. falciparum erythrocyte membrane protein-1 (PfEMP1) family (2). These polymorphic proteins are encoded by a multicopy gene family, var (3). Each individual parasite expresses a single var gene at a time, maintaining the remaining ∼60 var genes found in its genome in a transcriptionally silent state (4, 5). As the antibody response against the single expressed PfEMP1 develops, small subpopulations of parasites switch expression to alternative forms of PfEMP1, avoid the antibody response, and re-establish the infection (6). Immune evasion and the maintenance of long-term chronic infections by P. falciparum through antigenic variation depend on tight regulation of the parasites' ability to express only a single var gene at a time and then to switch expression to another gene that also is expressed in a mutually exclusive manner. Mutually exclusive expression and antigenic switching of var genes is epigenetically regulated at the level of transcription and involves chromatin modifications, changes in subnuclear localization, and interactions between cis-regulatory elements (7, 8). Silencing of individual var genes is established epigenetically during S-phase of the cell cycle and depends on a cooperative interaction between each var upstream promoter and its associated intron promoter (9). Unlike allelic exclusion in mammalian systems in which the expression of a functional protein at the surface of the cell is required for mutually exclusive expression (10, 11), in P. falciparum this process is regulated solely at the level of transcription, and functional antigen production is not required for proper var gene regulation and viability of the parasite (12, 13). This phenomenon suggests that each individual var gene contains the regulatory elements that enable it to maintain a transcriptionally silent state, even while an adjacent gene may be active, and to be “counted” by the mutually exclusive expression mechanism.
Bioinformatic comparison of the 5′ UTR of all 60 var genes in the genome suggested that they can be classified into several subgroups (UpsA, UpsB, UpsC, and UpsE) based on the sequence similarity of their promoter regions (14, 15). Within these upstream regulatory regions DNA elements have been identified by deletion analysis of the 5′UTR and EMSA (16, 17). It was demonstrated that one of these motifs, SPE2, specifically binds a member of a newly discovered DNA-binding protein family, ApiAP2, which has a role in chromosome end biology (18). Recently, an additional protein-binding DNA element implicated in mutually exclusive locus recognition was identified in 44 of the var 5′ UTRs (17). Unlike var promoters, the DNA sequence of all intron promoters is highly conserved and cannot be classified into subgroups (15, 19). var introns also contain a specific protein-binding element that plays a role in positioning var genes at the nuclear periphery through interaction with nuclear actin (20).
It appears that promoter-pairing of the var and intron promoters within each gene is necessary for the proper regulation of var gene expression. A “free” var promoter that is unpaired with an intron promoter cannot be silenced and therefore is constitutively active (9, 21–23). In addition, this free var promoter is not counted as a var gene by the mechanism that ensures that only a single var gene is active at a time. That is, a free var promoter is not expressed in mutually exclusive manner, and therefore it can be actively transcribed simultaneously with another var gene in the nucleus of a single parasite (24). In addition, once a var promoter–intron pair is established, it is maintained for many generations, suggesting a possible role in epigenetic memory (23). The nature of this promoter–promoter interaction and how it regulates var silencing and mutually exclusive expression is still elusive. Here we report that insulator-like DNA elements are found within both upstream var promoter regions and that introns mediate this promoter pairing and regulate var silencing and mutually exclusive expression.
Results
DNA Element in the 3′ UTR of Plasmodium berghei Dihydrofolate Reductase-Thymidylate Synthase Disrupts the Intron’s Ability to Silence a var Promoter.
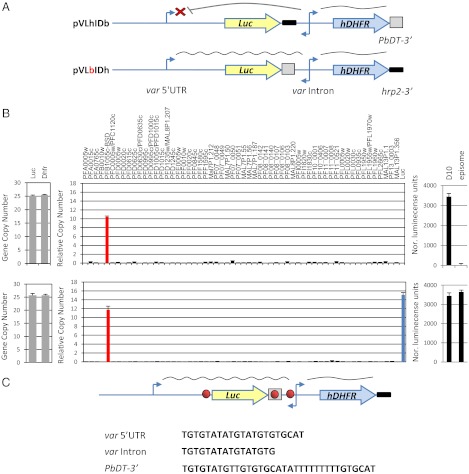
Silencing and mutually exclusive expression of var genes requires strict pairing between each var promoter and the promoter found within each var intron (22, 24). Although these noncoding regions are essential for proper regulation of var genes, the coding regions of PfEMP1 seem not to have a role in var gene regulation (12, 13). Therefore, it is possible to investigate the role of cis-regulatory elements using stably transfected plasmids in which var-regulatory elements control the expression of reporter genes. Previously the pVLhIDh construct was used to demonstrate that promoter pairing is required for silencing (22). In this construct a var promoter and upstream regulatory region was used to drive luciferase expression, and the intron promoter was used to drive expression of hdhfr as a positive selectable marker to enable stable transfection. However, in this construct both luciferase and hdhfr were terminated with the same hrp2 3′UTR, resulting in several recombination and rearrangement events that changed the transcriptional phenotype of the var promoter of the original plasmid. To avoid the possibility of rearrangement caused by plasmid recombination, we modified the pVLhIDh plasmid and created two plasmids in which the hrp2 3′UTRs of either luciferase or hdhfr were replaced with the 3′ UTR of Plasmodium berghei dhfr-thymidylate synthase (PbDT 3′) (25). These constructs were termed “pVLbIDh” and “pVLhIDb,” respectively (Fig. 1A). These constructs were transfected into the DC-J transgenic parasite line in which a particular endogenous var promoter (PFB1055c) can be selected for activation using blasticidin S, ensuring that the rest of the var repertoire is completely silent because of mutually exclusive expression (12) and thus allowing controlled examination of the recognition of our constructs. To our surprise, although the two plasmids were almost identical, and the transfected parasites carried plasmids that contained a 1:1 ratio of var promoter and intron (Fig. 1B), they had different luciferase expression phenotypes. Specifically, the var promoter in pVLhIDb was regulated properly and, as in pVLhIDh, was silent as expected because of the proper pairing with an intron. However, in pVLbIDh the var promoter was constitutively active and was not recognized by the mechanism that controls mutually exclusive expression. As shown in Fig. 1B, it was active at the same time as PFB1055c. Previously, a similar plasmid pVBbIDh containing bsd as a drug-selectable marker was transfected into a clonal population of NF54 parasites. When selected on 20 µg/mL blasticidin for activation of the episomal var promoter, the entire var gene family was silenced (12), suggesting that in this case that the promoter within the construct was recognized by the mechanism controlling mutually exclusive expression. However, to test whether this silencing actually was the result of the constraints of mutually exclusive expression or instead was caused by promoter titration (26), we repeated this transfection and found that without blasticidin selection or even with a low dose (2 µg/mL) the endogenous var gene (PFD1005c) and the episomal var promoter were active simultaneously (Fig. S1). These data indicate that, like the unregulated var promoter on pVLbIDh, the var promoter on the pVBbIDh plasmid is constitutively active and is not counted as a var gene for mutually exclusive expression. We reasoned that the insertion of PbDT 3′ between the var promoter and the intron could have disrupted the interaction between these two regulatory elements which is required for var gene silencing and for their being counted for mutually exclusive expression.
Fig. 1.
A DNA element found in the PbDT 3′ UTR disrupts the intron’s ability to silence a var promoter. Stably transfected parasite lines carried constructs mimicking var gene structure with luciferase as a reporter gene for var promoter expression and hdhfr as a positive selectable marker expressed by the var intron. (A) (Upper) Schematic of the pVLhIDb episome that contains a var promoter paired with a var intron. This var promoter is properly regulated and silent by default. (Lower) Schematic of the pVLbIDh episome, which is identical to pVLhIDb except for the replacement of the 3′ UTR that terminates luciferase. This replacement resulted in activation of the var promoter, which no longer is recognized by the mechanism that controls mutually exclusive expression. (B) Results of pVLhIDb (Upper) and pVLbIDh (Lower) in the DC-J parasite line. Quantification of the ratio between var promoter and intron by qRT-PCR (Left). Steady-state mRNA levels of each individual var gene measured by qRT-PCR are presented as copy number relative to the housekeeping genes arginyl-tRNA synthetase (PFL0900c) (Center) and the levels of luciferase expression (Right). The D10 parasite line (22) constitutively expressing luciferase from an endogenous var promoter was used as a positive control. All values presented are the average of at least two biological replicates. Error bars represent SE. (C) A similar TG-rich DNA element is found within the PbDT 3′ UTR, var promoter, and var intron. In the schematic of the construct, the TG-rich element is shown as a red circle, the PbDT 3′ UTR is boxed, and the hrp2 3′ UTR is shown as a bold black line.
Each var Gene Contains a Conserved TG Motif in its 5′UTR and Intron.
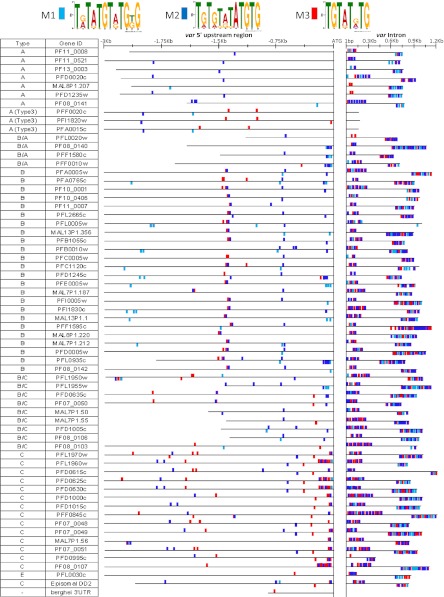
A closer look at these DNA elements revealed that the var promoter, intron, and PbDT 3′ all contain a similar TG-rich DNA sequence (Fig. 1C). Calderwood et al. (19) previously identified similar TG-rich conserved sequences in an alignment of 12 var introns of the Dd2 parasite line (19). However, alignments of the 5′ UTRs of the entire var gene family revealed that even though there is high level of sequence conservation among different var subtypes, no sequence was identified that is shared by all 60 genes (14, 15, 27). Nevertheless, because the interaction between the 5′ UTR and intron is important, and each var gene contains all the regulatory elements needed for its proper regulation, we hypothesized that similar motifs that enable the interaction between these two regulatory regions could be found in the 5′ UTR and intron of each var gene. Using the MEME motif-finding algorithm, we initially screened the introns of the entire var gene family and identified two 12-bp TG-rich motifs shared among all var introns (M1-2). We then used the MAST algorithm to map these motifs on the 5′ UTRs of the entire var gene family (Fig. 2). We found that each var gene contains similar motifs in its 5′ UTR and its intron. The 8-bp motif (M3) identified by Calderwood et al. (19) also was mapped on the entire var gene family and was found to be highly associated with M2. Interestingly, in type 3 var genes the motifs are found on the 5′ UTR but not on their truncated nonfunctional introns. As expected from the sequence asymmetry of var introns (19), we found an array of motifs repeated on both edges of the introns, although in the opposite orientations. We also noted that the distribution of the motifs in the 5′ UTRs is similar among members of the same var subtype.
Fig. 2.
Bioinformatic analysis of the entire var gene family revealed a unique TG-rich DNA motif in all var introns and 5′UTRs. The MEME motif-finding algorithm was used for the initial screen for TG-rich DNA motifs on all var introns of the 3D7 parasite line. Three T[G/A] motifs were identified, which were termed “M1-3” and are marked with light blue, blue, and red, respectively. The MAST algorithm was used to locate their presence in the 5′ UTRs of all var genes. This screen was limited to sequences with at least 25% G/C content. The presentation of these motifs above or below the genes indicates their orientation on the DNA strands.
Surprisingly, the PbDT 3′ used in our plasmids also contains the M3 motif. Moreover, the var promoter and intron used on our constructs contained an identical TG-rich sequence (Fig. S2). We performed an in silico screen of the 3D7 genome and identified this exact sequence only 131 times in the entire genome; in 121 of these instances it was found in the var-regulatory regions of 51 different var genes (Tables S1 and S2). We reasoned that these sequences might act as insulator-like pairing elements (PE) that could mediate the interaction between the var promoter and intron that is necessary for their silencing and mutually exclusive expression.
Deletion of the PE from a var Promoter or Insertion of an Additional Element Between a var Promoter and Intron Disrupts Silencing and Mutually Exclusive Expression.
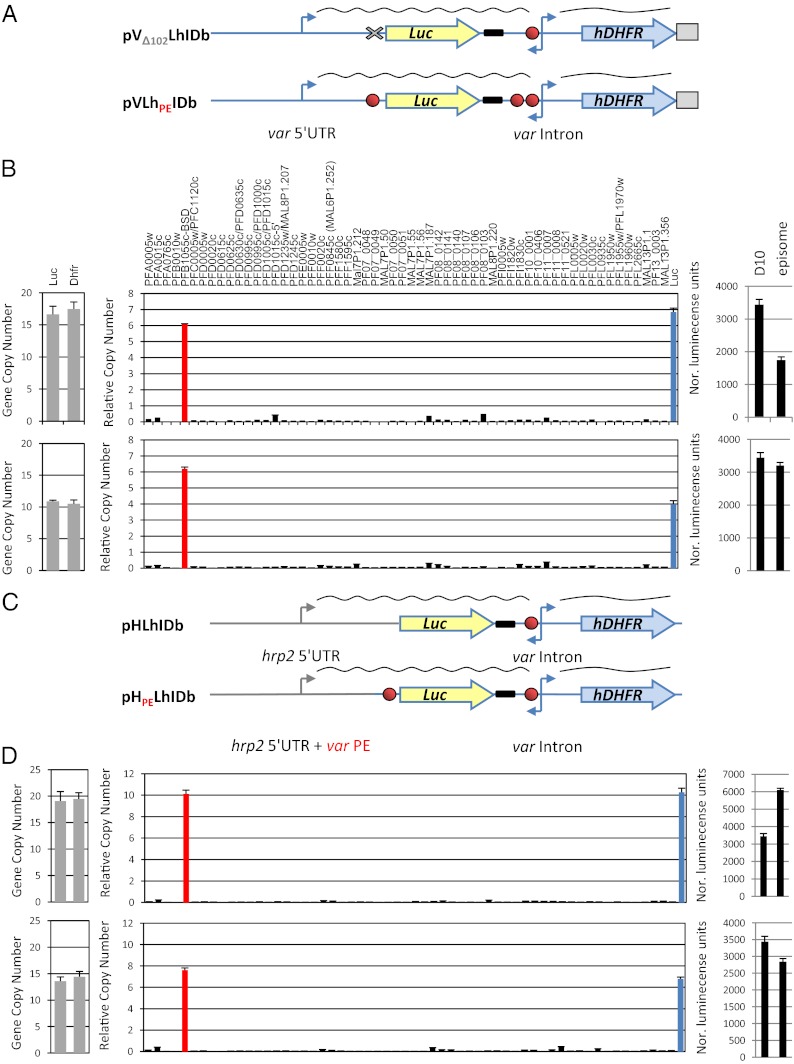
To show that these PEs are indeed essential for proper regulation of var genes, we modified the pVLhIDb construct, which is properly regulated, and deleted the last 102 bp of its 5′ UTR containing the PE, thus creating the pVΔ102LhIDb plasmid (Fig. 3A, Upper). In addition, we inserted an additional copy of the PE into pVLhIDb between the hrp2 3′ and the intron, creating a new plasmid termed “pVLhPEIDb” (Fig. 3A, Lower). Both plasmids were transfected into the DC-J parasite line, and the entire var transcription profile and luciferase expression levels were measured. We found that these modifications resulted in activation of the episomal var promoter even though both stably transfected parasite lines maintained a 1:1 ratio of var promoters and introns on the plasmids, indicating that no rearrangements or deletions had occurred. Moreover, these constitutively active promoters became uncounted by the mechanism that controls mutually exclusive expression, and both were simultaneously active with the endogenous var PFB1055c (Fig. 3B). We concluded that the PEs are regulatory elements required for pairing the var promoter with the intron and are essential for mediating var silencing and mutually exclusive expression.
Fig. 3.
DNA PEs regulate silencing and mutually exclusive expression of var genes through specific promoter–promoter interaction. The deletion of a PE or insertion of an extra PE results in the activation of a var gene. In the schematic of the construct, the TG-rich element is shown as a red circle, the PbDT 3′ UTR is boxed, and the hrp2 3′ UTR is shown as a bold black line. (A) (Upper) Schematic of the pVΔ102LhIDb in which the last 102 bp of the var promoter containing the PE were deleted. (Lower) Schematic of the pVLhPEIDh episome, which is identical to pVLhIDb except that an additional PE is inserted upstream of the var intron. These modifications resulted in activation of the var promoter, which no longer is recognized by the mechanism that controls mutually exclusive expression. (B) Results of pVΔ102LhIDb (Upper) and pVLhPEIDb (Lower) in the DC-J parasite line. Quantification of the ratio between var promoter and intron by qRT-PCR (Left), steady-state mRNA levels of each individual var gene measured by qRT-PCR presented as relative copy number to the housekeeping genes arginyl-tRNA synthetase (PFL0900c) (Center), and the levels of luciferase expression (Right). The D10 parasite line (22) constitutively expressing luciferase from an endogenous var promoter was used as a positive control. (C) The interaction between the two DNA elements is specific to var genes. Schematic of the pHLhIDb (Upper) and pHPELhIDb (Lower) constructs made by replacing the var promoter from pVLhIDb with the hrp2 promoter with or without the last 102 bp of the var promoter that contains the PE. The promoters of both plasmids were constitutively active simultaneously with the endogenous var gene in the DC-J parasite line. (D) Results of pHLhIDb (Upper) and pHPELhIDb (Lower) in the DC-J parasite line. (Left) Quantification of the ratio between var promoter and intron by qRT-PCR. Steady-state mRNA levels of each individual var gene measured by qRT-PCR are presented as relative copy number to the housekeeping genes arginyl-tRNA synthetase (PFL0900c) (Center) and the levels of luciferase expression (Right). The D10 parasite line (22) constitutively expressing luciferase from an endogenous var promoter was used as a positive control. All values presented are the average of at least two biological replicates. Error bars represent SEs.
Silencing and Mutually Exclusive Expression Through PE Interactions Is var Specific.
Cooperative silencing of var promoters through interactions with their introns was shown to be specific to var promoters, whereas other promoters such as hrp2 could not be silenced by the presence of an adjacent var intron (9). The promoter region of hrp2 does not contain the var PE identified here, and, therefore, if the PEs are required for these interactions as we hypothesize, it is reasonable that pairing with a var intron would not silence the hrp2 promoter. We were interested in testing whether the insertion of the PE to the hrp2 promoter would be sufficient to achieve intron-mediated silencing and whether such a chimeric gene would be counted for mutually exclusive var expression. To perform this experiment, we created two constructs in which we replaced the var promoter of pVLhIDb with hrp2 promoter, thus creating constructs similar to those that were silenced by the intron (Fig. 1) except that luciferase was driven by the hrp2 promoter. In one of these constructs we maintained the last 102 bp of the var 5′ UTR containing the PE at the same location as in the silent pVLhIDb plasmid. These two constructs were named “pHLhIDb” and “ pHPELhIDb,” respectively (Fig. 3C). In parasite populations that stably carried these constructs, the episomal hrp2 promoter was constitutively active simultaneously with the endogenous var gene (PFB1055c), even though they maintained a 1:1 ratio of the hrp2 promoter and var intron on the plasmid. These results indicated that cooperative silencing and mutually exclusive expression mediated by the PE is specific to var genes and probably is determined by additional elements found specifically within var 5′ UTRs.
var PEs Form Specific DNA–Protein Complexes.
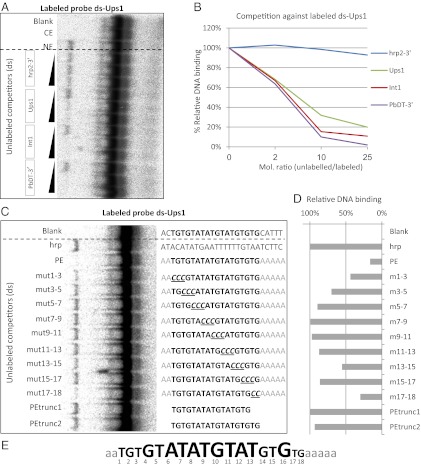
Interactions between distant regulatory elements that lead to changes in transcriptional activation often are mediated through the action-specific proteins (28). We hypothesized that the PE-dependent promoter–promoter interactions of var genes could be mediated by the binding of the PEs by specific mediator proteins. We performed EMSA with radiolabeled probes containing the PEs incubated with nuclear extracts. The radiolabeled probe containing the PE of the var promoter (Ups1) forms a specific protein–DNA complex only when incubated with nuclear extracts (Fig. 4A). A competition assay indicated that an unlabeled Ups1 probe interferes with the formation of the complex in a dose-dependent manner. However, formation of this complex was not affected by competition using the hrp2 3′ probe that does not contain the PE and therefore did not interfere with var gene regulation on our plasmid. These results indicate that this complex forms from the specific binding to the Ups1 probe. Moreover, competition assays using probes containing the PE of the intron (Int1) and the PbDT 3′ showed that those probes disrupted complex formation in a dose-dependent manner similar to the Ups1 probe. To verify these results, we performed the reciprocal experiment using a radiolabeled probe containing the PE of the var intron (Int1) (Fig. S3). Similar to the results obtained using the Ups1 probe, this DNA element also forms a specific DNA–protein complex only when incubated with nuclear extract. All the probes containing the PE (i.e., Int1, Ups1, and PbDT 3′) specifically interrupted the formation of the complex in a dose-dependent manner, but the hrp2 3′ probe did not.
Fig. 4.
The var PEs form a specific DNA–protein complex. (A) EMSA of extracts using a radiolabeled (32P) DNA ligand containing the PE of the var 5′UTR (Ups1) shows specific DNA–protein complex formation when incubated with a nuclear extract. Specific competition assays were performed with increasing concentrations of unlabeled DNA ligands (2×, 10×, and 25× of the labeled ligand, respectively) containing the PEs found in a var 5′UTR (Ups1), in a var intron (Int1), and in the PbDT-3′UTR (PbDT-3′). The DNA sequence from the hrp2-3′ UTR was used as nonspecific competitor (hrp2-3′). Blank, no protein was added; CE, cytoplasmic extract; NE, nuclear extract. (B) Phosphorimaging quantification of the EMSA data presented in A. The percentage of complex formation measured is presented relative to the value measured in the absence of competitive ligand, which was considered to be 100%. (C) Competition EMSA of radiolabeled Ups1 ligand with various mutated nonlabeled Ups1 ligands. The various mutated ligands are named “mut1-3,” “mut3-5,” and so forth, relative to the replacement of the original PE sequence with cytosine bases. The PE sequence is marked in bold. Base-pair exchange is underlined in the PE and is marked in gray in the flanking regions. Blank represents the original sequence of the PE and its flanking regions. (D) Phosphorimaging quantification of the EMSA data presented in C was performed, and the relative DNA-binding percentage was calculated as in B. (E) Schematic description of the importance of each nucleotide position in the PE for formation of the DNA–protein complex. Nucleotides shown in larger type have a great influence on binding.
To identify the nucleotides that are important for the interaction between the PEs and nuclear proteins, we performed competition EMSA with competitor ligands containing different mutations along the PE and its flanking sequence (Fig. 4C). In these mutants we performed serial substitutions of three nucleotides of the core PE with three cytosine bases. This mutants were named “mut1-3,” “mut3-5,” and so forth, according to the location of these replacements. In addition we used ligands in which the flanking regions of the PE were mutated. We found that the PE without its flanking regions (PEtrunc1-2) does not compete for protein binding, but the PE with a different sequence at its flanking region competes very well, indicating that the flanking region is essential for protein binding without sequence specificity. In addition base-pair replacement along the PE indicated that any change in the core sequence of the PE (base pairs 6–13) reduced its ability to form a complex, and therefore these ligands did not compete for binding. Mutated PE ligands with sequence changes at base pairs 1–3 and 17–18 still could compete for binding, indicating that the sequence at these positions has less effect on binding (Fig. 4 C–E). Taken together, these data indicate that the PEs found in var promoters and introns form specific DNA–protein complexes with nuclear proteins.
Discussion
The epigenetic control of var gene transcription involves complex, multilayer levels of regulation. The primary regulatory determinants of var transcription are found within the noncoding DNA regions in var upstream promoters and in their introns. The interaction between these two cis elements is essential for coordinated regulation of var expression in a mutually exclusive manner. Without an adjacent intron a free var promoter is constitutively active and is not counted as a member of the var gene family (29). Here we showed that the interactions between the intronic and upstream regulatory regions are mediated by specific protein-binding DNA elements. These PEs are found in both var promoters and introns. Deletion of this element from the var promoter or insertion of an additional element adjacent to the one found within the intron disrupts these promoter–promoter interactions and effectively leads to an unregulated var promoter similar to a free var promoter that has been separated from an intron (22, 24). In the current study the promoter activity of the intron previously shown to be essential for its function as a silencer of var promoters (19, 21) was not disrupted and was used to drive a drug-selectable marker. Nevertheless, repositioning the PEs by the genetic manipulations described above caused the transcriptionally silent var promoter to revert to an active one even though it still was adjacent to a functional intron promoter. These results indicate that, in addition to the promoter activity of the intron, which is a prerequisite for var silencing, the promoter–promoter interactions are dependent on and are mediated by these DNA elements. Similar interference with promoter–promoter interaction was observed in a transgenic parasite line in which an entire selectable marker cassette was inserted in between the var promoter and its intron. This rearrangement in the original gene structure of var2sca caused the loss of silencing of the endogenous var promoter, which became constitutively active (30). Nevertheless, constitutively active var promoters also can silence the entire var gene family in a mechanism that is different from mutually exclusive expression. It is possible to force transfected parasites to have up to 20 copies of active var promoters on episomes using different levels of drug selection (13, 26, 31). These multiple active var promoters clearly are not counted by the mechanism that controls mutually exclusive expression, but they cause complete silencing of the entire var gene family, possibly by titration of a limited nuclear factor required for var gene activation. Recently, Brancucci et al. (17) performed an elegant and thorough promoter deletion analysis and identified a novel protein-binding motif (MEE) that is found in 44 var 5′ UTRs. In their careful deletion analyses they showed that the MEE motif is essential to achieve complete PfEMP1 knockdown in parasites selected to carry active episomal var promoters. This knockdown could be caused by the recognition of the episomal var promoters by the mechanism that controls mutually exclusive expression. Alternatively, it is possible that the entire var gene family was silenced by titration by the competing episomes through binding of the MEE to the nuclear proteins required for var gene activation. Further investigation is needed to differentiate between these alternative hypotheses.
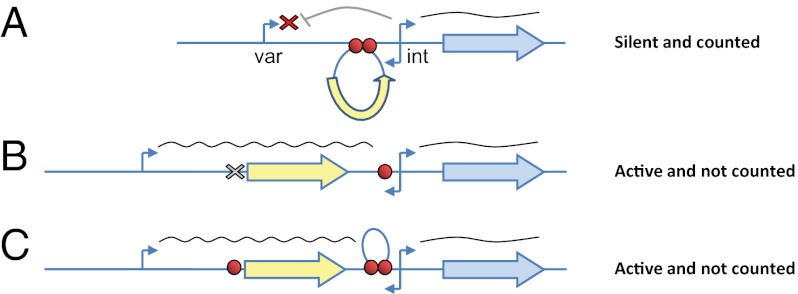
The effect of cis rearrangement of the var PEs on the transcriptional activation of a var promoter has many similarities to the effect of cis rearrangement of Su(Hw) chromatin insulators on enhancer-blocking activity reported in several Drosophila transgenes (32, 33). Chromatin insulators are specialized DNA-regulatory elements that affect gene expression by marking the boundaries of chromatin domains and limiting the range of interaction of enhancers and silencers with promoters (34). A model was proposed by which insulators can regulate gene expression by changes in chromatin conformation that would facilitate the spatial interactions between distant regulatory elements, such as enhancers or silencers, with a particular promoter. Although the PEs described here could not be considered chromatin insulators per se, a similar model that proposes long-range interactions of gene-regulatory elements dependent on a specific cis arrangement of DNA elements could explain the var promoter silencing and activation we observed in our constructs (see summary Fig. P1). The intron promoter can interact with a var promoter and act as its silencer only if the PEs are found both on the intron and the var promoter. Thus, the PEs define the interactions between cis-regulatory elements which are located 9–11 kb from each other. The absence of one PE or interruption of proper pairing by an additional PE leads to a “silencer-blocking effect” and prevents the intron from silencing. It is possible that these promoter–promoter interactions are mediated by the DNA-binding proteins that specifically bind to the PEs and contribute to the formation of possible 3D chromatin structures that would enable the intron to silence its var promoter. Recent studies have implicated enhancer-mediated noncoding RNAs (ncRNAs) in transcriptional regulation. The significance of these ncRNAs is still unknown, but they were postulated to recruit chromatin-modifying factors to their interacting promoters (35–39). It is possible that var intron promoters, which give rise to long ncRNAs, function as silencers in a similar way. However, these hypotheses require further experimental validation.
Long-range interactions between promoters and their distant regulatory elements occur in many gene loci. These long-range interactions require the formation of chromatin loops that allow direct association between distant sequences found on the same or even on another chromosome (28). Such long-range interactions that physically connect enhancers and core promoters and thus affect gene functions were found in the β-globin gene and its locus control region (40), between the olfactory receptors in mammals and the H enhancers (41), in the activation of oct4 in murine embryonic stem cells (42), and upon differentiation of TH2 and TH1 T cells (43, 44), as well as in human Hep3B cells (45). Several mediator proteins, such as CCCTC-binding factor and cohesin, which bind both enhancers and promoters, play a role in the maintenance of chromatin loops that enable these long-range interactions.
The proximity of coregulated genes and their regulatory elements with the components of their transcriptional machinery at distinct nuclear locations has been termed “chromatin hubs” or “transcriptional factories” (28). This proximity ensures efficient transcription and associated dynamics in nuclear architecture with transcription regulation. Dynamics in subnuclear organization also have been implicated in the regulation of var gene expression. Silent var genes seem to be associated with telomeric clusters at the nuclear periphery (46) and, when activated, reposition to a different location at the nuclear periphery (47). var positioning at the nuclear periphery was shown to be mediated by the interaction of nuclear actin and a conserved element found in var introns (20). In parasites in which mutually exclusive expression was partially disrupted by deletion of one intron, two active var genes colocalized to a distinct focus at the nuclear periphery. This result suggests that an element within var promoters directs the active var genes to a specific subnuclear var expression site (24). Similarly, Joergensen et al. (48) have isolated a parasite line in which two PfEMP1 types are expressed by a single parasite and showed that the two simultaneously active var genes colocalized at the nuclear periphery. In addition, an active rif gene, which belongs to another multicopy gene family, colocalized with an active var gene, suggesting that the expression site is shared with other gene families (31). Recently it was shown that the active var gene colocalizes with a histone methyltransferase, PfSET10, which has been implicated in the maintenance of the epigenetic memory at its “poised” state (49). These data support the role of subnuclear organization in var gene regulation, possibly by formation of transcriptional factories that contain the genes' regulatory elements and the transcriptional machinery that regulates gene expression.
var genes often are found in clusters in chromosomal loci and sometimes are separated from each other by as little as 3 kb. In these var clusters the intron of one gene can be closer to the promoter of the gene downstream than to its own promoter. It has been shown that an intron integrated upstream to a free var promoter will silence it (22). In addition, recent work on constructs having two var promoters and one intron showed that the single intron could silence in both orientations and alternate in silencing each of the two var promoters at a given time (23). However, in its native chromosomal location, each var gene is regulated as a separate independent unit, and the restrictions of their mutually exclusive expression ensure that, when one gene is active, its neighboring genes remain transcriptionally silent. This mutual exclusion would imply that distinct boundary elements separate var genes; however, to our knowledge neither boundary elements nor chromatin insulators have yet been identified in Plasmodium. The discovery of the PEs and their crucial role in the interactions between var-regulatory elements will contribute to our understanding of the spatial organization of the genome and the dynamics in nuclear architecture involved in the regulation of var gene expression.
Materials and Methods
Details of materials and methods used, including plasmid construction and probes used for EMSA, are found in SI Materials and Methods. Parasite culture, transfection, and selection were described previously (19, 50, 51). Genomic DNA extraction and RNA extraction and synthesis were done as described (12, 26, 52). Transcript copy numbers were measured by quantitative RT-PCR (qRT-PCR) and were determined using equation 2−ΔΔCT in Applied Biosystems User Bulletin 2 and as previously published (12). Luciferase assay protocols are described in SI Materials and Methods. The D10 parasite line (22) constitutively expressing luciferase from the endogenous var promoter was used as a positive control, and the DC-J parasite line (12) was used as negative control. For gel shift assays the competitive unlabeled ligands were added in increasing concentrations as indicated. Parasites' protein extracts were added last to ensure that the probe and its competitors have the same probability of binding the nuclear extract. The reaction mixtures were incubated on ice for 30 min; then samples were loaded on a 6% (wt/vol) native polyacrylamide gel in TAE buffer [6.7 mM Tris⋅acetate, 3.3 mM sodium acetate, 1 mM EDTA (pH 7.5)]. Electrophoresis was conducted at 2–4 °C and 16 V/cm for 1 h. Protein–DNA complexes were visualized and quantified by a Bio Imaging Analyzer (BAS1000; Fuji).
Supplementary Material
Acknowledgments
This work was supported by Grant 660/09 from the Israeli Academy for Science. R.D. also is supported by the Jacob and Lena Joels Memorial Foundation Senior Lectureship for Excellence in the Life and Medical Sciences. I.A. is supported by an Einstein Kaye Student Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 21196 (volume 109, number 52).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214572109/-/DCSupplemental.
References
- 1.WHO 2010. World Malaria Report 2010. (World Health Organization, Geneva)
- 2.Baruch DI, Gormely JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93(8):3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JD, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82(1):101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherf A, et al. Antigenic variation in malaria: In situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 1998;17(18):5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, et al. Developmental selection of var gene expression in Plasmodium falciparum. Nature. 1998;394(6691):392–395. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- 6.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415(6872):673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 7.Scherf A, Lopez-Rubio JJ, Riviere L. Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol. 2008;62:445–470. doi: 10.1146/annurev.micro.61.080706.093134. [DOI] [PubMed] [Google Scholar]
- 8.Dzikowski R, Deitsch KW. Genetics of antigenic variation in Plasmodium falciparum. Curr Genet. 2009;55(2):103–110. doi: 10.1007/s00294-009-0233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deitsch KW, Calderwood MS, Wellems TE. Malaria. Cooperative silencing elements in var genes. Nature. 2001;412(6850):875–876. doi: 10.1038/35091146. [DOI] [PubMed] [Google Scholar]
- 10.Corcoran AE. Immunoglobulin locus silencing and allelic exclusion. Semin Immunol. 2005;17(2):141–154. doi: 10.1016/j.smim.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 2004;20(12):648–653. doi: 10.1016/j.tig.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Dzikowski R, Frank M, Deitsch K. Mutually exclusive expression of virulence genes by malaria parasites is regulated independently of antigen production. PLoS Pathogens. 2006;2(3):e22. doi: 10.1371/journal.ppat.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss TS, et al. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439(7079):1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- 14.Kraemer SM, Smith JD. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol Microbiol. 2003;50(5):1527–1538. doi: 10.1046/j.1365-2958.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- 15.Lavstsen T, Salanti A, Jensen ATR, Arnot DE, Theander TG. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar J. 2003;2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voss TS, Kaestli M, Vogel D, Bopp S, Beck HP. Identification of nuclear proteins that interact differentially with Plasmodium falciparum var gene promoters. Mol Microbiol. 2003;48(6):1593–1607. doi: 10.1046/j.1365-2958.2003.03528.x. [DOI] [PubMed] [Google Scholar]
- 17.Brancucci NM, Witmer K, Schmid CD, Flueck C, Voss TS. Identification of a cis-acting DNA-protein interaction implicated in singular var gene choice in Plasmodium falciparum. Cell Microbiol. 2012 doi: 10.1111/cmi.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flueck C, et al. A major role for the Plasmodium falciparum ApiAP2 protein PfSIP2 in chromosome end biology. PLoS Pathog. 2010;6(2):e1000784. doi: 10.1371/journal.ppat.1000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calderwood MS, Gannoun-Zaki L, Wellems TE, Deitsch KW. Plasmodium falciparum var genes are regulated by two regions with separate promoters, one upstream of the coding region and a second within the intron. J Biol Chem. 2003;278(36):34125–34132. doi: 10.1074/jbc.M213065200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, et al. A critical role of perinuclear filamentous actin in spatial repositioning and mutually exclusive expression of virulence genes in malaria parasites. Cell Host Microbe. 2011;10(5):451–463. doi: 10.1016/j.chom.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gannoun-Zaki L, Jost A, Mu JB, Deitsch KW, Wellems TE. A silenced Plasmodium falciparum var promoter can be activated in vivo through spontaneous deletion of a silencing element in the intron. Eukaryot Cell. 2005;4(2):490–492. doi: 10.1128/EC.4.2.490-492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank M, et al. Strict pairing of var promoters and introns is required for var gene silencing in the malaria parasite Plasmodium falciparum. J Biol Chem. 2006;281(15):9942–9952. doi: 10.1074/jbc.M513067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swamy L, Amulic B, Deitsch KW. Plasmodium falciparum var gene silencing is determined by cis DNA elements that form stable and heritable interactions. Eukaryot Cell. 2011;10(4):530–539. doi: 10.1128/EC.00329-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzikowski R, et al. Mechanisms underlying mutually exclusive expression of virulence genes by malaria parasites. EMBO Rep. 2007;8(10):959–965. doi: 10.1038/sj.embor.7401063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duraisingh MT, Triglia T, Cowman AF. Negative selection of Plasmodium falciparum reveals targeted gene deletion by double crossover recombination. Int J Parasitol. 2002;32(1):81–89. doi: 10.1016/s0020-7519(01)00345-9. [DOI] [PubMed] [Google Scholar]
- 26.Dzikowski R, Deitsch KW. Active transcription is required for maintenance of epigenetic memory in the malaria parasite Plasmodium falciparum. J Mol Biol. 2008;382(2):288–297. doi: 10.1016/j.jmb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell TL, De Silva EK, Olszewski KL, Elemento O, Llinás M. Identification and genome-wide prediction of DNA binding specificities for the ApiAP2 family of regulators from the malaria parasite. PLoS Pathog. 2010;6(10):e1001165. doi: 10.1371/journal.ppat.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong CT, Corces VG. Enhancer function: New insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12(4):283–293. doi: 10.1038/nrg2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank M, Deitsch K. Activation, silencing and mutually exclusive expression within the var gene family of Plasmodium falciparum. Int J Parasitol. 2006;36(9):975–985. doi: 10.1016/j.ijpara.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Viebig NK, et al. Disruption of var2csa gene impairs placental malaria associated adhesion phenotype. PLoS ONE. 2007;2(9):e910. doi: 10.1371/journal.pone.0000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howitt CA, et al. Clonally variant gene families in Plasmodium falciparum share a common activation factor. Mol Microbiol. 2009;73(6):1171–1185. doi: 10.1111/j.1365-2958.2009.06846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai HN, Shen P. Effects of cis arrangement of chromatin insulators on enhancer-blocking activity. Science. 2001;291(5503):493–495. doi: 10.1126/science.291.5503.493. [DOI] [PubMed] [Google Scholar]
- 33.Muravyova E, et al. Loss of insulator activity by paired Su(Hw) chromatin insulators. Science. 2001;291(5503):495–498. doi: 10.1126/science.291.5503.495. [DOI] [PubMed] [Google Scholar]
- 34.Bell AC, West AG, Felsenfeld G. Insulators and boundaries: Versatile regulatory elements in the eukaryotic genome. Science. 2001;291(5503):447–450. doi: 10.1126/science.291.5503.447. [DOI] [PubMed] [Google Scholar]
- 35.Kim TK, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465(7295):182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei EP, Corces VG. RNA interference machinery influences the nuclear organization of a chromatin insulator. Nat Genet. 2006;38(8):936–941. doi: 10.1038/ng1850. [DOI] [PubMed] [Google Scholar]
- 37.Tsai MC, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang KC, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472(7341):120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao H, et al. Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes Dev. 2010;24(22):2543–2555. doi: 10.1101/gad.1967810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295(5558):1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 41.Lomvardas S, et al. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126(2):403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 42.Kagey MH, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467(7314):430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spilianakis CG, Flavell RA. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nat Immunol. 2004;5(10):1017–1027. doi: 10.1038/ni1115. [DOI] [PubMed] [Google Scholar]
- 44.Hadjur S, et al. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460(7253):410–413. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishiro T, et al. Architectural roles of multiple chromatin insulators at the human apolipoprotein gene cluster. EMBO J. 2009;28(9):1234–1245. doi: 10.1038/emboj.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freitas-Junior LH, et al. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature. 2000;407(6807):1018–1022. doi: 10.1038/35039531. [DOI] [PubMed] [Google Scholar]
- 47.Ralph SA, Scheidig-Benatar C, Scherf A. Antigenic variation in Plasmodium falciparum is associated with movement of var loci between subnuclear locations. Proc Natl Acad Sci USA. 2005;102(15):5414–5419. doi: 10.1073/pnas.0408883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joergensen L, et al. Surface co-expression of two different PfEMP1 antigens on single plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathog. 2010;6(9):e1001083. doi: 10.1371/journal.ppat.1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volz JC, et al. PfSET10, a Plasmodium falciparum methyltransferase, maintains the active var gene in a poised state during parasite division. Cell Host Microbe. 2012;11(1):7–18. doi: 10.1016/j.chom.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Aley SB, Sherwood JA, Howard RJ. Knob-positive and knob-negative Plasmodium falciparum differ in expression of a strain-specific malarial antigen on the surface of infected erythrocytes. J Exp Med. 1984;160(5):1585–1590. doi: 10.1084/jem.160.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deitsch KW, Driskill CL, Wellems TE. Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Res. 2001;29(3):850–853. doi: 10.1093/nar/29.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyes S, Pinches R, Newbold C. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol Biochem Parasitol. 2000;105(2):311–315. doi: 10.1016/s0166-6851(99)00193-0. [DOI] [PubMed] [Google Scholar]