Abstract
Background
Corticolimbic circuits, including direct projections from prefrontal cortex to nucleus accumbens (NAc), permit “top-down” control of intense motivations generated by subcortical circuits. In rats, localized disruptions of glutamate signaling within medial shell of NAc generate desire or dread, anatomically organized along a rostrocaudal gradient analogous to a limbic “keyboard”. At rostral locations in shell these disruptions generate appetitive eating, but at caudal locations the disruptions generate progressively fearful behaviors (distress vocalizations, escape attempts and antipredator reactions). Here we asked whether medial prefrontal cortex can modulate intense motivations generated by subcortical NAc disruptions.
Methods
We used simultaneous microinjections in medial prefrontal cortex regions and in NAc shell to examine whether the desire or dread generated by NAc shell disruptions is modulated by activation/inhibition of three specific regions of prefrontal cortex: medial orbitofrontal cortex, infralimbic cortex (homologous to area 25 or subgenual anterior cingulate in the human), or prelimbic cortex (midventral anterior cingulate).
Results
We found that activation of medial orbitofrontal cortex biased intense bivalent motivation in an appetitive direction by amplifying generation of eating behavior by middle to caudal NAc disruptions, without altering fear. In contrast, activation of infralimbic prefrontal cortex powerfully and generally suppressed both appetitive eating and fearful behaviors generated by NAc shell disruptions.
Conclusions
These results suggest that corticolimbic projections from discrete prefrontal regions can either bias motivational valence or generally suppress subcortically-generated intense motivations of desire or fear.
Keywords: eating, fear, accumbens, prefrontal, medial orbitofrontal, infralimbic
Introduction
Motivations or emotions generated by subcortical circuits involving the nucleus accumbens (NAc) may be powerfully modulated by “top-down” corticolimbic controls from prefrontal cortex (1–2). In humans, successful voluntary suppression of subjective cravings or emotional responses is accompanied by activation of prefrontal cortical areas and simultaneous reduction of subcortical activity in NAc, amygdala and ventral tegmentum (1, 3–6).
Here, we sought to probe corticolimbic modulation of intense unconditioned appetitive and defensive behaviors generated by disruptions in medial shell of NAc in the rat. The medial shell of NAc is an important node in the generation of both positive desire and aversive dread (7–13). Localized disruptions of glutamate transmission in medial shell, via microinjections of the AMPA antagonist DNQX, generate intense unconditioned appetitive and/or fearful behaviors organized along a rostrocaudal gradient (probably involving disinhibition of ventral pallidum, hypothalamus, and related targets from GABAergic suppression, thus releasing motivation-generating circuits) (14–18). Rostral disruptions in NAc shell evoke purely appetitive behaviors like voracious eating (19–20). Caudal disruptions instead evoke increasingly fearful behaviors including audible distress vocalizations, escape attempts and spontaneous defensive treading, an innate anti-predator reaction in which rodents toss debris at a threatening stimulus (e.g., rattlesnake) (21–26). Intermediate disruptions produce ambivalent mixtures of appetitive and fearful behaviors (27–28).
The valence of motivation produced by glutamate disruption at many shell sites can be retuned by changes in external ambience, which might reflect top-down influences from cortex and related structures that send glutamate inputs to NAc (27–28). This study probed three regions of prefrontal cortex that send direct glutamate projections to the medial shell. First, the medial orbitofrontal cortex (Brodmann’s area 10) (29–30, but see 31), which is implicated in pleasure and emotion (32–33). Second, infralimbic cortex (34), which is homologous to human subgenual or deeply ventral anterior cingulate cortex (Brodmann’s area 25) (35–37), and has been suggested to suppress motivated behaviors, such as reinstatement of cocaine and food seeking (38–39) and conditioned fear (40). Third, prelimbic cortex (potentially homologous to Brodmann’s areas 24 and 32 of anterior cingulate cortex) (35–37) projects to NAc shell and core, and has been suggested to participate in appetitive and fearful motivations (41–43) and in some forms of inhibitory control (44–45). Here, we tested the effects of reversible activation versus inhibition of medial orbitofrontal, infralimbic, and prelimbic cortex on the ability of glutamate disruptions within NAc shell to produce unconditioned appetitive and fearful motivated behaviors.
Materials and Methods
Experimental design
To investigate whether activity in infralimbic, prelimbic, and orbitofrontal regions of medial prefrontal cortex modulates the generation of strong unconditioned motivations by NAc shell glutamate disruptions, we simultaneously generated eating or fearful defensive behaviors via microinjections in medial shell of a low dose of the AMPA antagonist DNQX (6,7-dinotroquinoxaline-2,3(1H,4H)-dione; 250 ng/ 0.2 µl per side in 50% saline/50% DMSO), while temporarily either activating or inhibiting each prefrontal region (Figure 1A). Temporary activation of prefrontal cortex was produced by microinjections of the GABA-A antagonist bicuculline (.1 µg / .2 µl side in ACSF), which by preventing local inhibition induces relative activation of neurons (46). Using bicuculline allowed receptor-based comparison to the opposite polarity effects of GABA agonist microinjections at the same prefrontal sites (muscimol/baclofen microinjections). We conducted a careful slow motion video analysis of behavior to ensure that no seizure indicators were ever produced by bicuculline in prefrontal cortex, which is consistent with previous reports of no seizure manifestations after bicuculline injected into cortex at doses similar to ours or even higher (47–52). Temporary inhibition of cortex was produced by a mixture of GABAA and GABAB agonists, muscimol (5 ng per side) and baclofen (65 ng per side in .2 µl ACSF) (38). Microinjection spread was assessed by Fos plumes surrounding drug microinjections. We have previously found that the diameter of drug-induced Fos plumes shrinks after several microinjections (28), and therefore we used a dedicated Fos group measured after a single microinjection to capture maximal diameter.
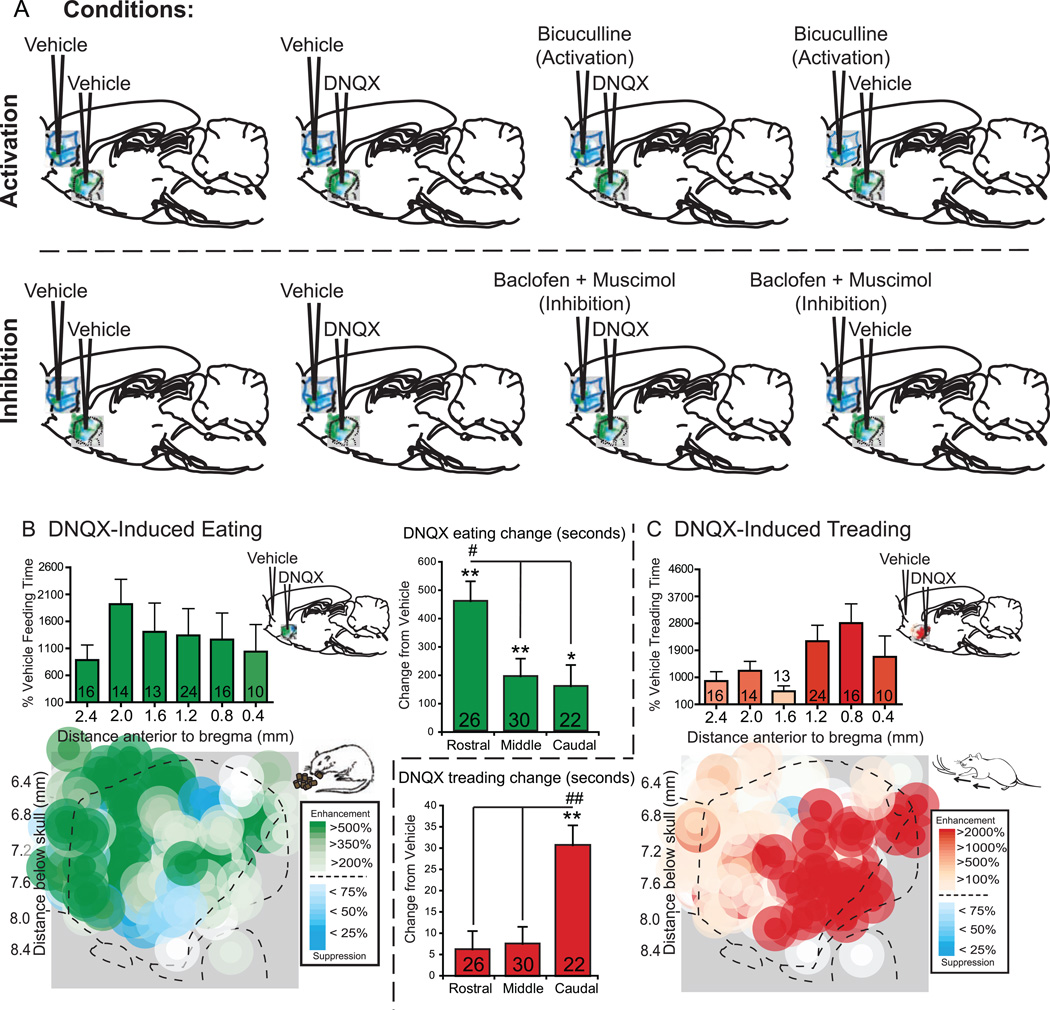
Figure 1. Microinjection conditions and DNQX effects.
Rats received the following microinjections (A): rats in the activation group (top, n=68) received either DNQX or vehicle in NAc shell and bicuculline or vehicle into prefrontal cortex, and rats in the inactivation group (bottom, n=30) received either DNQX or vehicle in NAc shell and a baclofen plus muscimol combination or vehicle in prefrontal cortex. Fos plume maps show the effects of DNQX alone (vehicle in prefrontal cortex) on eating (B, green) or defensive treading behavior (C, red). Histogram bars above the maps show mean behaviors as a percent of vehicle at each rostrocaudal level (errors bars = SEM). Summary bar graphs show the DNQX induced eating (B) and treading (C) as change from vehicle at rostral (n=26), middle (n=30) and caudal (n=22) locations in NAc shell; data is given as seconds per hour, ** p < .01, * p < .05 versus vehicle, ## p < .01, # p < .05 subregion difference, with Sidak corrections for multiple comparisons.
Subjects
Male Sprague-Dawley rats [n = 124; Prefrontal Activation, n = 68, Prefrontal Inhibition, n = 30, Fos analysis, n = 26), 300 – 400 grams prior to surgery] were housed at ~21 C° on a reverse 12:12 light:dark cycle, with ad libitum access to both food and water. All experiments were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Surgery and drug microinjections
Rats received bilateral implantation of cranial cannulae aimed at points throughout the rostrocaudal extent of medial shell of NAc, and a separate pair aimed at either the infralimbic, prelimbic, or medial orbitofrontal regions of prefrontal cortex. Following one week of recovery, each rat (n = 68; infralimbic, n = 26; prelimbic, n=11; orbitofrontal, n=29) in the prefrontal activation group was tested after the following 4 drug conditions for spontaneous motivated behavior (see Supplement for more detail): 1) vehicle in prefrontal cortex and vehicle in NAc, 2) bicuculline in prefrontal cortex and vehicle in NAc, 3) vehicle in prefrontal cortex and DNQX in NAc, and 4) bicuculline in prefrontal cortex and DNQX in NAc. Each rat in the prefrontal inhibition group (n = 30; infralimbic, n=9; prelimbic, n=5; orbitofrontal, n=16) received the following 4 drug conditions: 1) vehicle in prefrontal cortex and vehicle in NAc, 2) baclofen plus muscimol in prefrontal cortex and vehicle in NAc, 3) vehicle in prefrontal cortex and DNQX in NAc, and 4) baclofen plus muscimol in prefrontal cortex and DNQX in NAc (Figure 1A). Each rat in the Fos group received comparable single microinjections of one above condition.
Behavioral coding
Observers blind to drug treatment scored each 60 minute session for the total time (seconds) spent in each of the following behaviors: appetitive behaviors such as eating (mouth on the food or engaged in chewing action) and drinking (licking the spout of the water bottle), fearful behavior consisting of defensive treading (spraying or pushing of bedding by rapid alternating thrusts of the forepaws), and grooming (a stereotyped sequence described in (53)). Observers scored the total number for behaviors which tended to occur as discrete events, including appetitive behaviors such as food carrying (transportation of food pellets in the mouth) and food sniffs (sniffing near the food for at least 1 second), and two general motor activities: rearing (forepaws at least one inch off the floor) and cage crosses (forepaws and head cross the halfway point of the cage). Observers also looked for any indicators of seizure including: behavioral arrest or akinesia (freezing), stereotyped behaviors including repetitive blinking or rhythmic jaw-opening, head nodding, head shaking, wet dog shakes (repetitive shaking of the entire trunk), tonic seizures (sudden-onset tonic extension or flexion of the head, trunk and/or extremities for several seconds), sudden loss of posture (falling over), and myoclonic or clonic twitches (brief arrhythmic or rhythmic jerking of a muscle group).
Fos-like protein immunohistochemistry
Rats used for Fos analysis (n=26) were anesthetized with an overdose of sodium pentobarbital and transcardially perfused 90 minutes after bilateral microinjection of either 1) vehicle in prefrontal cortex and vehicle in NAc (n = 6), 2) bicuculline in prefrontal cortex and vehicle in NAc (n = 2), 3) vehicle in prefrontal cortex and DNQX in NAc (n = 8), or 4) bicuculline in prefrontal cortex and DNQX in NAc (n = 4) for Fos plumes analysis, and 5) vehicle in prefrontal cortex and no injection in NAc (n = 3), or 6) bicuculline in prefrontal cortex and no injection in NAc (n = 3), for analysis of the effect of prefrontal activation on baseline NAc Fos (see Supplement for more detail).
Results
Local glutamate disruptions in medial shell of NAc induce appetitive and defensive behavior organized along a rostrocaudal gradient
When prefrontal cortex received no drug manipulation (vehicle microinjection), localized glutamate disruptions in medial shell generated intense appetitive eating and/or fearful behaviors as expected, organized by valence along the usual rostrocaudal gradient (Figure 1B). Medial shell microinjections of a moderate dose of DNQX produced robust stimulation of eating and food consumption to above five-times vehicle control levels, with most intense eating (of up to 10 grams) occurring from the most rostral sites (Figure 1B; average of 611 seconds +/− 65 SEM eating after rostral DNQX versus 154 seconds on vehicle control; eating time: drug×placement, F(1,74) = 5.415, p = .006; average of 5.5 grams +/− .4 SEM grams consumed after rostral DNQX versus 1.1 grams on vehicle control; food intake: drug×placement, F(1,74) = 7.557, p = .001). At caudal sites the same microinjections produced active fearful behaviors, including audible distress vocalizations and escape attempts to touch (vocalizations: 59% of rats on DNQX vs. 0% on vehicle, McNemar’s test, p < .001; escape: 27% of rats on DNQX vs. 0% on vehicle, p = .031) and spontaneous defensive treading at 60-times vehicle control levels (Figure 1C; average of 31.3 seconds +/− 6.13 SEM after caudal DNQX versus .49 seconds on vehicle control; drug×placement, F(1,74) = 9.550, p = < .001).
Medial orbitofrontal activation specifically enhances appetitive motivation produced by NAc shell
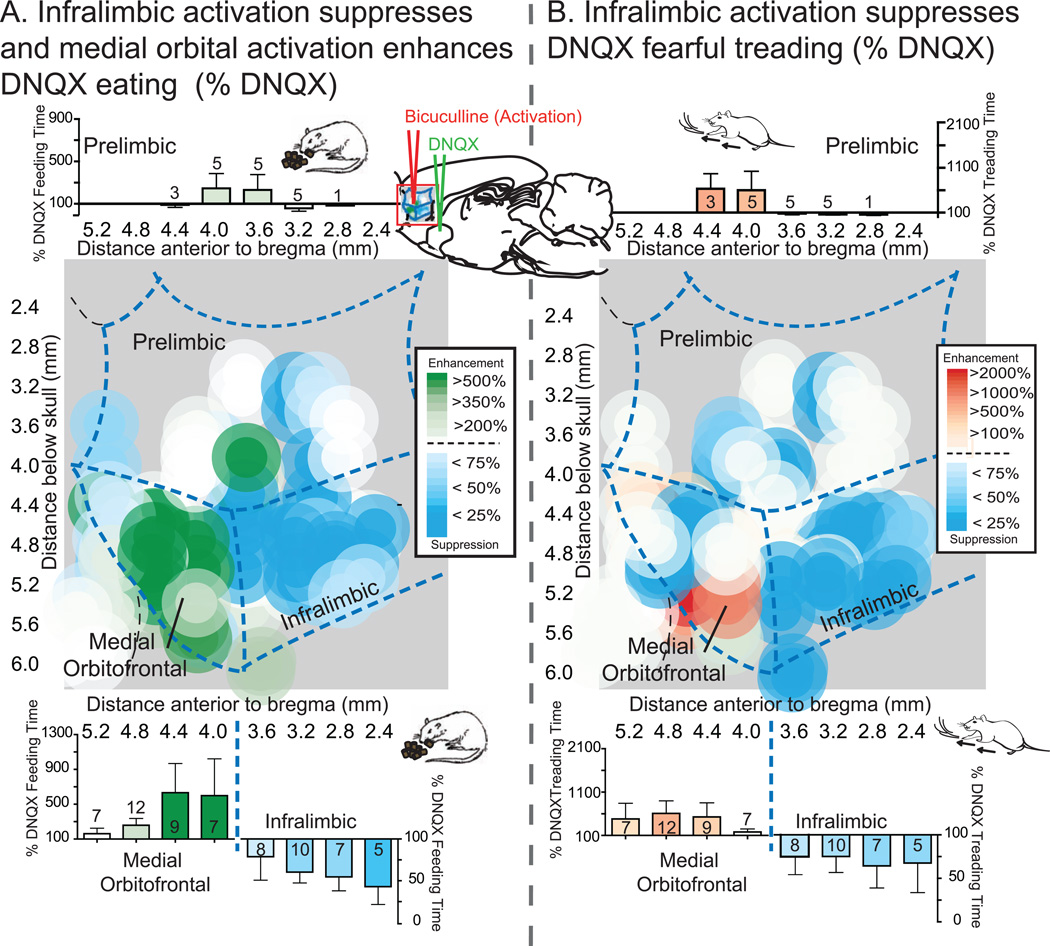
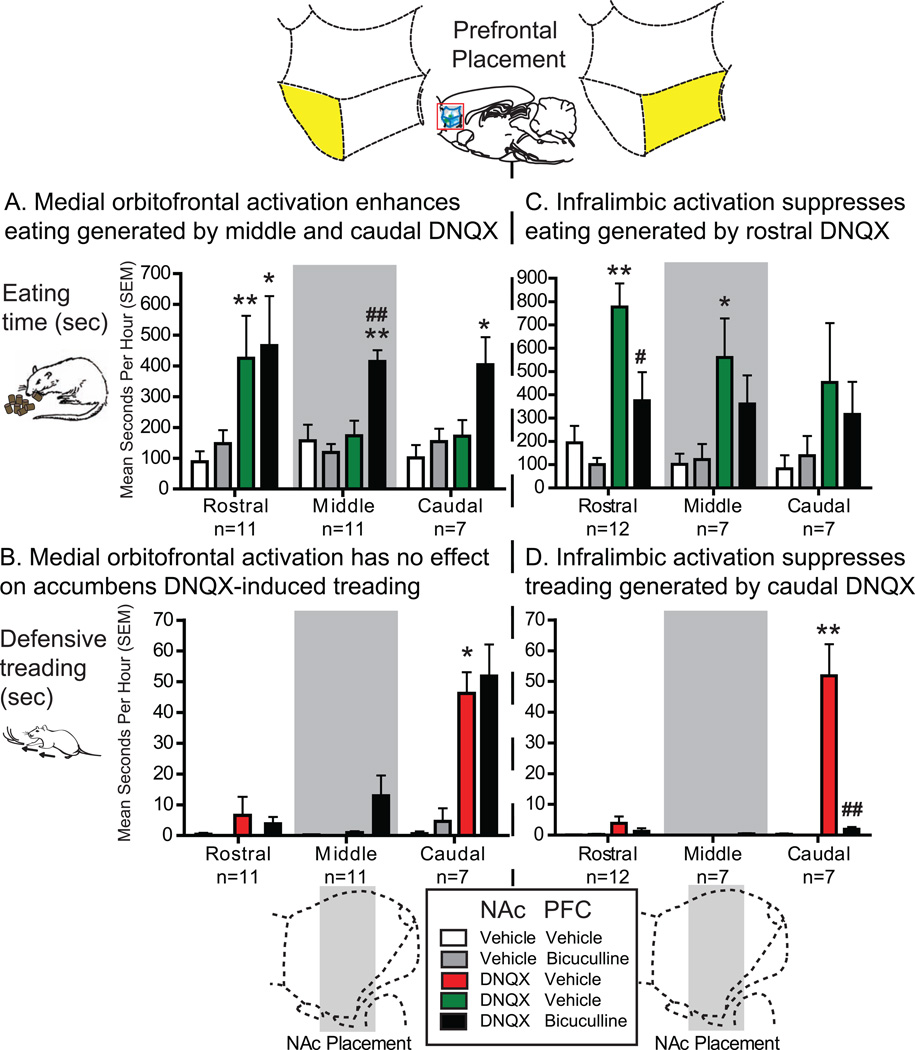
Co-activation of medial orbitofrontal cortex (immediately rostral to infralimbic cortex) selectively enhanced eating induced by NAc DNQX at middle and caudal sites that otherwise produced only fear (Figures 2A and 3A). Orbitofrontal activation made caudal DNQX generate levels of eating that were 250% of levels without orbitofrontal activation, and as high as any eating generated at rostral DNQX sites (up to 9.5 grams; eating time: DNQX×bicuculline, F(1,20) = 4.483, p = .047; DNQX×bicuculline, F(1,21) = 4.376, p = .049; Figure 3A). At more rostral NAc sites for DNQX, which generated high levels of food intake to begin with (>5-times control), robust eating remained unchanged by orbitofrontal co-activation (Figure 3A). Orbitofrontal activation never altered DNQX generation of fearful behaviors (Figures 2B and 3B; medial orbitofrontal cortex: defensive treading, DNQX×bicuculline, F<1; DNQX×bicuculline X NAc placement, F(2,19) = 1.764, p = .198; vocalizations and escape attempts, p = .375 to 1.00). Likewise medial orbitofrontal activation had no effect on nonvalenced motor behaviors such as grooming (Fs<1), cage crosses (F(1,14) = 2.788, p = .117) or rears (F(1,14) = 1.424, p = .253).
Figure 2. Maps of prefrontal activation effects on NAc shell DNQX generated eating and defensive treading.
Maps show the effects of prefrontal activation (n=68) on DNQX-induced eating (A, left) or defensive treading (B, right) at sites mapped on the sagittal plane of prefrontal cortex, color-coded for changes in behavior as a percent of DNQX. Histograms bars show mean behavior as percent of DNQX at each rostrocaudal level, split by dorsal (prelimbic, top; n=11) and ventral (medial orbitofrontal, n=29, and infralimbic, n=26, bottom) areas of prefrontal cortex (error bars = SEM).
Figure 3. Motivated behavior graphs.
Graphs demonstrating the specific effects of medial orbitofrontal activation (left) and infralimbic activation (right) on appetitive eating (top) and defensive treading (bottom), depending on particular rostrocaudal location (rostral, middle or caudal). Simultaneous microinjections of bicuculline in medial orbitofrontal (n=29) with DNQX in NAc shell (black, left) produced enhancement of DNQX induced eating (green, A), specifically at more middle (n=11) and caudal (n=7) locations but not rostral (n=11), and had no effect on DNQX induced treading (red, B). Microinjections of bicuculline in infralimbic cortex (n=26) with simultaneous DNQX in NAc shell (black, right) produced suppression of both DNQX-induced eating (green) from rostral sites (n=12) and treading (red) from caudal sites (n=7; middle sites, n=7). Data is given as seconds per hour, errors bars indicate SEM, * p < .05 versus vehicle, ** p < .01 versus vehicle, # p < .05 versus DNQX, ## p < .01 versus DNQX, pairwise comparison using Sidak corrections.
Infralimbic activation suppressed eating and fear generated by NAc shell glutamate disruption
Infralimbic activation nonspecifically suppressed all intense motivated behaviors generated by NAc disruptions, appetitive and fearful. Bicuculline-induced activation roughly cut in half the high level of eating behavior and food intake otherwise induced by DNQX in rostral sites of NAc shell (Figures 2A and 3C; eating time, DNQX×bicuculline, F(1,20) = 4.563, p = .045; food intake, DNQX×bicuculline, F(1,23) = 10.903, p = .003; DNQX×bicuculline X NAc placement, F(2,23) = 3.522, p = .046). Activation of infralimbic cortex similarly nearly abolished fearful distress vocalizations, escape attempts, and defensive treading behavior otherwise produced by DNQX at caudal shell sites (at least 96% reduction; Figure 2B and 3D; defensive treading, DNQX×bicuculline, F(1,23) = 37.906, p < .001; DNQX×bicuculline×NAc placement, F(2,23) = 31.177, p < .001; vocalizations, McNemar’s test, DNQX alone versus DNQX plus bicuculline, p = .021).
Infralimbic suppression is specific to DNQX-induced levels of motivation
Infralimbic cortex activation did not interfere with baseline levels of spontaneous appetitive and defensive behaviors after vehicle microinjection in NAc. Infralimbic microinjections of bicuculline did not suppress moderate baseline levels of eating or drinking (Fs<1, Figure 3C), nor change baseline defensive behaviors, which remained near zero (Figure 3D; defensive treading, F<1, vocalizations and escape attempts, McNemar’s tests, p = 1.00). It also did not prevent NAc DNQX microinjections from stimulating nonvalenced activities such as grooming (F<1), and even slightly enhanced locomotion (cage crosses, main effect of bicuculline, F(1,16) = 5.125, p = .038; rears, main effect of bicuculline, F(1,16) = 7.981, p = .012).
Prelimbic activation has no effect on motivated behaviors generated by NAc shell DNQX
By contrast to co-activation of infralimbic cortex, which suppressed DNQX appetitive and fearful behaviors, co-activation of the immediately dorsal region of prelimbic cortex failed to alter NAc DNQX-induced eating or food intake (Figure 2A; eating time, DNQX×bicuculline, F<1; DNQX×bicuculline×NAc placement, F<1; food intake, DNQX×bicuculline, F(1,5) = 2.330, p = .187; DNQX×bicuculline×NAc placement, F<1). Prelimbic cortex co-activation also failed to alter defensive treading behavior, distress calls or escape attempts induced by glutamate disruptions in NAc caudal shell (Figure 2B; prelimbic cortex: defensive treading, Fs<1). Finally, co-activation of prefrontal cortex had no impact on baseline defensive behaviors in the absence of NAc DNQX, which remained near zero (defensive treading, F<1, McNemar’s Test, vocalizations and escape attempts, p = 1.00).
No behavioral indicators of seizure were observed after microinjections of bicuculline in either prelimbic, infralimbic or medial orbitofrontal regions. Thus we conclude that seizures were not induced by prefrontal activations.
Inhibition of all prefrontal cortex regions leaves unchanged levels of unconditioned appetitive or defensive behaviors produced by NAc shell glutamate disruption
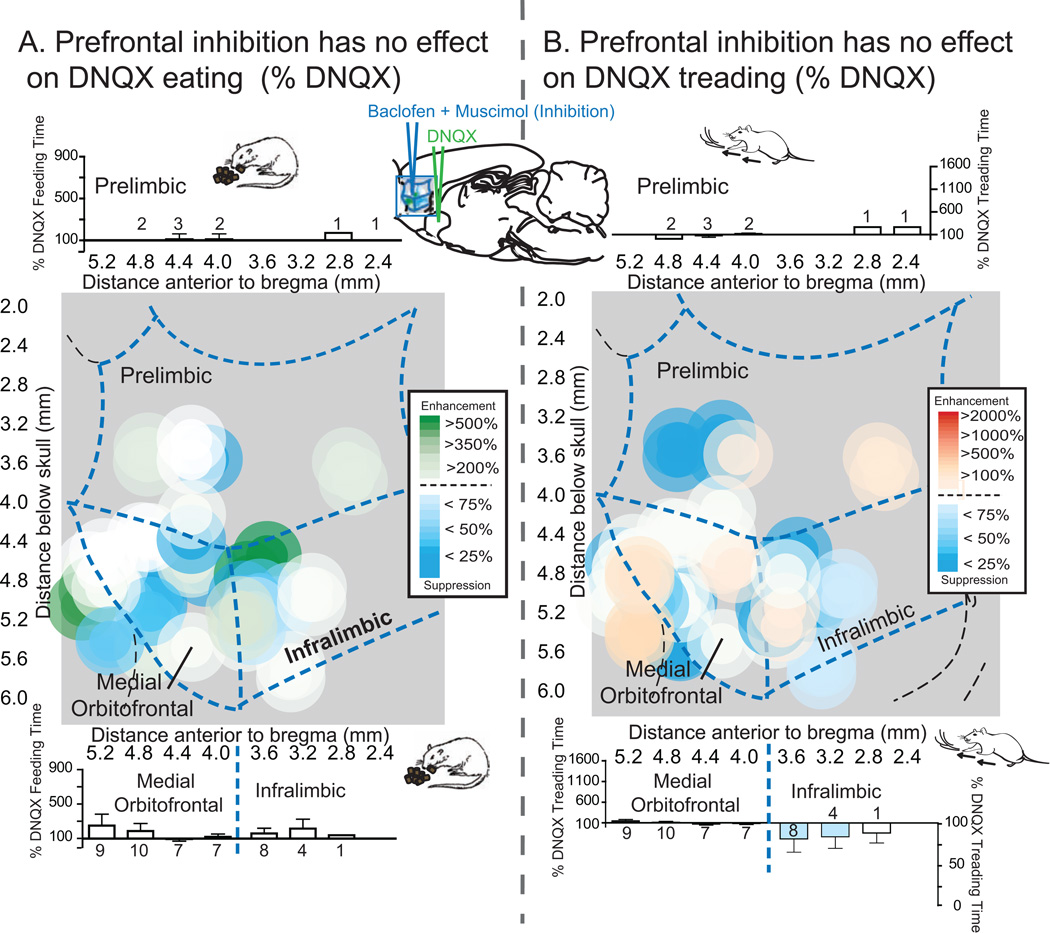
Inhibition of infralimbic, prelimbic or medial orbitofrontal regions of prefrontal cortex, via combined microinjection of GABA agonists baclofen and muscimol, all failed to alter the DNQX NAc shell generation of intense levels of appetitive or defensive behaviors, regardless of prefrontal subregion (Figure 4A and B; eating time: Fs<1; food intake: DNQX×baclofenmuscimol, F(1,14) = 2.580, p = .131; DNQX×baclofen-muscimol×prefrontal placement, F<1; defensive treading, DNQX×baclofen-muscimol, F<1; DNQX×baclofen-muscimol×prefrontal placement, F(2,14) = 2.536, p = .115; vocalizations and escape attempts, p = 1.00; Figures 2 and 3). Inhibition of prefrontal cortex also had no impact on baseline levels of eating or defensive behaviors (food intake, Fs<1; eating, baclofen-muscimol, F(1,14) = 1.833, p = .197; baclofen plus muscimol×prefrontal placement, F(2,14) = 1.241, p = .319; defensive treading, Fs<1).
Figure 4. Maps of prefrontal inhibition effects on NAc shell DNQX generated eating and defensive treading.
Maps show the effects of prefrontal inhibition (n=30) on DNQX-induced eating (A, left) or defensive treading (B, right) at sites mapped on the sagittal plane of prefrontal cortex, color-coded for changes in behavior as a percent of DNQX. Histograms bars show mean behavior as percent of DNQX at each rostrocaudal level, split by dorsal (prelimbic, top; n=5) and ventral (medial orbitofrontal, n=16, and infralimbic, n=9, bottom) areas of prefrontal cortex (error bars = SEM).
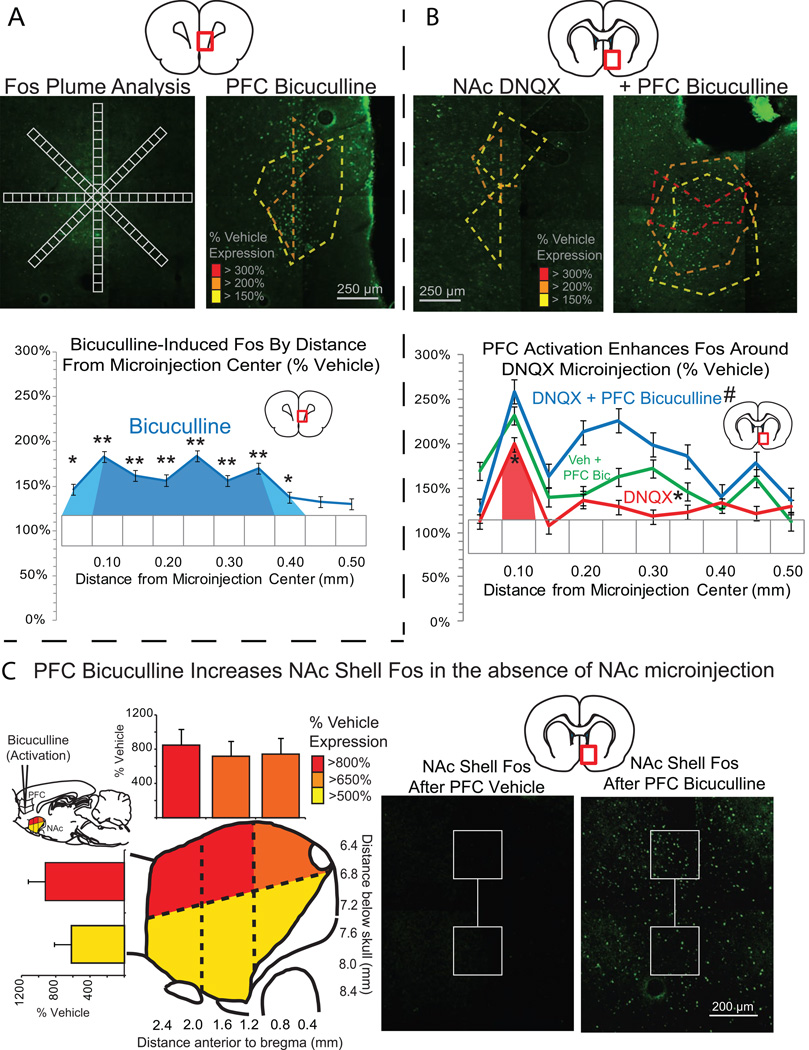
Fos plume analysis: determining functional microinjection drug spread
We assessed Fos plumes produced by DNQX microinjections in NAc and bicuculline microinjections in orbitofrontal and infralimbic prefrontal cortex, and also assessed whether prefrontal bicuculline modulated distant Fos plumes in NAc induced by DNQX in medial shell (Figure 5). DNQX microinjections in NAc shell produced plumes containing a small .008 mm3 volume center where Fos expression was more than doubled (radius = .125 mm), surrounded by a larger .056 mm3 sphere of mildly elevated Fos expression between 1.5 to 2 times vehicle levels (radius = .24 mm; Figure 5B). Prefrontal bicuculline microinjections similarly produced enhancement of Fos expression surrounding the microinjection. Infralimbic and orbitofrontal plumes contained a small excitatory .00075mm3 center of tripled Fos expression (radius = .056 mm), surrounded by a larger .03 mm3 middle zone of more than doubled Fos expression (radius = .19 mm) and an outer .15 mm3 halo of mildly elevated Fos expression between 1.5 to 2 times vehicle (radius = .33 mm) (Figure 5A). In addition, bicuculline activation of either infralimbic or medial orbitofrontal cortex amplified distant Fos expression in medial shell (main effect of bicuculline, F(1,8) = 7.737, p = .024; bicuculline×prefrontal placement, F(1,8) = 1.484, p = .258; Figure 5). Fos expression surrounding NAc shell DNQX microinjections was elevated to 3-times DNQX alone levels in a radius of .15 mm from microinjection center (volume = .0014 mm3) and 2-times DNQX levels up to .26 mm away (volume = .073 mm3) following bicuculline microinjections. Prefrontal bicuculline enhanced distant Fos in uninjected NAc shell to between 550 and 1150% over normal levels (main effect of drug, F(33,2) = 15.895, p < .001), with greatest effects in dorsal shell and in particular in the most rostral and dorsal portion of medial shell (bicuculline×dorsoventral location, F(1,10) = 8.566, p = .015; bicuculline×rostrocaudal location×dorsoventral location, F(2,30 = 4.914, p = .014; Fig. 5C).
Figure 5. Fos plume analysis and NAc-prefrontal interactions.
Fos plumes were analyzed for functional drug spread of bicuculline in prefrontal cortex (A) and DNQX in NAc shell (B). Fos labeled cells were individually counted within successive blocks (50 µm×50 µm), along 8 radial arms emanating from the center of the site, with 10x magnification (A). Colors indicate levels of Fos expression of 3x (red), 2x (orange) and 1.5x (yellow) vehicle level Fos expression. Line graphs show levels of Fos expression following bicuculline (blue, A) and DNQX (red, B), as well as the impact of prefrontal bicuculline on levels of Fos expression in NAc shell following either DNQX (blue, B) or vehicle (green, B) microinjections in NAc shell. Analysis of Fos expression in uninjected NAc shell (C) showed that bicuculline elevated NAc shell Fos even in the absence of NAc shell microinjections at levels more than 500% (yellow), 650% (orange), and 800% (red) of vehicle. Bar graphs indicate levels of elevated Fos at three rostrocaudal and two dorsoventral levels. * p < .05, ** p < .01 versus vehicle, # p < .05, ## p < .01 versus DNQX.
Discussion
Medial orbitofrontal amplification of subcortically generated eating
We found that activation of medial orbitofrontal cortex biased the valence of motivation generated by NAc DNQX in an appetitive direction. Activation of medial orbitofrontal neurons by bicuculline microinjection specifically enhanced the eating generated by microinjections in caudal NAc shell, which otherwise produced mostly fearful behaviors. The intensity of eating generated at caudal NAc sites rose to levels equaling those normally produced by DNQX microinjections at more rostral sites, which produced almost purely appetitive valence. However, orbitofrontal activation never generated behavior on its own, nor further enhanced the already intense eating generated by DNQX in rostral NAc. That pattern suggests that orbitofrontal cortex specifically modulates intense eating generated by NAc, up to the level of a response ceiling. Alternatively, it is possible that orbitofrontal co-activation can enhance positive valence only in the presence of a pre-existing state of negatively-valenced fear or anxiety.
In human studies of sensory rewards, neuroimaging activation of a specific mid-anterior region of orbitofrontal cortex (32) is specifically associated with subjective pleasure for food, as well as drugs, music, etc. (32, 54–58). A special role for orbitofrontal cortex in coding human pleasure seems consistent with our finding that orbitofrontal activation specifically enhanced positive incentive motivation generated by some sites of NAc (32, 59).
Infralimbic cortex suppresses subcortically generated fear and eating
We found that activation of infralimbic cortex (corresponding in humans to deeply ventral or subgenual anterior cingulate cortex; area 25) generally inhibited the intensity of both positive and negative motivations produced by glutamate disruptions in NAc shell. Thus, infralimbic activation acted primarily as a nonspecific brake: suppressing appetitive behavior elicited by disruption at rostral shell sites, and suppressing fearful behaviors elicited at caudal shell sites. Our findings therefore support the hypothesis that infralimbic cortex activation might generally regulate or inhibit the subcortical generation of intense motivations of either positive or negative valence.
This hypothesis may fit with neuroimaging evidence from humans. For instance, anterior cingulate cortex is activated when people successfully engage in voluntary efforts to suppress their aversive emotional reactions to distressing photos or to suppress their appetitive cravings to images of palatable foods, and those anterior cingulate activations are accompanied by reductions of activity in NAc, ventral tegmental area and extended amygdala otherwise triggered by viewing the same images (4–5). Infralimbic suppression of subcortically-generated motivation is also consistent with findings in rodent studies that infralimbic cortex suppresses reinstated seeking of cocaine and food rewards (38–39, 60–62), and similarly suppresses reinstatement of conditioned fear responses (41, 43, 63). Our findings extend infralimbic suppression to include intense unconditioned appetitive and fearful behaviors, which do not depend on learning nor explicitly require top-down control to be generated.
Prefrontal cortical excitation modulates but is not necessary for enhanced motivation
Our results also showed an asymmetrical role of cortical excitation versus inhibition in modulating motivations generated by inhibition of NAc shell. Excitation of medial orbitofrontal and infralimbic cortex modulated motivations released by NAc shell DNQX microinjections as described above, but inhibition of the same orbitofrontal or infralimbic cortex regions failed to alter NAc shell desire or dread in any detectable way. This asymmetrical pattern suggests that unconditioned motivations elicited by NAc shell disruptions may not need input from prefrontal cortex, but that supra-normal levels of prefrontal activation are nonetheless able to modulate these motivations. Additionally, our finding that neither prefrontal excitation nor inhibition affected normal levels of baseline eating and fearful behavior may indicate that normal levels of unconditioned eating and fear do not require prefrontal control.
Overall, our results are consistent with the notion that infralimbic cortex and orbitofrontal cortex hierarchically control NAc production of intense desire and dread (64). Prefrontal cortex acted here as hierarchically superior in the functional sense of being able to suppress and/or modulate the valence of robust motivations triggered by disruptions of NAc. At the same time, while subordinate, the NAc still possessed a degree of autonomy that is characteristic of a hierarchical element. That is, only manipulations of NAc and not prefrontal cortex were capable of producing intense levels of motivated behaviors. Such features of suppression/modulation by a hierarchically superior unit, combined with semi-autonomy of a subordinate unit, have been suggested to characterize functional hierarchies (64).
Neurobiological bases of infralimbic versus orbitofrontal top-down regulation
Local AMPA blockade by DNQX likely produces relative hyperpolarizations in NAc neurons by reducing glutamatergic depolarizations (65). This likely reduces firing and GABA release by NAc projection neurons, and thus disinhibits downstream targets neurons in ventral pallidum, lateral hypothalamus and ventral tegmentum to generate intense levels of motivated behaviors (14–18).
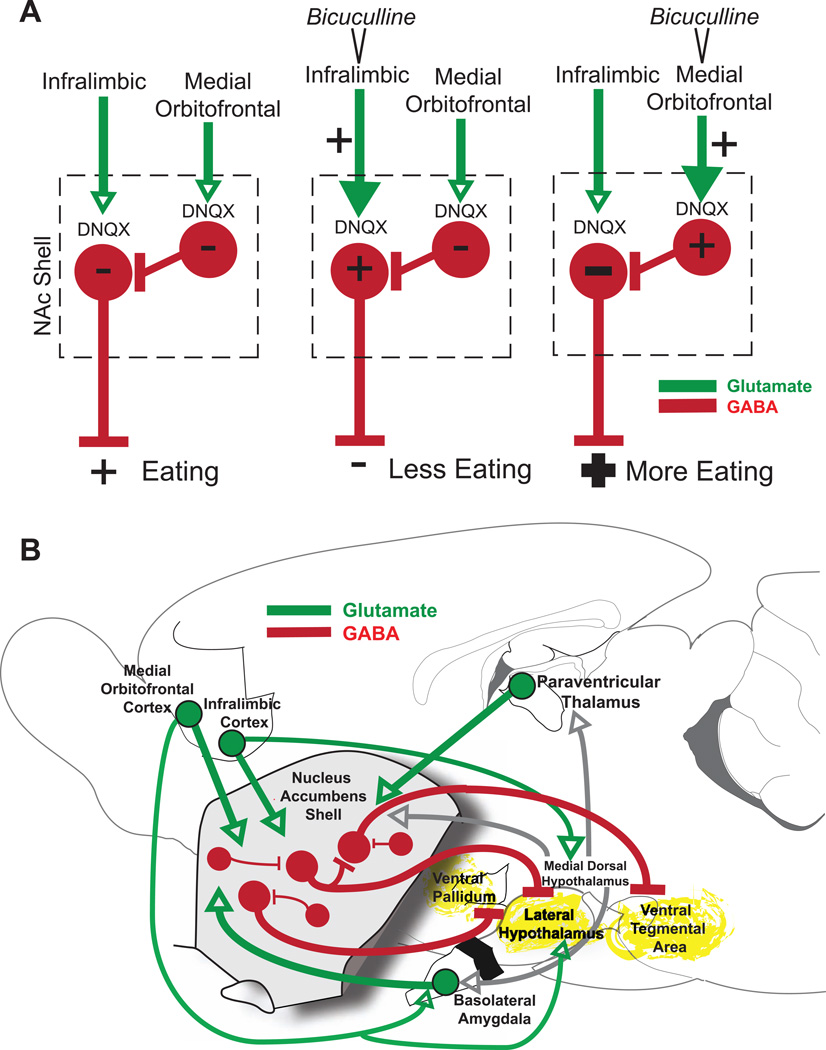
How does prefrontal cortex activation interact with NAc release of intense motivations? There may be a relatively straightforward explanation for the effects of infralimbic cortex activation, which directly opposed or suppressed NAc DNQX-induced motivation. If infralimbic activation increases glutamate release on NAc neurons that are hyperpolarized by DNQX, that elevated glutamate level may compete with and perhaps partially overcome the local hyperpolarization, thus reducing the intensity of DNQX-elicited motivations. The explanation of orbitofrontal modulation of NAc DNQX-generated eating is probably more complex. One possibility is that orbitofrontal activation may inhibit some NAc shell neurons by activation of inhibitory GABAergic interneurons. NAc shell neurons are reported to be excited by either orbitofrontal activation or infralimbic activation, but not both (66). That is, if a particular NAc neuron is excited by infralimbic cortex, it may be inhibited by medial orbitofrontal cortex (66). Conversely, if a NAc neuron is excited by orbitofrontal cortex, it is inhibited by infralimbic cortex. This mutual exclusivity suggests two parallel corticolimbic channels, with mutual inhibition between them. Thus, orbitofrontal activation may inhibit some NAc shell neurons (specifically those excited by infralimbic activation). If inhibition of these NAc shell neurons contributes to intense appetitive behaviors, then orbitofrontal activation may increase the intensity of eating by augmenting their hyperpolarization (Figure 6A). If this hypothesis is correct, then future pharmacological or optogenetic inactivation of local interneurons might modulate orbitofrontal-NAc interactions in producing intense motivated behaviors.
Figure 6. Potential mechanisms of prefrontal modulation of DNQX generated motivated behaviors.
Proposed direct prefrontal to NAc shell mechanism (A) mediating opposite infralimbic versus orbitofrontal effects on DNQX-induced eating. Bicuculline infusions excite glutamate inputs (green) from infralimbic or medial orbitofrontal cortex. DNQX alone inhibits rostral NAc shell projection neurons (red), resulting in disinhibition of downstream targets and intense eating. Infralimbic activation may overcome DNQX inhibition of these same neurons, suppressing eating. Medial orbitofrontal activation may instead activate GABAergic interneurons, which further inhibit neurons already inhibited by DNQX, potentiating eating. A circuit diagram (B) shows prefrontal and NAc shell projections to relevant third-party structures that may mediate larger circuit interactions. DNQX microinjections likely inhibit neurons in NAc shell, disinhibiting downstream structures such as ventral pallidum, lateral hypothalamus, and ventral tegmental area (yellow) via GABAergic projections neurons (red). Medial orbitofrontal and infralimbic activation may act to modulate DNQX-induced behaviors via direct glutamate (green) projections to NAc shell or parallel projections to structures such as medial dorsal hypothalamus, lateral hypothalamus and basolateral amygdala.
A second category of explanation for infralimbic or medial orbitofrontal effects goes beyond direct projections to involve indirect modulations via wider mesocorticolimbic networks, whereby prefrontal activations could recruit third-party structures to modulate the valence or intensity of intense motivations produced by NAc inhibition (including brain structures such as basolateral amygdala, lateral and medial dorsal hypothalamus or brainstem) (Figure 6B) (30, 67–70). Combined manipulations of these other subcortical structures could be used in future to test their roles in modulating prefrontal-NAc shell interactions.
Clinical implications
Improved top-down control could help deal with maladaptively intense emotions in a variety of psychopathologies involving corticolimbic circuitry, including addiction, schizophrenia and PTSD (71–74). Speculatively, treatments aimed at enhancing medial orbitofrontal function might conceivably help increase positively-valenced emotion or shift the balance away from negatively-valenced emotions. Reduced orbitofrontal volume is found in patients with schizophrenia, panic disorders, PTSD and OCD (75–79). Extrapolation of our results to such conditions would suggest that enhancing orbitofrontal activity might help add to positive appetitive motivation in cases where intense but negative valenced emotion already exists, such as in pathological anxiety or fearful paranoia.
In contrast, reduced activity in subgenual anterior cingulate cortex (homologous to infralimbic cortex in our study) has been reported to leave some patients with reduced ability to regulate certain unwanted emotions, such as in post-traumatic stress disorder (80). Additionally, abnormalities in area 25 in cocaine addicts are associated with reduced top-down control and poor decision making (81). Again speculatively applying our findings, these results support the view that activation of deep anterior cingulate area 25, homologous to infralimbic cortex here, might suppress the levels of unwanted intense emotions, regardless of whether the valence of the pathological emotion was appetitive or fearful.
In conclusion, our results suggest orbitofrontal cortex may play an important role in enhancing the positive valence of intense emotional states that might otherwise be purely fearful or anxious. Additionally, deep anterior cingulate or infralimbic cortex may be important in suppressing intense emotional states involving either desire or dread. These demonstrations of top-down hierarchical control over intense motivations generated by subcortical neural events in nucleus accumbens illustrate corticolimbic mechanisms that may contribute to regulating normal emotional well-being.
Supplementary Material
Acknowledgements
This research was supported by National Institutes of Health Grants (DA015188 and MH63649 to KCB) and by a National Research Service Award fellowship to JMR (MH090602). We thank Aaron Garcia and Stephen Burwell for assistance with immunohistochemistry, and Alexandra DiFeliceantonio and Benjamin Saunders for comments on an earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kompus K, Hugdahl K, Ohman A, Marklund P, Nyberg L. Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci Lett. 2009;467:76–80. doi: 10.1016/j.neulet.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacology Biochemistry and Behavior. 2008;90:236–249. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. Fighting food temptations: The modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage. 2012;60:213–220. doi: 10.1016/j.neuroimage.2011.12.067. [DOI] [PubMed] [Google Scholar]
- 5.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- 8.Muschamp JW, Van't Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, et al. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard JM, Berridge KC. Metabotropic glutamate receptor blockade in nucleus accumbens shell shifts affective valence towards fear and disgust. Eur J Neurosci. 2011;33:736–747. doi: 10.1111/j.1460-9568.2010.07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive tastereactivity responses by an auditory conditioned stimulus in a devaluation task: A FOS and behavioral analysis. Learn Mem. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taber MT, Fibiger HC. Feeding-evoked dopamine release in the nucleus, accumbens: regulation by glutamatergic mechanisms. Neuroscience. 1997;76:1105–1112. doi: 10.1016/s0306-4522(96)00450-2. [DOI] [PubMed] [Google Scholar]
- 15.Kelley AE. Neural integrative activities of nucleus accumbens subregions in relation to learning and motivation. Psychobiology. 1999;27:198–213. [Google Scholar]
- 16.Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–4756. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. Journal of Neuroscience. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study. Behavioural Brain Research. 1997;89:107–113. doi: 10.1016/s0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 21.Coss RG, Owings DH. Snake-Directed Behavior By Snake Naive and Experienced California Ground Squirrels in a Simulated Burrow. Zeitschrift Fur Tierpsychologie-Journal of Comparative Ethology. 1978;48:421–435. [Google Scholar]
- 22.Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacology, Biochemistry & Behavior. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. Journal of Neuroscience. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste "liking"/"disliking" reactions, place preference/avoidance, and fear. Journal of Neuroscience. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- 26.Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. J Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- 30.Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- 31.Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 33.Kringelbach ML. The hedonic brain: A functional neuroanatomy of human pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford U.K.: Oxford University Press; 2010. pp. 202–221. [Google Scholar]
- 34.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolusvulgaris leucoagglutinin. Journal of Comparative Neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 35.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 36.Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11:611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- 43.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLeod JE, Bucci DJ. Contributions of the subregions of the medial prefrontal cortex to negative occasion setting. Behav Neurosci. 2010;124:321–328. doi: 10.1037/a0019344. [DOI] [PubMed] [Google Scholar]
- 45.Hayton SJ, Lovett-Barron M, Dumont EC, Olmstead MC. Target-specific encoding of response inhibition: increased contribution of AMPA to NMDA receptors at excitatory synapses in the prefrontal cortex. J Neurosci. 2010;30:11493–11500. doi: 10.1523/JNEUROSCI.1550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 48.Markham CM, Luckett CA, Huhman KL. The medial prefrontal cortex is both necessary and sufficient for the acquisition of conditioned defeat. Neuropharmacology. 2012;62:933–939. doi: 10.1016/j.neuropharm.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, et al. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology (Berl) 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qu CL, Tang JS, Jia H. Involvement of GABAergic modulation of antinociception induced by morphine microinjected into the ventrolateral orbital cortex. Brain Res. 2006;1073–1074:281–289. doi: 10.1016/j.brainres.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 51.Dang YH, Zhao Y, Xing B, Zhao XJ, Huo FQ, Tang JS, et al. The role of dopamine receptors in ventrolateral orbital cortex-evoked anti-nociception in a rat model of neuropathic pain. Neuroscience. 2010;169:1872–1880. doi: 10.1016/j.neuroscience.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 52.Paine TA, Slipp LE, Carlezon WA., Jr Schizophrenia-like attentional deficits following blockade of prefrontal cortex GABAA receptors. Neuropsychopharmacology. 2011;36:1703–1713. doi: 10.1038/npp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aldridge JW, Berridge KC, Herman M, Zimmer L. Neuronal coding of serial order: Syntax of grooming in the neostriatum. Psychological Science. 1993;4:391–395. [Google Scholar]
- 54.Grabenhorst F, Rolls ET. Different representations of relative and absolute subjective value in the human brain. Neuroimage. 2009;48:258–268. doi: 10.1016/j.neuroimage.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 55.de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- 56.de Araujo IE, Rolls ET. Representation in the Human Brain of Food Texture and Oral Fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vollm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, et al. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology. 2004;29:1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- 58.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Ghazizadeh A, Ambroggi F, Odean N, Fields HL. Prefrontal cortex mediates extinction of responding by two distinct neural mechanisms in accumbens shell. J Neurosci. 2012;32:726–737. doi: 10.1523/JNEUROSCI.3891-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishikawa A, Ambroggi F, Nicola S, Fields H. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008;28:5088–5098. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 64.Gallistel CR. In: The organization of action: a new synthesis. Hillsdale NJ, editor. L. Erlbaum Associates; 1980. [Google Scholar]
- 65.Hu XT, White FJ. Glutamate receptor regulation of rat nucleus accumbens neurons in vivo. Synapse. 1996;23:208–218. doi: 10.1002/(SICI)1098-2396(199607)23:3<208::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 66.Asher A, Lodge DJ. Distinct prefrontal cortical regions negatively regulate evoked activity in nucleus accumbens subregions. Int J Neuropsychopharmacol. 2011:1–8. doi: 10.1017/S146114571100143X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 68.Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- 69.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 70.Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30:14102–14115. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baare WF, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry. 1999;45:1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- 76.Asami T, Yamasue H, Hayano F, Nakamura M, Uehara K, Otsuka T, et al. Sexually dimorphic gray matter volume reduction in patients with panic disorder. Psychiatry Res. 2009;173:128–134. doi: 10.1016/j.pscychresns.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- 78.Roppongi T, Nakamura M, Asami T, Hayano F, Otsuka T, Uehara K, et al. Posterior orbitofrontal sulcogyral pattern associated with orbitofrontal cortex volume reduction and anxiety trait in panic disorder. Psychiatry Clin Neurosci. 2010;64:318–326. doi: 10.1111/j.1440-1819.2010.02085.x. [DOI] [PubMed] [Google Scholar]
- 79.Sobanski T, Wagner G, Peikert G, Gruhn U, Schluttig K, Sauer H, et al. Temporal and right frontal lobe alterations in panic disorder: a quantitative volumetric and voxel-based morphometric MRI study. Psychol Med. 2010;40:1879–1886. doi: 10.1017/S0033291709991930. [DOI] [PubMed] [Google Scholar]
- 80.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biological Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.