Abstract
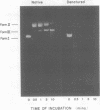
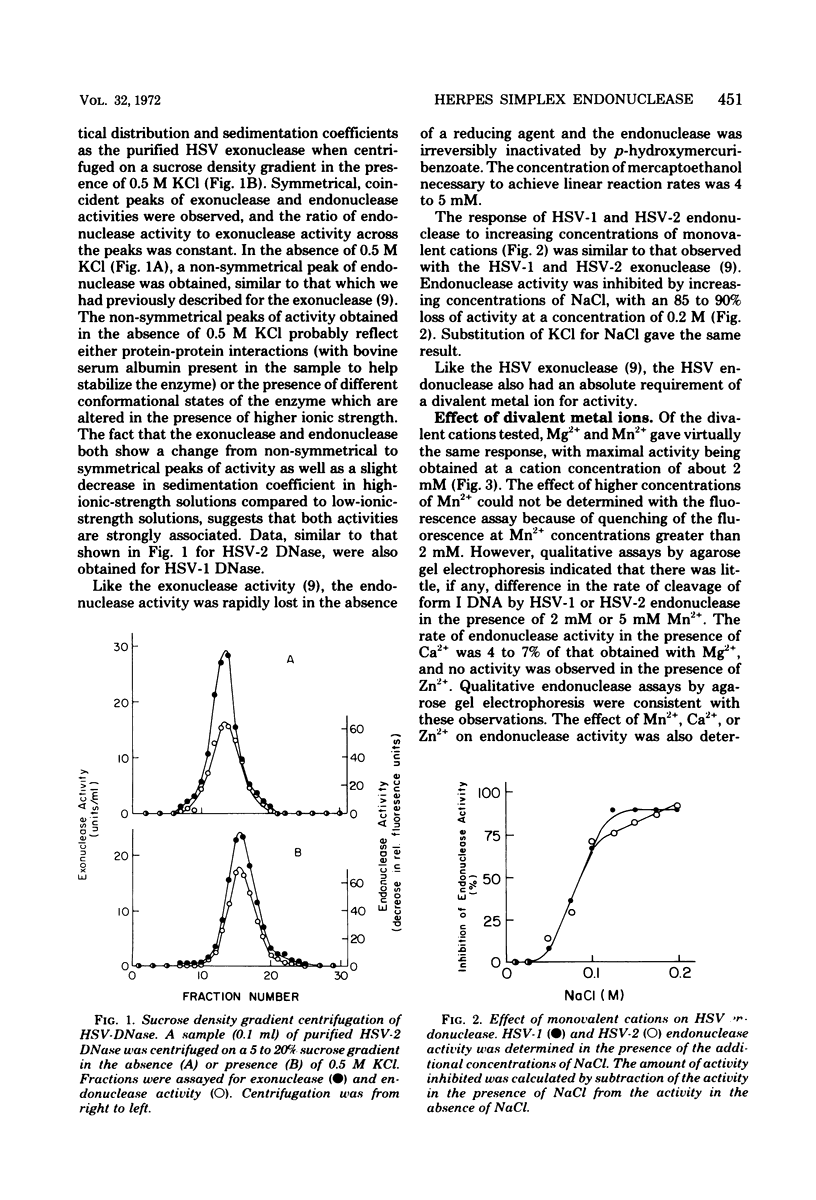
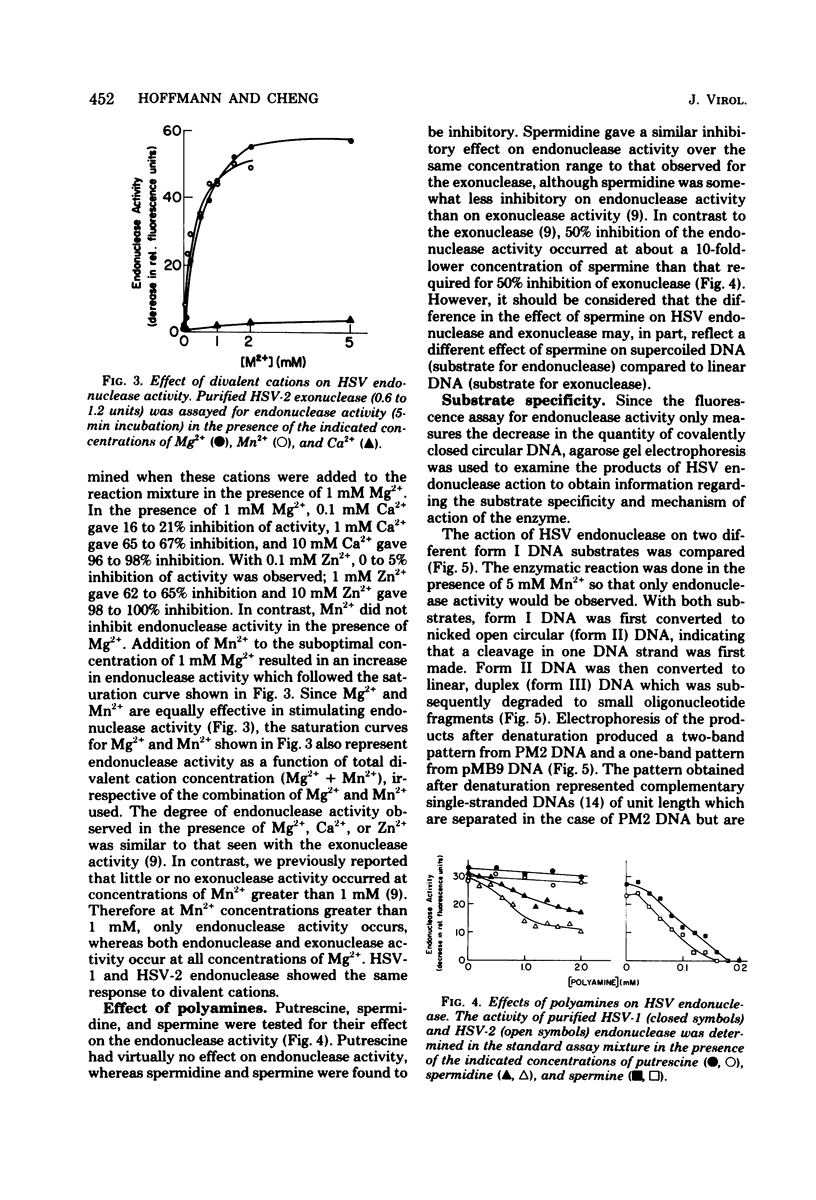
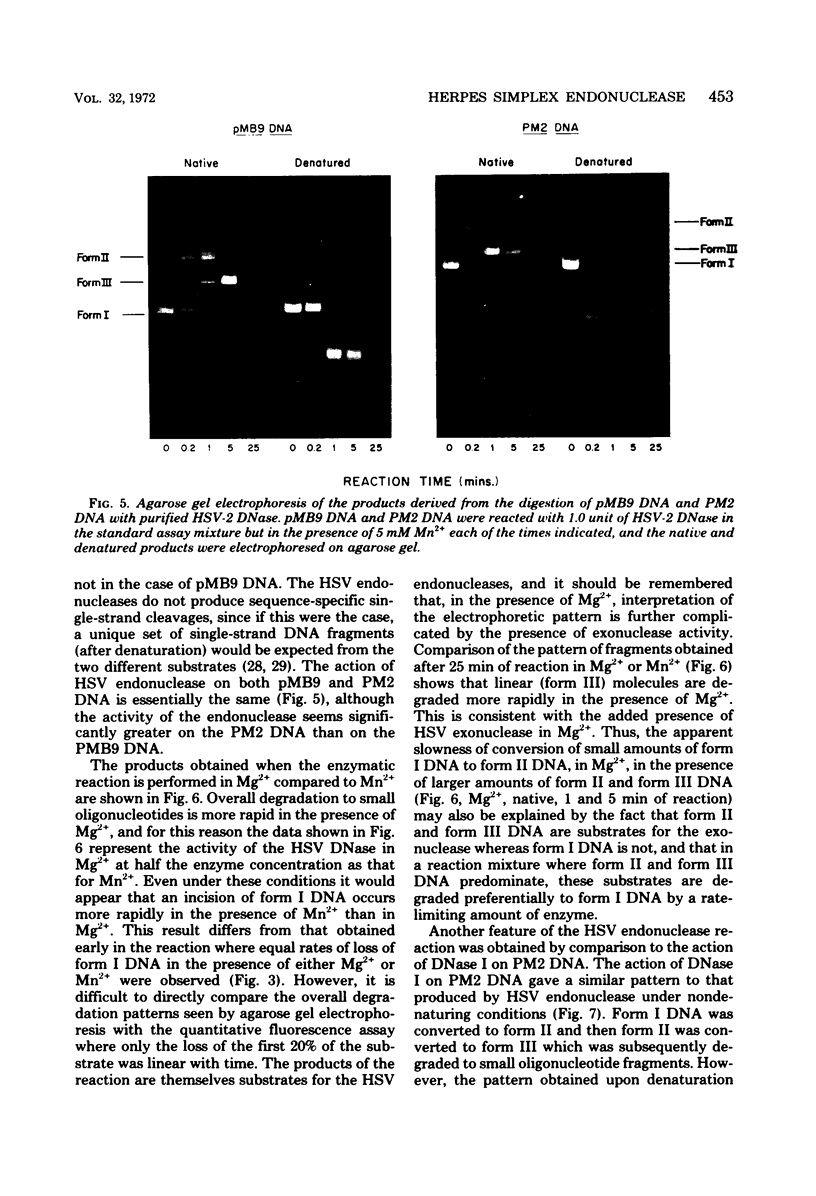
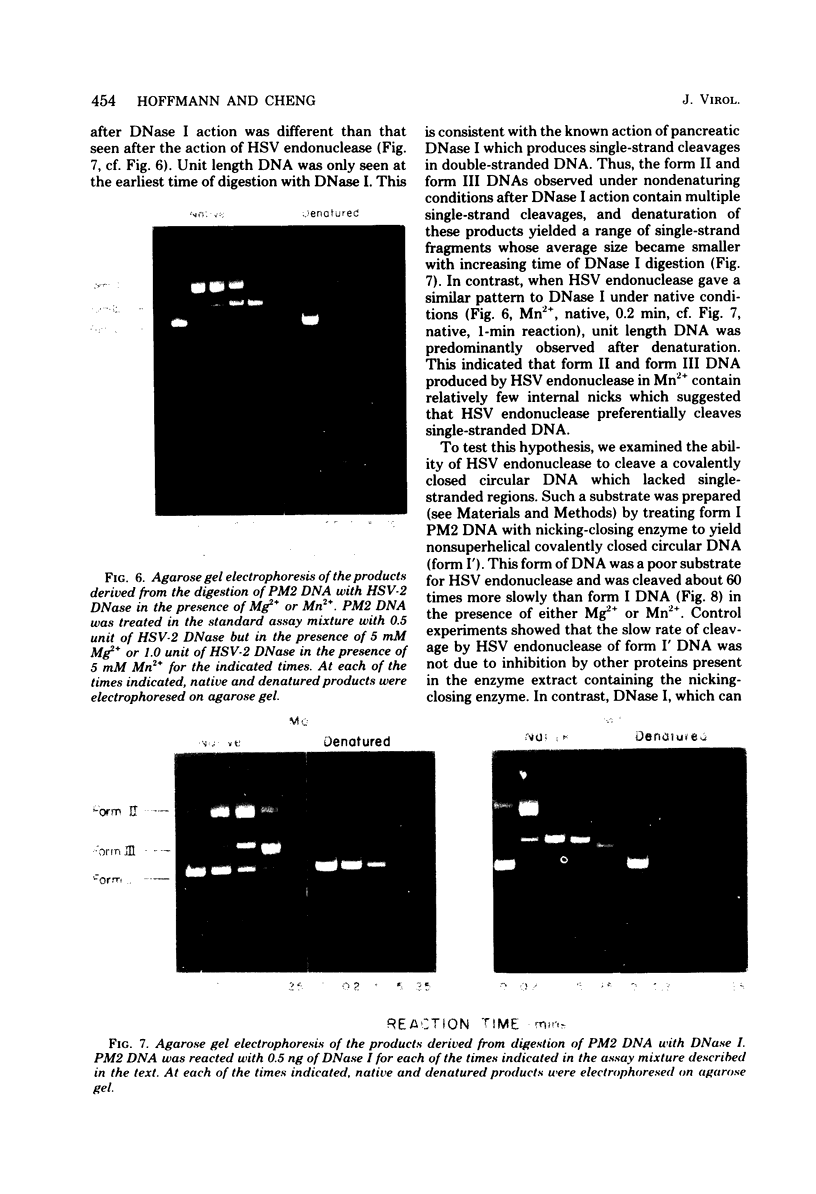
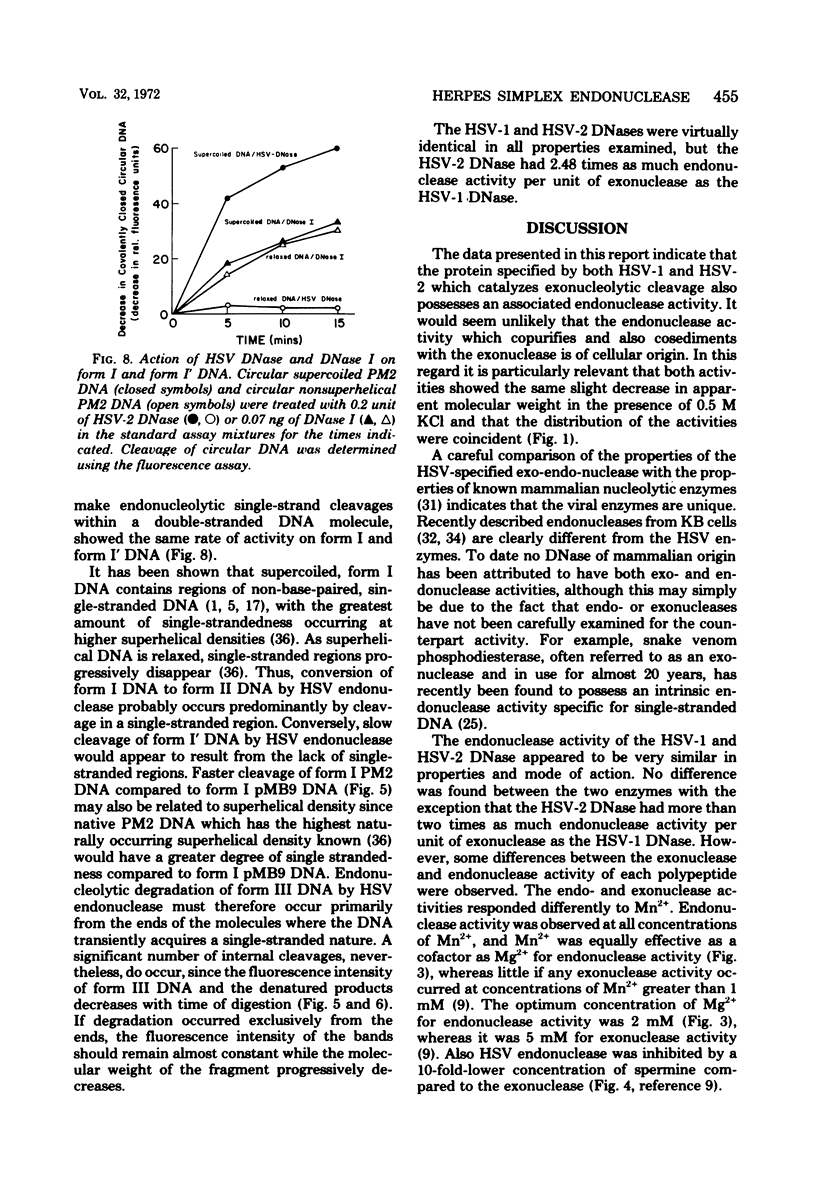
Purified preparations of the “exonuclease” specified by herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) possess an endonuclease activity. The exonuclease and endonuclease activities copurify and cosediment in a sucrose density gradient. Endonuclease activity is only observed in the presence of a divalent cation, and Mg2+ or Mn2+ is equally effective as a cofactor with an optimal concentration of 2 mM. A slight amount of endonuclease activity is observed in the presence of Ca2+, whereas no activity occurs in the presence of Zn2+. In the presence of Mg2+, Ca2+ and Zn2+ are inhibitory. Comparison of exonuclease and endonuclease activity in the presence of various divalent cations revealed that, at concentrations of Mn2+ greater than 1 mM, only endonuclease activity occurs whereas endonuclease and exonuclease activity occur at all concentrations of Mg2+. The endonuclease was affected by putrescine and spermidine to the same extent as the exonuclease activity, but in marked contrast the endonuclease was inhibited by a 10-fold-lower concentration of spermine compared to the exonuclease. The activity specified by HSV-1 and HSV-2 has very similar properties. HSV-1 and HSV-2 endonuclease cleave covalently closed circular DNA to yield, firstly, nicked circles and then linear DNA which is subsequently hydrolyzed to small oligonucleotides. Cleavage does not appear to be base sequence specific. Conversion of nicked circles to linear DNA and subsequent degradation of linear DNA occurs more rapidly in the presence of Mg2+ than Mn2+ presumably by virtue of the presence of the exonuclease activity. Nonsuperhelical covalently closed circular duplex DNA is cleaved by the endonucleases at a rate 60 times slower than the rate observed on the supercoiled form. These data indicate that the HSV-1 and HSV-2 endonuclease preferentially recognize single-stranded DNA regions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beerman T. A., Lebowitz J. Further analysis of the altered secondary structure of superhelical DNA. Sensitivity to methylmercuric hydroxide a chemical probe for unpaired bases. J Mol Biol. 1973 Sep 25;79(3):451–470. doi: 10.1016/0022-2836(73)90398-7. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Tokazewski S. A. Replication of herpesvirus DNA. II. Sedimentation characteristics of newly synthesized DNA. Virology. 1977 Jun 15;79(2):292–301. doi: 10.1016/0042-6822(77)90356-7. [DOI] [PubMed] [Google Scholar]
- Bode V. C. Single-strand scissions induced in circular and linear lambda DNA by the presence of dithiothreitol and other reducing agents. J Mol Biol. 1967 May 28;26(1):125–129. doi: 10.1016/0022-2836(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Dean W. W., Lebowitz J. Partial alteration of secondary structure in native superhelical DNA. Nat New Biol. 1971 May 5;231(18):5–8. [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. The deoxyribonuclease induced after infection of KB cells by herpes simplex virus type 1 or type 2. I. Purification and characterization of the enzyme. J Biol Chem. 1978 May 25;253(10):3557–3562. [PubMed] [Google Scholar]
- Hsu M., Berg P. Altering the specificity of restriction endonuclease: effect of replacing Mg2+ with Mn2+. Biochemistry. 1978 Jan 10;17(1):131–138. doi: 10.1021/bi00594a019. [DOI] [PubMed] [Google Scholar]
- Jacob R. J., Morse L. S., Roizman B. Anatomy of herpes simplex virus DNA. XII. Accumulation of head-to-tail concatemers in nuclei of infected cells and their role in the generation of the four isomeric arrangements of viral DNA. J Virol. 1979 Feb;29(2):448–457. doi: 10.1128/jvi.29.2.448-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R. J., Roizman B. Anatomy of herpes simplex virus DNA VIII. Properties of the replicating DNA. J Virol. 1977 Aug;23(2):394–411. doi: 10.1128/jvi.23.2.394-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean J. H., Blankenship M. L., Ben-Porat T. Replication of herpesvirus DNA. I. Electron microscopic analysis of replicative structures. Virology. 1977 Jun 15;79(2):281–291. doi: 10.1016/0042-6822(77)90355-5. [DOI] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Johnston J. V., Nichols B. P., Donelson J. E. Distribution of "minor" nicks in bacteriophage T5 DNA. J Virol. 1977 May;22(2):510–519. doi: 10.1128/jvi.22.2.510-519.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Effect of neocarzinostatin-induced strand scission on the template activity of DNA for DNA polymerase I. Biochemistry. 1977 Feb 8;16(3):479–485. doi: 10.1021/bi00622a022. [DOI] [PubMed] [Google Scholar]
- Kato A. C., Bartok K., Fraser M. J., Denhardt D. T. Sensitivity of superhelical DNA to a single-strand specific endonuclease. Biochim Biophys Acta. 1973 Apr 21;308(7):68–78. doi: 10.1016/0005-2787(73)90123-8. [DOI] [PubMed] [Google Scholar]
- McGuire M. S., Center M. S., Consigli R. A. Purification and properties of an endonuclease from nuclei of uninfected and polyoma-infected 3T3 cells. J Biol Chem. 1976 Dec 25;251(24):7746–7752. [PubMed] [Google Scholar]
- Melgar E., Goldthwait D. A. Deoxyribonucleic acid nucleases. II. The effects of metals on the mechanism of action of deoxyribonuclease I. J Biol Chem. 1968 Sep 10;243(17):4409–4416. [PubMed] [Google Scholar]
- Morgan A. R., Pulleyblank D. E. Native and denatured DNA, cross-linked and palindromic DNA and circular covalently-closed DNA analysed by a sensitive fluorometric procedure. Biochem Biophys Res Commun. 1974 Nov 27;61(2):396–403. doi: 10.1016/0006-291x(74)90970-x. [DOI] [PubMed] [Google Scholar]
- Morrison J. M., Keir H. M. A new DNA-exonuclease in cells infected with herpes virus: partial purification and properties of the enzyme. J Gen Virol. 1968 Dec;3(3):337–347. doi: 10.1099/0022-1317-3-3-337. [DOI] [PubMed] [Google Scholar]
- Paddock G. V., Heindell H. C., Salser W. Deoxysubstitution in RNA by RNA polymerase in vitro: a new approach to nucleotide sequence determinations. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5017–5021. doi: 10.1073/pnas.71.12.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard A. E., Kowalski D., Laskowski M., Sr An endonuclease activity of venom phosphodiesterase specific for single-stranded and superhelical DNA. J Biol Chem. 1977 Dec 10;252(23):8652–8659. [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. Partial purification of "omega" protein from calf thymus. Biochemistry. 1975 Nov 18;14(23):5205–5209. doi: 10.1021/bi00694a029. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Rogers S. G., Rhoades M. Bacteriophage T5-induced endonucleases that introduce site-specific single-chain interruptions in duplex DNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1576–1580. doi: 10.1073/pnas.73.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salditt M., Braunstein S. N., Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. X. Improved techniques for the purification of bacteriophage PM2. Virology. 1972 Apr;48(1):259–262. doi: 10.1016/0042-6822(72)90133-x. [DOI] [PubMed] [Google Scholar]
- Sierakowska H., Shugar D. Mammalian nucleolytic enzymes. Prog Nucleic Acid Res Mol Biol. 1977;20:59–130. doi: 10.1016/s0079-6603(08)60470-5. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Arens M., Padmanabhan R., Green M. An endodeoxyribonuclease of human KB cells. Purification and properties of the enzyme. J Biol Chem. 1978 May 25;253(10):3400–3407. [PubMed] [Google Scholar]
- Vosberg H. P., Vinograd J. Purification and demonstration of the enzymatic character of the nicking-closing protein from mouse L cells. Biochem Biophys Res Commun. 1976 Jan 26;68(2):456–464. doi: 10.1016/0006-291x(76)91167-0. [DOI] [PubMed] [Google Scholar]
- Wang E. C., Furth J. J., Rose J. A. Purification and characterization of a DNA single strand specific endonculease from human cells. Biochemistry. 1978 Feb 7;17(3):544–549. doi: 10.1021/bi00596a027. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Woodworth-Gutai M., Lebowitz J. Introduction of interrupted secondary structure in supercoiled DNA as a function of superhelix density: consideration of hairpin structures in superhelical DNA. J Virol. 1976 Apr;18(1):195–204. doi: 10.1128/jvi.18.1.195-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]