Abstract
The inability to generate broadly neutralizing antibody (bnAb) responses to the membrane proximal external region (MPER) of HIV-1 gp41 using current vaccine strategies has hampered efforts to prevent the spread of HIV. To address this challenge, we investigated a novel hypothesis to help improve the anti-MPER antibody response. Guided by structural insights and the unique lipid reactivity of anti-MPER bnAbs, we considered whether amino acid side chain modifications that emulate hydrophilic phospholipid head groups could contribute to the generation of 2F5-like or 4E10-like neutralizing anti-MPER antibodies. To test this hypothesis, we generated a series of chemically modified MPER immunogens through derivatization of amino acid side chains with phosphate or nitrate groups. We evaluated the binding affinity of the chemically modified peptides to their cognate monoclonal antibodies, 2F5 and 4E10, using surface plasmon resonance. The modifications had little effect on binding to the antibodies and did not influence epitope secondary structure when presented in liposomes. We selected five of the chemically modified sequences to immunize rabbits and found that an immunogen containing both the 2F5 and 4E10 epitopes and a phosphorylated threonine at T676 elicited the highest anti-peptide IgG titers, although the high antipeptide titers did not confer higher neutralizing activity. These data indicate that side chain modifications adjacent to known neutralizing antibody epitopes are capable of eliciting antibody responses to the MPER but that these chemically modified gp41 epitopes do not induce neutralizing antibodies.
INTRODUCTION
An effective HIV vaccine will require both humoral and cellular immune responses to prevent infection (1–3). Progress toward an HIV vaccine has been slow; the Merck trial of a recombinant adenoviral vaccine failed to protect against infection (4), and efforts in the RV144 Thai trial to elicit neutralizing antibodies showed only modest efficacy (5). A small number of broadly neutralizing antibodies (bnAbs) isolated from HIV-infected patients have guided the rational design of immunogens that might be suitable for an HIV vaccine (6–12). Three of the more potent bnAbs (2F5, 4E10, and Z13) are directed to the membrane proximal external region (MPER) of gp41, comprised of 35 amino acids N terminal to the transmembrane domain (Fig. 1) (13). The MPER is conserved across viral clades and essential for virus-cell fusion (14–16). However, with the exception of a few recent reports (17–19), MPER immunogens have failed to elicit antibodies, and none have had the breadth or potency of patient-derived bnAbs (13).
Fig 1.
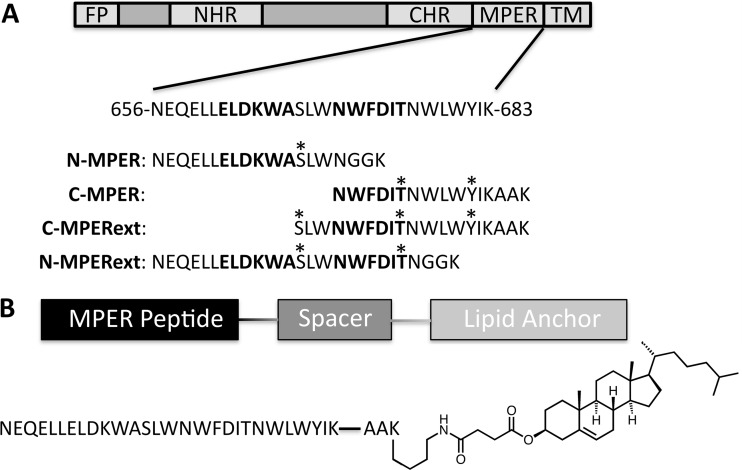
MPER peptides and MPER-CHEMS lipopeptides. (A) N-MPER and C-MPER contained the nominal epitopes of monoclonal antibodies 2F5 and 4E10, respectively, with additional flanking sequences previously reported to improve binding (43, 58). The C terminus is amended with a two-residue linker and a lysine for on-resin lipid conjugation. Residues that were modified with phospho or nitro groups are indicated with asterisks. (B) Lipopeptide structure denoting the peptide, linker, and cholesteryl hemisuccinate lipid anchor. FP, fusion protein; NHR, N-heptad region; CHR, C-heptad region; TM, transmembrance domain.
Weak antibody responses and a lack of structural definition are primary concerns with MPER-based immunogens (13). MPER-specific antibodies are rare in infected patients, and highly immunogenic scaffolds grafted with MPER sequences have failed to elicit detectable MPER reactivity in animals (20–22). Haynes and Alam (23) and Zwick (24) have suggested that antibody responses to the MPER are limited by tolerance mechanisms, which is supported by the cross-reactivity of MPER-targeted bnAbs with phospholipids (25–28). Moreover, these antibodies contain unusually long, hydrophobic heavy-chain complementarity-determining region 3 (CDRH3) sequences, which are necessary for viral neutralization by these bnAbs (29). In humans, antibodies with long CDRH3 segments are typically deleted in the bone marrow due to their autoreactive character, which could explain the rarity of 2F5-like and 4E10-like bnAbs (30). However, therapeutic use of 2F5 and 4E10 has shown no immunological side effects and an excellent overall safety profile (31–36). Furthermore, the interaction of 2F5 with unilamellar phospholipid vesicles is dependent on the presence of the MPER sequence in the bilayer (37). Alternative explanations for the rarity of MPER antibodies may include the immunodominance of the gp120 variable loops (38), the rapidity of conformational changes that expose the MPER (39), masking by nonneutralizing cluster II epitopes (40), or a bias in the germ line antibody repertoire (41). However, responses from B-cell clones against gp41 are distributed across clusters I, II, and IV, suggesting that epitope masking is not the cause for failure to neutralize the virus (42).
Lipid cross-reactivity is essential for broad neutralization by MPER-specific antibodies, but vaccine delivery strategies employing MPER-containing peptides or recombinant proteins formulated in lipid bilayers have not resulted in robust neutralization (13, 27, 43). We considered a novel alternative approach to the rational design of MPER immunogens: incorporation of amino acid side chain modifications that emulate hydrophilic phospholipid head groups. Moreover, posttranslational modifications (PTMs) such as phosphorylation and nitration are known to augment immune responses against antigens in cancer, autoimmunity, and infectious diseases (44–49). Although these modifications have not been observed during HIV infection, it is conceivable that PTMs resulting from the inflammatory milieu during HIV infection could contribute to altered anti-MPER antibody responses in a subset of patients.
The Szoka laboratory previously reported MPER-derived lipopeptides that are potently immunogenic when presented in lipid bilayer vesicles (50, 51). In the present study, we generated a series of MPER lipopeptide immunogens bearing anionic side chain modifications that could mimic hydrophilic phospholipid head groups or inflammation-associated PTMs. Phosphorylated, nitrated, and carboxylated peptides were investigated for binding to monoclonal antibodies 2F5 and 4E10. The peptides were then presented in liposomal formulations to analyze the effects of each modification on differences in secondary structure and the ability to elicit anti-MPER neutralizing antibodies in vivo.
MATERIALS AND METHODS
Amino acids, resins, and coupling agents were obtained from Novabiochem (Darmstadt, Germany), Anaspec (San Jose, CA), or ChemPep (Miami, FL). Cholesterol, dimyristoylphosphatidylcholine (DMPC), and dimyristoylphosphatidylglycerol (DMPG) were obtained from Avanti Polar Lipids (Alabaster, AL). Cholesteryl hemisuccinate (CHEMS; C6512) and monophosphoryl lipid A derived from Escherichia coli (MPL; L6638) were obtained from Sigma-Aldrich (St. Louis, MO). Peptides and lipopeptides were synthesized and formulated in liposomes as described previously (51). Phosphorylated and nitrated residues were incorporated by addition of amino acids with side chains derivatized with O-benzyl-protected phosphate or 9-fluorenylmethoxy carbonyl–3-nitro-tyrosine-OH (Bachem, Torrence, CA), respectively.
Surface plasmon resonance (SPR) kinetic binding analysis.
A Biacore T100 instrument (GE Healthcare) was used in all experiments. Monoclonal antibodies (MAbs) 2F5 and 4E10 (NIH AIDS Research & Reference Reagent Program) were covalently coupled to Biacore CM5 series S sensor chips at final densities of ∼4,000 response units (RU). Peptides were flowed over as analytes with 2-fold dilutions ranging from 200 to 12.5 nM when evaluating peptide affinity for 2F5 and 2,000 to 125 nM when evaluating peptide affinity for 4E10. Peptides were prepared in running buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.01% Tween 20) and evaluated at a flow rate of 30 μl/min for 90 s, followed by injection of running buffer for 2 min. Binding was analyzed using BiaEvaluation software (GE Healthcare).
Circular dichroism spectroscopy.
Stock liposome solutions containing 5 mM carrier lipid and 500 μM lipopeptide were prepared in 10 mM phosphate, pH 7.4. To minimize light scattering, liposomes were prepared by bath sonication under argon until a size of less than 100 nm was obtained. For analysis, samples were diluted to 5 μM lipopeptide in 10 mM phosphate buffer containing 1 mM carrier lipid. Spectra were obtained with a J-715 spectropolarimeter (Jasco, Easton, MD), and data were processed using Jasco software. Data were acquired in continuous scanning mode with a 1-cm path length, a 0.1-nm interval, and a 1-nm/s scan speed. Each spectrum represents an average of three scans in triplicate. A background spectrum of empty liposomes in buffer was subtracted from each sample spectrum. Percent helicity was estimated from the mean residue ellipticity at 222 nm according to the method of Taylor and Kaiser (52).
Rabbit immunizations.
Rabbit studies were performed by Covance Inc. (Denver, PA). Specific-pathogen-free New Zealand White rabbits received subcutaneous immunizations on days 0, 21, 42, 63, 84, and 105. Blood was collected from the ear vein on days 31, 52, 73, 94, and 115. Injections on day 0 contained 250 μg lipopeptide, 125 μg MPL, and 10 μmol lipid vehicle in 250 μl sterile phosphate-buffered saline emulsified in 250 μl Freund's complete adjuvant. Subsequent injections contained 125 μl of the formulation described above emulsified in 125 μl Freund's incomplete adjuvant. Alving and coworkers have demonstrated that liposomes are compatible with oil-in-water emulsions (53). After blood draw, cells were removed by centrifugation and sera were sent to the University of California, San Francisco (UCSF), on dry ice and stored at −80°C.
Enzyme-linked immunosorbent assays (ELISAs) were performed on antisera as previously described (51). The titer was defined as the reciprocal dilution of antisera yielding an optical density twice that of the background. Samples were assayed in duplicate. Statistical significance was assessed by analysis of variance with Bonferroni posttest analysis, and values were considered significant when P was <0.05. Data analyses were performed using Prism software (GraphPad Software, Inc.).
Virus neutralization assay.
Neutralization was determined by a reduction in luciferase reporter gene expression after infection of either TZM-bl cells (54) or A3R5 cells. Neutralization in TZM-bl cells tested a virus pseudotyped with the Env of a clade B tier 1 virus (SF162.LS); murine leukemia virus Env-pseudotyped virus was used as a negative control. Assays in A3R5 cells utilized four clade B tier 2 viruses (SC22.3C2, RHPA, CH77, CH58) as infectious molecular clones containing a Tat-regulated Renilla luciferase reporter gene (IMC.LucR viruses) (55). Neutralization titer was defined as the sample dilution at which relative luminescence units (RLUs) were reduced by 50% compared to the numbers of RLUs in virus control wells after subtraction of background RLUs in cell control wells (50% infectious dose). Sera were heat inactivated (60 min, 56°C) before use.
RESULTS AND DISCUSSION
The MPER of gp41 has been investigated as a potential target for vaccine development (13). However, antibody responses to MPER immunogens, particularly the C terminus, have been weak, and few studies have reported elicitation of neutralizing antibodies with MPER immunogens (17, 56, 57). In an attempt to overcome the weak immune responses to the MPER and generate neutralizing antibodies, we designed a series of lipopeptide immunogens bearing anionic side chain modifications that may recapitulate the immunogenic propensity of phospholipid head groups or inflammation-associated PTMs and thereby elicit 2F5-like or 4E10-like bnAbs.
Design of chemically modified MPER peptides and lipopeptides.
The crystal structures of bnAbs 2F5 (Protein Data Bank [PDB] accession number 1TJG) (43) and 4E10 (PDB accession number 2FX7) (58) in complex with their corresponding peptide epitopes suggest that modifications of the epitopes at selected amino acids may promote favorable contacts with the antibody's extended CDRH3 loops capable of interacting with such a modification. Furthermore, the binding pocket of 2F5 is largely cationic, with arginine and lysine residues surrounding the epitope, suggesting that an anionic charge may improve affinity (Fig. 2A). Likewise, 4E10 has an arginine at the base of the epitope-binding pocket, which appears to be too distant from the threonine in the 4E10 epitope to hydrogen bond (Fig. 2B); however, a phosphate group may introduce additional charge interactions. These structural insights were used to design a series of chemically modified MPER peptides and lipopeptides corresponding to the 2F5 epitope (N-MPER and N-MPERext) and the 4E10 epitope (C-MPER and C-MPERext) (summarized in Fig. 1A) with side chain modifications incorporated at available residues (serine, threonine, and tyrosine). A summary of the nomenclature and molecular weight data for the peptides and lipopeptides are reported in Table 1.
Fig 2.
Crystal structures of 2F5 and 4E10 with corresponding epitopes. (A) Monoclonal antibody 2F5 with epitope showing arginines at positions 58.H and 95.H and histidine at position 96.L in the main chains of the antibodies and 100H.H in the CDRH3 loop. Adapted from PDB accession number 1TJG (43). (B) Monoclonal antibody 4E10 with epitope showing arginines at positions 73.H and 94.H, lysines at 100E.H and 32.L, and histidine at position 102.H. Adapted from PDB accession number 2FX7 (58). Antibodies are denoted in red, with bound epitopes denoted in green. Cationic R groups surrounding the antibody-binding pocket are shown in cyan, with nitrogen atoms shown in blue. Serine and threonine in the respective antibodies are shown in cyan, with oxygen shown in red.
Table 1.
Nomenclature and molecular weights of peptides and lipopeptides
| Name | Sequence | Linker | Modification | Mol wt |
|||
|---|---|---|---|---|---|---|---|
| Free peptide |
CHEMS conjugated |
||||||
| Experimental | Observed | Experimental | Observed | ||||
| C-MPER | 671–683 | AA | None | 2,537.1 | 2,539.6 | ||
| C-MPERext | 667–683 | AA | None | 2,526.0 | 2,550.1 (Na+) | 2,994.6 | 2,993.9 |
| C-MPERext(S-PO3) | 667–683 | AA | S-PO3 | 2,605.0 | 2,609.3 | 3,077.6 | 3,075.2 |
| C-MPER(Y-PO3) | 671–683 | AA | Y-PO3 | 2,147.4 | 2,151.0 | 2,615.1 | 2,617.0 |
| C-MPER(Y-NO2) | 671–683 | AA | Y-NO2 | 2,113.4 | 2,106.7 | 2,585.2 | 2,583.2 |
| N-MPER | 656–671 | GG | None | 2,271.5 | 2,273.5 | 2,698.3 | 2,718.2 (Na+) |
| N-MPER(D) | 656–677 | GG | S668D | 2,299.5 | 2,307.2 | ||
| N-MPER(E) | 656–677 | GG | S668E | 2,313.6 | 2,336.8 (Na+) | ||
| N-MPER(S-PO3) | 656–671 | GG | S-PO3 | 2,308.5 | 2,313.6 | 2,776.2 | 2,776.7 |
| N-MPERext(T-PO3) | 656–677 | GG | T-PO3 | 3,085.4 | 3,087.3 | 3,554.0 | 3,556.3 |
Surface plasmon resonance kinetic binding studies.
Previous studies have identified MPER residues necessary for binding through natural amino acid mutations (59), and unnatural modifications that introduce structural constraints have also been investigated (60, 61). However, immunogens bearing phosphate, nitrate, and carboxylate chemical modifications of gp41 have not been reported. We measured the effects of these modifications on the binding affinity of MPER peptides to bnAbs 2F5 and 4E10 using SPR. Peptides containing the 2F5 epitope bound to 2F5 with ∼10 nM affinity, while peptides containing the 4E10 epitope bound to 4E10 with ∼50 nM affinity (Table 2). The binding affinities for the unmodified peptides were consistent with those reported previously (58, 62). Side chain modifications generally did not influence binding affinity, with one notable exception: phosphorylation of the 4E10 epitope T676 completely inhibited binding to MAb 4E10. Representative sensorgrams are shown in Fig. S1 in the supplemental material.
Table 2.
Kinetic binding data obtained from surface plasmon resonance analysis of peptides interacting with MAbs 2F5 and 4E10
| MAb | Name | Sequence | Modification | KDa (nM) |
|---|---|---|---|---|
| 2F5 | N-MPER | 656–671 | None | 8 |
| N-MPER(D) | 656–677 | S668D | 9 | |
| N-MPER(E) | 656–677 | S668E | 7 | |
| N-MPER(S-PO3) | 656–671 | S-PO3 | 3 | |
| N-MPERext(T-PO3) | 656–677 | T-PO3 | 34 | |
| 4E10 | N-MPERext(T-PO3) | 656–677 | T-PO3 | DNBb |
| C-MPERext | 667–683 | None | 61 | |
| C-MPERext(S-PO3) | 667–683 | S-PO3 | 40 | |
| C-MPER(Y-PO3) | 671–683 | Y-PO3 | 50 | |
| C-MPER(Y-NO2) | 671–683 | Y-NO2 | 121 |
KD, affinity constant.
DNB, does not bind.
Effect of side chain modification on peptide secondary structure.
We then determined if the modifications altered the secondary structure of the lipopeptides when presented in a lipid bilayer. The 4E10 epitope binds to the antibody in an alpha helical conformation, while the 2F5 antibody binds to a beta turn consisting of the DKW motif (43, 58). Presentation of structures similar to those that induced 2F5 and 4E10 may lead to a more robust neutralizing response. We have previously shown that the selection of the lipid anchor and lipid conjugation site used to present the peptides in the liposomes affects helicity (51). Here, we show that the side chain modifications have little effect on the secondary structure of MPER peptides, as summarized in Table 3 and Fig. S2 in the supplemental material. In general, unmodified lipopeptides had very little helical content, and side chain modifications did not significantly impact helicity. The lipopeptides exhibiting the highest helical content were N-MPER-CHEMS and C-MPER(Y-PO3)-CHEMS, both of which contained greater than 25% helical character when formulated in phospholipid bilayer vesicles.
Table 3.
Percent helicity of lipopeptides presented in liposomes determined by circular dichroism
| Lipopeptide | θ222a | % helicity |
|---|---|---|
| N-MPER-CHEMS | −8,411 ± 287 | 29 |
| N-MPER(S-PO3)-CHEMS | −4,493 ± 80 | 19 |
| N-MPERext(T-PO3)-CHEMS | −3,571 ± 85 | 17 |
| C-MPER-CHEMS | −824 ± 113 | 10 |
| C-MPER(Y-PO3)-CHEMS | −6,918 ± 202 | 25 |
| C-MPER(Y-NO-2)-CHEMS | −2,170 ± 294 | 13 |
| C-MPERext-CHEMS | −4,019 ± 114 | 18 |
| C-MPERext(S-PO3)-CHEMS | −1,389 ± 5 | 11 |
Mean ellipticity at 222 nm.
Effect of side chain modifications on antibody responses to MPER lipopeptides in New Zealand White rabbits.
Five chemically modified MPER lipopeptides were selected to evaluate whether side chain modifications influence the immunogenicity of MPER lipopeptides in rabbits. The peptides used for rabbit immunizations included an unmodified peptide containing both epitopes (N-MPERext), two peptides containing the 4E10 epitope with modifications N terminal [C-MPERext(S-PO3)] and C-terminal [C-MPER(YNO2)] to the epitope, and two peptides containing the 2F5 epitope with C-terminal modifications adjacent to [N-MPER(S-PO3)] or distant from [N-MPER(T-PO3)] the epitope. Furthermore, we included N-MPERext(T-PO3) in the rabbit immunizations to evaluate a peptide to which the 4E10 MAb failed to bind but that could potentially induce modified epitope-specific polyclonal antibodies.
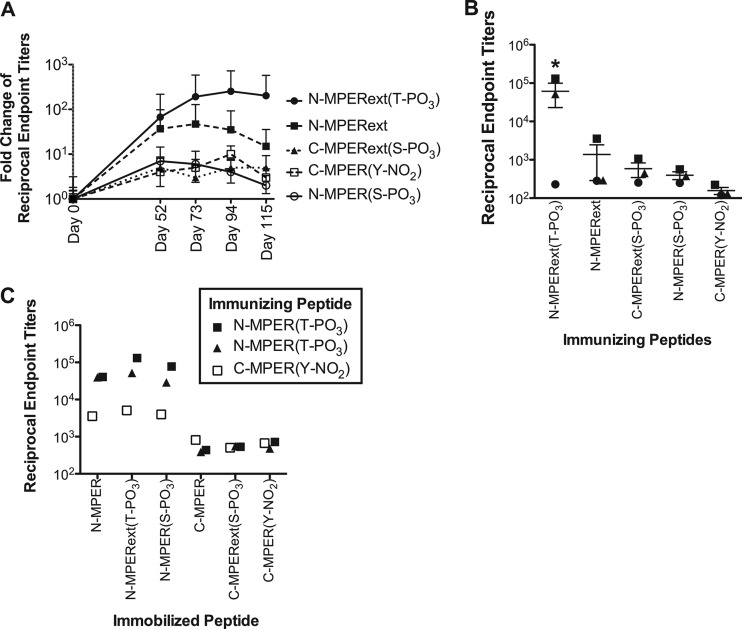
Antibody responses against the extended epitope containing a phosphorylated threonine [N-MPERext(T-PO3)] provided the highest response, with 2 of 3 rabbits responding with a mean titer of 6.1 × 104. For each group, the fold change in average antibody titers compared to day 0 titers over time are shown in Fig. 3A, and the endpoint antibody titers of individual animals are shown in Fig. 3B. Antiserum titers over time postimmunization are shown in Fig. S3 in the supplemental material. It is important to note that one rabbit from the N-MPERext(T-PO3) group, with an endpoint titer of 1.3 × 105, had an unexpectedly high epitope-specific antibody titer prior to immunization. It is unclear why this animal had an elevated preimmunization titer, but as a result, this animal exhibited a 160-fold increase in titer over the course of the study, compared to a >1,500-fold increase in the other responder (endpoint titer, 5.2 × 104) from this group. The analogous unmodified peptide, N-MPERext, elicited a 110-fold increase in just one rabbit (3.6 × 103), while the two other rabbits failed to respond. All other rabbits showed less than a 15-fold increase over preimmunization titers. Furthermore, all rabbit sera failed to bind recombinant gp140 in an ELISA (data not shown). Antibody responses from the three rabbits with high titers showed a response directed toward the N-MPER peptide (Fig. 3C) but not the C-MPER peptide. Furthermore, the titers were unaffected when the serine adjacent to the epitope was phosphorylated, as determined by ELISA. Antibody responses toward each peptide immunogen are shown in Fig. S4 in the supplemental material. Endpoint sera from all rabbits were then evaluated for neutralization activity using a luciferase-based assay, but all samples failed to neutralize the virus. Thus, although strong antipeptide antibody responses were achieved using side chain-modified lipopeptides, the antibodies generated are not neutralizing.
Fig 3.
Anti-MPER IgG titers in New Zealand White rabbits immunized with side chain-modified MPER lipopeptides. (A) Antibody responses directed against the immunizing epitope. Antibody responses against N-MPER(T-PO3) were significantly increased at days 94 and 115 over those on day 0 (P < 0.05), while other modifications failed to induce a significant response. Average antipeptide reciprocal endpoint titers are calculated at each day and normalized to day 0 preimmune titers. Responses are shown as the fold change over day 0 preimmune titers. (B) Antibody responses from rabbits at day 115 elicited by their respective lipopeptides. Antibody responses to N-MPER(T-PO3) were significantly different from those to all other lipopeptides (P < 0.05). (C) Antibody responses from rabbits with high titers tested against all other immobilized immunogens at day 115. Rabbit sera showed strong responses toward 2F5 epitopes with and without S668 phosphorylation but lacked responses toward modified and unmodified 4E10 epitopes.
Conclusions.
In this study, the biophysical and immunogenic properties of side chain-modified MPER lipopeptides were evaluated. MPER peptides bearing anionic side chain modifications bound to anti-MPER bnAbs 2F5 and 4E10 with the same affinity as analogous unmodified peptides. When the peptides were attached to a cholesteryl anchor and the lipopeptides were formulated in liposomes, circular dichroism revealed very little structural difference between the side chain-modified lipopeptides and unmodified analogues. Three of the four modified peptides failed to induce high-titer antibodies in rabbits compared to those in sera prior to immunization (day 0). The N-MPERext(T-PO3) lipopeptide formulation induced high antipeptide antibodies in rabbits, with titers being statistically significantly higher than those elicited by its unmodified lipopeptide analogue. Although neutralizing antibodies were not achieved, the improved immune response with one of the modified lipopeptides is consistent with the role of PTMs in breaking tolerance (46, 47, 63).
In summary, the key findings of these studies are 2-fold. First, chemical anionic side chain modifications of MPER peptides do not alter binding to human bnAbs 2F5 and 4E10, as determined by surface plasmon resonance. Second, specific modifications of amino acid side chains can modulate the antibody response toward MPER epitopes [N-MPERext(T-PO3) versus N-MPERext] but do not enable the generation of neutralizing antibodies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jay Levy (UCSF) and Anthony DeFranco (UCSF) for helpful discussions and Gary Fujii (Molecular Express, Inc., Rancho Dominguez, CA) for helpful suggestions regarding the liposome formulation. We thank Christina Ochsenbauer and John Kappes for the IMC.LucR viruses, and we thank Robert McLinden and Jerome Kim for the A3R5 cell line. We also thank Nancy Miller (NIH) and Alan Schultz (NIH) for their encouragement and support.
This work was supported by NIH grants R01 GM061851 and R21 AI093135, and partial funding was provided by NIH grant HHSN27201100016C. V. J. Venditto was funded by a grant from the National Institutes of Health, University of California, San Francisco—Gladstone Institute of Virology & Immunology Center for AIDS Research (P30-AI027763). D. S. Watson was supported by a U.S. Department of Homeland Security (DHS) graduate fellowship, administered by the Oak Ridge Institute for Science and Education (ORISE) under U.S. Department of Energy (DOE) contract number DE-AC05-00OR22750.
The opinions expressed herein do not necessarily reflect the policies or views of DHS, DOE, or ORISE.
Footnotes
Published ahead of print 31 October 2012
Supplemental material for this article may be found at 10.1128/CVI.00615-12.
REFERENCES
- 1. Liu J, O'Brien K, Lynch D, Simmons N, La Porte A, Riggs A, Abbink P, Coffey R, Grandpre L, Seaman M, Landucci G, Forthal D, Montefiori DC, Carville A, Mansfield K, Havenga M, Pau M, Goudsmit J, Barouch D. 2009. Immune control of an SIV challange by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 206:431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walker B, Burton DR. 2008. Toward an AIDS vaccine. Science 320:760–764 [DOI] [PubMed] [Google Scholar]
- 4. Fauci AS. 2007. The release of new data from the HVTN 502 (STEP) HIV vaccine study. In National Institutes of Health (NIH) news, 7 November National Institutes of Health, Bethesda, MD [Google Scholar]
- 5. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, Investigators MOPH-TAVEG. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 6. Bianchi E, Joyce JG, Miller MD, Finnefrock AC, Liang X, Finotto M, Ingallinella P, McKenna P, Citron M, Ottinger E, Hepler RW, Hrin R, Nahas D, Wu C, Montefiori DC, Shiver JW, Pessi A, Kim PS. 2010. Vaccination with peptide mimetics of the gp41 prehairpin fusion intermediate yields neutralizing antisera against HIV-1 isolates. Proc. Natl. Acad. Sci. U. S. A. 107:10655–10660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corti D, Langedjik JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, Silacci C, Pinna D, Jarrossay D, Balla-Jhagjhoorsingh S, Willems B, Zekveld MJ, Dreja H, O'Sullivan E, Prade C, Orkin C, Jeffs SA, Montefiori DC, Davis D, Weissenhorn W, McKnight A, Heeney JL, Sallusto F, Sattentau QJ, Weiss RA, Lanzavecchia A. 2010. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1 infected individuals. PLoS One 5:e8805 doi:10.1371/journal.pone.0008805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karlsson Hedestam G, Fouchier R, Phogat S, Burton D, Sodroski J, Wyatt R. 2008. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat. Rev. Microbiol. 6:143–155 [DOI] [PubMed] [Google Scholar]
- 9. Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliviera TYK, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulard F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Gross JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu Z, Qin HR, Chen W, Zhao Q, Shen X, Schutte R, Wang Y, Ofek G, Streaker E, Prabakaran P, Fouda GG, Liao HX, Owens J, Louder M, Yang Y, Klaric KA, Moody MA, Mascola JR, Scott JK, Kwong PD, Montefiori D, Haynes BF, Tomaras GD, Dimitrov DS. 2011. Cross-reactive HIV-1 neutralizing human monoclonal antibodies identified from a patient with 2F5-like antibodies. J. Virol. 85:11401–11408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Montero M, van Houten N, Wang X, Scott JK. 2008. The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design. Microbiol. Mol. Biol. 72:54–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manrique A, Rusert P, Joos B, Fischer M, Kuster H, Leemann C, Niederost B, Weber R, Stiegler G, Katinger H, Gunthard H, Trkola A. 2007. In vivo and in vitro escape from neutralizing antibodies 2G12, 2F5, and 4E10. J. Virol. 81:8793–8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salzwedel K, Johnston P, Roberts S, Dubay J, Hunter E. 1999. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J. Virol. 73:2469–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Steigler G, Katinger H, Burton DR, Parren PW. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892–10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnold G, Velasco P, Holmes A, Wrin T, Geisler S, Phung P, Tian Y, Resnick D, Ma X, Mariano T, Petropoulos C, Taylor J, Katinger H, Arnold E. 2009. Broad neutralization of HIV-1 elicited from human rhinoviruses that display the HIV-1 gp41 ELDKWA epitope. J. Virol. 83:5087–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matyas GR, Wieczorek L, Beck Z, Ochsenbauer-Jambor C, Kappes JC, Michael NL, Polonis VR, Alving CR. 2009. Neutralizing antibodies induced by liposomal HIV-1 glycoprotein 41 peptide simultaneously bind to both the 2F5 or 4E10 epitope and lipid epitopes. AIDS 23:2069–2075 [DOI] [PubMed] [Google Scholar]
- 19. Ye L, Wen Z, Dong K, Wang X, Bu Z, Zhang H, Compans RW, Yang C. 2011. Induction of HIV neutralizing antibodies against the MPER of the HIV envelope protein by HA/gp41 chimeric protein-based DNA and VLP vaccines. PLoS One 6:e14813 doi:10.1371/journal.pone.0014813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim M, Qiao L, Yu J, Montefiori DC, Reinherz EL. 2007. Immunogenicity of recombinant human immunodeficiency virus type 1-like particles expressing gp41 derivatives in a pre-fusion state. Vaccine 25:5102–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Law M, Cardoso RM, Wilson IA, Burton DR. 2007. Antigenic and immunogenic study of membrane-proximal external region-grafted gp120 antigens by a DNA prime-protein boost immunization strategy. J. Virol. 81:4272–4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Svehla K, Louder M, Wycuff D, Phogat S, Tang M, Migueles S, Wu X, Phogat X, Shaw GM, Connors M, Hoxie J, Mascola J, Wyatt R. 2009. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J. Virol. 83:1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haynes BF, Alam SM. 2008. HIV-1 hides an Achilles' heel in virion lipids. Immunity 28:10–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zwick M. 2005. The membrane-proximal external region of HIV-1 gp41: a vaccine target worth exploring. AIDS 19:1725–1737 [DOI] [PubMed] [Google Scholar]
- 25. Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, Chen B. 2009. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 106:20234–20239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown BK, Karasavvas N, Beck Z, Matyas GR, Birx DL, Polonis VR, Alving CR. 2007. Monoclonal antibodies to phosphatidylinositol phosphate neutralize human immunodeficiency virus type 1: role of phosphate-binding subsites. J. Virol. 81:2087–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haynes BF, Fleming J, St Clair E, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 208:1906–1908 [DOI] [PubMed] [Google Scholar]
- 28. Matyas G, Beck Z, Karasavvas N, Alving CR. 2008. Lipid binding properties of 4E10, 2F5, and WR304 monoclonal antibodies that neutralize HIV-1. Biochim. Biophys. Acta 1788:660–665 [DOI] [PubMed] [Google Scholar]
- 29. Ofek G, McKee K, Yang Y, Yang ZY, Skinner J, Guenaga FJ, Wyatt R, Zwick MB, Nabel GJ, Mascola JR, Kwong PD. 2010. Relationship between antibody 2F5 neutralization of HIV-1 and hydrophobicity of its heavy chain third complementarity-determining region. J. Virol. 84:2955–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meffre E, Milili M, Blanco-Betancourt C, Antunes H, Nussenzweig M, Schiff C. 2001. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J. Clin. Invest. 108:879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Armbruster C, Stiegler GM, Vcelar BA, Jager W, Michael NL, Vetter N, Kattinger HW. 2002. A phase I trial with two human monoclonal antibodies (hMAb 2F5, 2G12) against HIV-1. AIDS 16:227–233 [DOI] [PubMed] [Google Scholar]
- 32. Armbruster C, Stiegler GM, Vcelar BA, Jager W, Koller U, Jilch R, Ammann CG, Pruenster M, Stoiber H, Katinger HW. 2004. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J. Antimicrob. Chemother. 54:915–920 [DOI] [PubMed] [Google Scholar]
- 33. Hessell AJ, Rakasz EG, Tahrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. 2010. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84:1302–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joos B, Trkola A, Kuster H, Aceto L, Fischer M, Steigler G, Armbruster C, Vcelar B, Katinger H, Gunthard HF. 2006. Long-term multiple dose pharmacokinetics of human monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 envelope gp120 (MAb 2G12) and gp41 (MAbs 4E10 and 2F5). Antimicrob. Agents Chemother. 50:1773–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mehandru S, Vcelar B, Wrin T, Steigler G, Joos B, Mohri H, Boden D, Galovich J, Tenner-Racz K, Racz P, Carrington M, Petropoulos C, Katinger H, Markowitz M. 2007. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J. Virol. 81:11016–11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vcelar B, Steigler G, Wolf HM, Muntean W, Leschnik B, Mehandru S, Markowitz M, Armbruster C, Kunert R, Eibl MM, Katinger H. 2007. Reassessment of autoreactivity of the broadly neutralizing HIV antibodies 4E10 and 2F5 and retrospective analysis of clinical safety data. AIDS 21:2161–2170 [DOI] [PubMed] [Google Scholar]
- 37. Apellaniz B, Garcia-Saez AJ, Huarte N, Kunert R, Vorauer-Uhl K, Katinger H, Schwille P, Nieva JL. 2010. Confocal microscopy of giant vesicles supports the absence of HIV-1 neutralizing 2F5 antibody reactivity to plasma membrane phospholipids. FEBS Lett. 584:1591–1596 [DOI] [PubMed] [Google Scholar]
- 38. Xu J, Gorny MK, Palker T, Karwowska S, Zolla-Pazner S. 1991. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J. Virol. 65:4832–4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan DC, Kim PS. 1998. HIV entry and its inhibition. Cell 93:681–684 [DOI] [PubMed] [Google Scholar]
- 40. Alam SM, Scearce RM, Parks RJ, Plonk K, Plonk SG, Sutherland LL, Gorny MK, Zolla-Pazner S, Vanleeuwen S, Moody MA, Xia SM, Montefiori DC, Tomaras GD, Weinhold JK, Karim SA, Hicks CB, Liao HX, Robinson J, Shaw GM, Haynes BF. 2008. Human immunodeficiency virus type 1 gp41 antibodies that mask the membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J. Virol. 82:115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiao X, Chen W, Feng Y, Zhu Z, Prabakaran P, Wang Y, Zhang MY, Longo NS, Dimitrov DS. 2009. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochem. Biophys. Res. Commun. 390:404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pietzsch J, Scheid JF, Mouquet H, Seaman MS, Broder CC, Nussenzweig MC. 2010. Anti-gp41 antibodies cloned from HIV-infected patients with broadly neutralizing serologic activity. J. Virol. 84:5032–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. 2004. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 78:10724–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anderton S. 2004. Post-translational modifications of self antigens: implications for autoimmunity. Curr. Opin. Immunol. 16:753–758 [DOI] [PubMed] [Google Scholar]
- 45. Dhiman M, Estrada-Franco JG, Pando JM, Ramirez-Aguilar FJ, Spratt H, Vazquez-Corzo S, Perez-Molina G, Gallegos-Sandoval R, Moreno R, Garg NJ. 2009. Increased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas' disease. Clin. Vaccine Immunol. 16:660–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Doyle H, Mamula M. 2001. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 22:443–449 [DOI] [PubMed] [Google Scholar]
- 47. Ohmori H, Oka M, Nishikawa Y, Shigemitsu H, Takeuchi M, Magari M, Kanayama N. 2005. Immunogenicity of autologous IgG bearing the inflammation-associated marker 3-nitrotyrosine. Immunol. Lett. 96:47–54 [DOI] [PubMed] [Google Scholar]
- 48. Semballa S, Geffard M, Daulouede S, Malvy D, Veyret B, Lemesre J, Holzmuller P, Mnaimneh S, Vincendeau P. 2004. Antibodies directed against nitrosylated neoepitopes in sera of patients with human African trypanosomiasis. Trop. Med. Int. Health 9:1104–1110 [DOI] [PubMed] [Google Scholar]
- 49. Wegner N, Lundberg K, Kinloch A, Fischer B, Malmstrom V, Feldmann M, Venables PJ. 2010. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol. Rev. 233:34–54 [DOI] [PubMed] [Google Scholar]
- 50. Watson DS, Platt VM, Cao L, Venditto VJ, Szoka FC. 2011. Antibody response in mice to polyhistidine-tagged peptide and protein antigens attached to liposomes via lipid-linked nitrilotriacetic acid. Clin. Vaccine Immunol. 18:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Watson D, Szoka FC. 2009. Role of lipid structure in the humoral immune response in mice to covalent lipid-peptides from the membrane proximal region of HIV-1 gp41. Vaccine 27:4672–4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taylor J, Kaiser E. 1987. Structure-function analysis of proteins through the design, synthesis and study of peptide models. Methods Enzymol. 154:473–498 [DOI] [PubMed] [Google Scholar]
- 53. Muderwha JM, Matyas GR, Spitler LE, Alving CR. 1999. Oil-in-water liposomal emulsions: characterization and potential use in vaccine delivery. J. Pharm. Sci. 88:1332–1339 [DOI] [PubMed] [Google Scholar]
- 54. Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BM, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Edmonds TG, Ding H, Yuan X, Conway JA, Smith K, West J, Montefiori DC, Kappes JC, Ochsenbauer C. 2010. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marusic C, Rizza P, Lattanzi L, Mancini C, Spada M, Belardelli F, Benvenuto E, Capone I. 2001. Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J. Virol. 75:8434–8439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. 1994. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 68:4031–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cardoso RM, Brunel FM, Ferguson S, Zwick M, Burton DR, Dawson PE, Wilson IA. 2007. Structural basis of enhanced binding of extended and helically constrained peptide epitopes of the broadly neutralizing HIV-1 antibody 4E10. J. Mol. Biol. 365:1533–1544 [DOI] [PubMed] [Google Scholar]
- 59. Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, Haynes BF. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1-envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 178:4424–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cardoso RMF, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. 2005. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22:163–173 [DOI] [PubMed] [Google Scholar]
- 61. Ingale S, Gach JS, Zwick MB, Dawson PE. 2010. Synthesis and analysis of the membrane proximal external region epitopes of HIV-1. J. Pept. Sci. 16:716–722 [DOI] [PubMed] [Google Scholar]
- 62. Kim M, Sun ZY, Rand KD, Shi X, Song L, Cheng Y, Fahmy AF, Majumdar S, Ofek G, Yang Y, Kwong PD, Wang JH, Engen JR, Wagner G, Reinherz EL. 2011. Antibody mechanics on a membrane-bound HIV segment essential for GP41-targeted viral neutralization. Nat. Struct. Mol. Biol. 18:1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gauba V, Grunewald J, Gourney V, Deaton LM, Kang M, Bursulaya B, Ou W, Lerner RA, Schmedt C, Geierstanger BH, Schultz PG, Ramirez-Montagut T. 2011. Loss of CD4 T-cell-dependent tolerance to proteins with modified amino acids. Proc. Natl. Acad. Sci. U. S. A. 108:12821–12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.