Abstract
Rhodotorula species are emergent fungal pathogens capable of causing invasive infections, primarily fungemia. They are particularly problematic in immunosuppressed patients when using a central venous catheter. In this study, we evaluated the species distribution of 51 clinical and 8 environmental Rhodotorula species isolates using the ID32C system and internal transcribed spacer (ITS) sequencing. Antifungal susceptibility testing and biofilm formation capability using a crystal violet staining assay were performed. Using ITS sequencing as the gold standard, the clinical isolates were identified as follows: 44 R. mucilaginosa isolates, 2 R. glutinis isolates, 2 R. minuta isolates, 2 R. dairenensis isolates, and 1 Rhodosporidium fluviale isolate. The environmental isolates included 7 R. mucilaginosa isolates and 1 R. slooffiae isolate. Using the ID32C system, along with a nitrate assimilation test, only 90.3% of the isolates tested were correctly identified. In the biofilm formation assay, R. mucilaginosa and R. minuta exhibited greater biofilm formation ability compared to the other Rhodotorula species; the clinical isolates of R. mucilaginosa showed greater biofilm formation compared to the environmental isolates (P = 0.04). Amphotericin B showed good in vitro activity (MIC ≤ 1 μg/ml) against planktonic cells, whereas voriconazole and posaconazole showed poor activity (MIC50/MIC90, 2/4 μg/ml). Caspofungin and fluconazole MICs were consistently high for all isolates tested (≥64 μg/ml and ≥ 4 μg/ml, respectively). In this study, we emphasized the importance of molecular methods to correctly identify Rhodotorula species isolates and non-R. mucilaginosa species in particular. The antifungal susceptibility profile reinforces amphotericin B as the antifungal drug of choice for the treatment of Rhodotorula infections. To our knowledge, this is the first study evaluating putative differences in the ability of biofilm formation among different Rhodotorula species.
INTRODUCTION
Rhodotorula species are basidiomycetous yeasts that are widely distributed in nature. They have been isolated from a variety of environmental sources, including soil, air, aquatic ecosystems, plants, and fruits. In humans, these yeasts have been isolated from the nails, skin, sputum, urine, feces, and hands of health care workers (1–3). Rhodotorula species were traditionally considered to be nonvirulent saprophytes and common contaminant microorganisms. However, in the last 2 decades, these yeasts have emerged as opportunistic pathogens (4–7).
The increase in invasive fungal infections caused by emergent pathogens is related to several factors, including the increased occurrence of degenerative and malignant diseases in different populations, as well as the increased number of patients that undergo organ transplantation, immunosuppressive therapies, broad-spectrum antibiotic therapy, and invasive medical procedures (5, 6, 8). In addition, the availability of new tools for the identification of microorganisms has certainly played a role in the ever-increasing capability of labs to recognize emergent pathogens.
Invasive infections caused by Rhodotorula species are mostly associated with underlying immunosuppression or cancer and with the use of central venous catheters (CVCs) and other implantable medical devices. The most frequent infection caused by Rhodotorula species is fungemia, followed by eye infections, peritonitis, and meningitis (4, 7, 9–11).
Although Rhodotorula species are responsible for a small percentage of all nosocomial acquired fungemia, this pathogen has been reported as the third most common yeast isolated from blood cultures (9) and as the fourth most common infectious fungus according to the ARTEMIS Global Antifungal Surveillance Program (12). Overall, R. mucilaginosa, R. glutinis, and R. minuta have been recognized as the three most clinically relevant species of Rhodotorula isolated from blood cultures (4, 7, 12).
Conventional phenotypic methods are limited in accuracy and consistency for species identification of emergent pathogens, including Rhodotorula species (9, 13–17). Thus, limited published data are available on the species distribution of Rhodotorula species that cause invasive infections and the antifungal susceptibilities of Rhodotorula species isolates correctly identified by molecular methods.
In this study, we evaluated the species distribution of 51 clinical and 8 environmental Rhodotorula species isolates by using internal transcribed spacer (ITS) sequencing and by testing the antifungal susceptibilities of clinical Rhodotorula species isolates using the CLSI broth microdilution assay. In addition, we investigated the biofilm formation capabilities of different Rhodotorula species.
MATERIALS AND METHODS
Microorganisms.
We initially tested 59 yeast isolates previously identified as Rhodotorula species, including 51 clinical and 8 environmental isolates recovered from 14 different Brazilian hospitals during the period from 1995 to 2010. The 51 clinical strains included 39 samples obtained from blood cultures and 12 samples from different anatomical sites. Clinical strains were isolated from 50 different patients, and the yeasts were sent to the Laboratório Especial de Micologia, Universidade Federal de São Paulo, São Paulo, Brazil, for identification and antifungal susceptibility testing. Reference strains of R. mucilaginosa (CBS 329), R. pallida (CBS 320), and R. glutinis (CBS 20) were also included as control organisms in all laboratory tests, totaling 62 Rhodotorula species isolates.
Screening of Rhodotorula species by conventional methods.
Yeast isolates from stock cultures were initially plated on Sabouraud dextrose agar (SDA) (Difco; BD and Company) and incubated at 35°C for 48 h to ensure purity and viability. Rhodotorula species isolates were screened based on the texture and typical color exhibited by their colonies on SDA, as well as by their micromorphology after culturing each isolate on cornmeal-Tween 80 agar. All isolates that were able to produce carotenoid pigments conferring a salmon-pink to coral-red color to the colonies and presenting only spheroidal to oval budding cells without the rudimentary formation of hyphae were considered to belong to the genus Rhodotorula (18, 19).
Microorganism identification using amplification and sequencing of the ITS region of ribosomal DNA (rDNA).
The genomic DNA was extracted using mechanical disruption with glass beads combined with the SDS-based enzymatic lysis method adapted from Wach et al. (20). PCR and sequencing were performed with the universal primers ITS1 and ITS4 as described previously, and amplicons were sequenced using the dideoxynucleotide method in an ABI Prism 3100 automated sequencer (Applied Biosystems, CA) (21, 22). The DNA sequences generated were edited using Sequencher 4.1.4 (Genes Code Co., MI) to obtain contigs for each sample. Species identification was performed using the Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov); an E value of <10−5 was used as the cutoff for species identification.
Biochemical profile of yeasts evaluated by the ID32C galleries.
All of the yeasts were tested by the ID32C system (bioMérieux, Marcy l'Etoile, France), along with a nitrate assimilation test, for species-level identification of Rhodotorula isolates. The tests were performed rigorously according to the manufacturer's instructions. The galleries were incubated at 30°C for 72 h in an airtight box containing a small volume of water to create a humid atmosphere. The carbohydrate assimilation profile obtained for each tested isolate was compared to the database apiweb version 3.0 (bioMérieux, Marcy l'Etoile, France) to obtain the final yeast identification. The complementary nitrate assimilation tests were performed by culturing the isolates on yeast carbon agar, to which potassium nitrate and peptone were added as nitrogen test sources, at 30°C for up to 7 days (15, 23).
In vitro susceptibility testing.
Antifungal susceptibility testing was performed using the broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI) document M27-A3 (24). The antifungal agents and concentrations tested included the following: 0.125 to 64 μg/ml for fluconazole (FLC) (Pfizer, Inc., NY) and 0.03 to 16 μg/ml for amphotericin B (AMB) (Sigma Chemical Corporation, St. Louis, MO), caspofungin (CAS) (Merck & Co., Inc., Rahway, NJ), posaconazole (PSC) (Schering Plough Research Institute, Kenilworth, NJ), and voriconazole (VRC) (Pfizer Inc., NY). The antifungal compounds were provided as pure powders by the manufacturer. All of the plates were prepared with RPMI 1640 medium buffered with 0.165 M morpholinepropanesulfonic acid at pH 7.0 and frozen at −20°C until use.
The microplates were incubated at 35°C for 72 h. The MICs were determined visually after 48 h and 72 h of incubation. Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality control strains. The MIC endpoint for AMB was considered to be the lowest tested drug concentration able to prevent any visible growth. The MICs for CAS, FLC, PSC, and VRC were based on a prominent decrease (≥50%) in growth compared to that of the drug-free growth control. The MICs at which 50% (MIC50) and 90% (MIC90) of the tested isolates were inhibited for each drug after 72 h at 35°C were also determined.
Biofilm production assays. (i) Growth conditions and biofilm formation.
The biofilm formation assay was adapted from previously described methods (25). The strains initially cultured in SDA at 35°C for 48 h were further subcultured into RPMI 1640 broth and grown for 24 h with shaking at 200 rpm at 37°C. The cell cultures were harvested, washed twice with phosphate-buffered saline (PBS), and adjusted to a concentration of 107 cells/ml in RPMI 1640 medium. Biofilm formation was tested in sterile 96-well polystyrene flat-bottom plates (Techno Plastic Products, Switzerland).
For the attachment phase, 100 μl of the cell suspension was transferred to each well of the plates and then incubated at 37°C for 1 h 30 min at 75 rpm. Unattached cells were removed, the wells were washed twice with 150 μl of PBS, and 150 μl of fresh RPMI 1640 medium was added. The plate was incubated at 37°C for 72 h, with shaking at 75 rpm to allow biofilm growth. A test medium without cells was added to the final column of each plate and used as a negative control.
(ii) Biofilm quantification by CV staining.
After biofilm formation, each well was washed twice with 150 μl PBS, and the plate was dried for 20 min at 35°C. The washed biofilms were stained with 110 μl of 0.4% aqueous crystal violet (CV) solution for 45 min. Afterwards, the wells were washed three times with 200 μl of Milli-Q sterile water and destained with 200 μl of 95% ethanol. After 45 min, 100 μl of destaining solution from each sample was transferred to a new plate and measured with a spectrophotometer plate reader (model 680; Bio-Rad) at 570 nm. The absorbance values (A570) of the negative controls (containing no cells) were subtracted from the values of the test wells to minimize background interference. Each strain was tested five times on three different days, and the biofilm production quantities were reported as the arithmetic means ± standard deviations (SD) of the A570 values for 15 replicate tests.
(iii) Scanning electron microscopy (SEM).
Biofilms were formed on sterile polyvinyl chloride (PVC) strips (surface area, 0.5 cm2) and placed in 24-well microtiter plates (Techno Plastic Products, Switzerland) as described previously. Biofilms formed on PVC strips were fixed overnight at 4°C with 4% formaldehyde plus 2% glutaraldehyde buffered at pH 7.2 with 0.1 M sodium cacodylate. After fixation, the samples were treated with 1% osmium tetroxide in cacodylate buffer for 1 h. Subsequently, the samples were treated with 1% tannic acid for 45 min, washed three times with distilled water for 15 min, and treated again with 1% osmium tetroxide in cacodylate buffer for 1 h. The samples were dehydrated with a graded series of ethanol washes, critical-point dried in CO2, coated with gold, and examined with a JEOL JSM-5300 scanning electron microscope (26).
(iv) Statistical analysis.
For a comparison of the biofilm formation capability between clinical and environmental strains of R. mucilaginosa, mean A570 values obtained for each group were compared using Student's t test with the GraphPad Prism version 5.00 for Windows (GraphPad Software, CA). A P value of <0.05 was considered significant. Possible outliers were determined by Grubb's test using a P value of <0.05 as the cutoff with GraphPad software. The test was performed until no outliers were detected in the replicates. Outliers were excluded from the analysis.
Nucleotide sequence accession numbers. The sequences generated in this study have been deposited in the GenBank (NCBI) database under the following accession numbers: JX499189, JX512667, JX512677, JX512680, JX512681, JX512682, JX512683, JX512684, JX512685, JX512686, JX512687, JX512688, JX512689, JX512690, JX512691, JX512692, JX512693, JX512694, JX512695, JX512696, JX512697, JX512698, JX512699, JX512700, JX512701, JX512702, JX512703, JX512704, JX512705, JX512706, JX512707, JX512708, JX512709, JX512710, JX512711, JX512712, JX512713, JX512714, JX512715, JX499188, JX512668, JX512669, JX512670, JX512671, JX512672, JX512673, JX512674, JX512675, JX512676, JX512678, JX512679, JX272795, JX272796, JX494370, JX494371, JX494373, JX494375, JX494374, and JX494372.
RESULTS
Screening of Rhodotorula species by conventional methods.
All 59 yeast isolates tested in this study plus the three reference strains were pure and viable. The individual colonies exhibited a salmon-pink to coral-red color on SDA and presented only spheroidal to oval blastoconidia with the absence of hyphae on corn meal-Tween 80 agar. Thus, all isolates were considered to belong to the genus Rhodotorula.
Molecular identification of Rhodotorula species isolates by sequencing the ITS region of rDNA.
ITS sequencing was used as the gold standard for the species-level identification of all clinical and environmental Rhodotorula species isolates tested. DNA sequences of approximately 560 bp were obtained for all isolates tested. The sequences were subjected to BLAST searches to confirm the preliminary identification of the strains. BLAST alignments with the ITS sequences were successful in identifying all Rhodotorula species isolates, including the reference strains (100% query coverage, ≥99% identity, and an E value of 0.0 for all isolates). The 51 clinical isolates previously screened as Rhodotorula species were identified as the following: 44 R. mucilaginosa isolates, 2 R. glutinis isolates, 2 R. minuta isolates, 2 Rhodotorula dairenensis isolates, and 1 Rhodosporidium fluviale isolate. The environmental isolates were identified as R. mucilaginosa (n = 7) and Rhodotorula slooffiae (n = 1) (Table 1).
Table 1.
Species distribution of clinical and environmental isolates of Rhodotorula species identified by ITS sequencing
| Species | No. of isolates from: |
Total | ||
|---|---|---|---|---|
| Blood culture | Other sourcea | Environment | ||
| R. mucilaginosa | 34 | 10 | 7 | 51 |
| R. minuta | 2 | − | − | 2 |
| R. glutinis | − | 2 | − | 2 |
| R. dairenensis | 2 | − | − | 2 |
| R. slooffiae | − | − | 1 | 1 |
| Total | 38 | 12 | 8 | 58 |
Other sources include pleural fluid, bronchoalveolar lavage, skin biopsy, secretion from a fistula hand, nasal mucus, scalp, sole of the foot, and catheter.
Biochemical profile of Rhodotorula species isolates evaluated by ID32C and a nitrate assimilation test.
Using ITS identification as a reference, 56 out of the 62 (90.3%) isolates tested, including the reference strains, were correctly identified to the species level by the ID32C system combined with the nitrate assimilation test. However, even after the addition of the nitrate assimilation test, the ID32C resulted in discrepancies for 5 (8.1%) of the strains: 2 R. dairenensis strains were misidentified as R. mucilaginosa, 1 Rhodosporidium fluviale strain was misidentified as R. glutinis, 1 R. slooffiae strain was misidentified as R. minuta, and the reference R. pallida strain (CBS 320) did not assimilate nitrate and was identified with low discrimination between R. glutinis and the species Candida sphaerica and Candida sake (Table 2). This particular strain was phenotypically identified as a Rhodotorula species based on the salmon-pink color of the colonies and the compatible micromorphology. Moreover, 1 (1.6%) R. minuta isolate (2145) presented an unacceptable profile for final ID32C identification.
Table 2.
Concordance between the genotypic and phenotypic identification of 62 yeast isolates previously identified as Rhodotorula speciesa
| Isolate | Source | ITS sequencing |
ID32C system + nitrate assimilation |
||
|---|---|---|---|---|---|
| Yeast species | Accession no. | Yeast species | Identification code | ||
| 16 | Blood culture | R. mucilaginosa | JX499189 | R. mucilaginosa | 5461750113 |
| 17 | Blood culture | R. mucilaginosa | JX512667 | R. mucilaginosa | 5461750311 |
| 230* | Blood culture | R. dairenensis | JX512677 | R. mucilaginosa | 5061710113 |
| 281 | Blood culture | R. mucilaginosa | JX512680 | R. mucilaginosa | 5461750113 |
| 320 | Blood culture | R. mucilaginosa | JX512681 | R. mucilaginosa | 5461750111 |
| 369* | Blood culture | Rhodosporidium fluviale | JX512682 | R. glutinis | 5465750313 |
| 467 | Blood culture | R. mucilaginosa | JX512683 | R. mucilaginosa | 7461750111 |
| 701 | Blood culture | R. mucilaginosa | JX512684 | R. mucilaginosa | 5461750111 |
| 702 | Blood culture | R. mucilaginosa | JX512685 | R. mucilaginosa | 5461750113 |
| 717 | Blood culture | R. mucilaginosa | JX512686 | R. mucilaginosa | 5461750113 |
| 740 | Blood culture | R. mucilaginosa | JX512687 | R. mucilaginosa | 5461750111 |
| 755 | Blood culture | R. mucilaginosa | JX512688 | R. mucilaginosa | 5461750111 |
| 800 | Blood culture | R. mucilaginosa | JX512689 | R. mucilaginosa | 5461750111 |
| 803 | Blood culture | R. mucilaginosa | JX512690 | R. mucilaginosa | 5461750111 |
| 808 | Blood culture | R. mucilaginosa | JX512691 | R. mucilaginosa | 5461710113 |
| 1385 | Blood culture | R. mucilaginosa | JX512692 | R. mucilaginosa | 5461750111 |
| 1765 | Blood culture | R. mucilaginosa | JX512693 | R. mucilaginosa | 5461750111 |
| 2145* | Blood culture | R. minuta | JX512694 | Unacceptable profile | 5313514331 |
| 2265 | Blood culture | R. mucilaginosa | JX512695 | R. mucilaginosa | 5461750113 |
| 2334B | Blood culture | R. mucilaginosa | JX512696 | R. mucilaginosa | 5061550313 |
| 2413 | Blood culture | R. mucilaginosa | JX512697 | R. mucilaginosa | 5461750111 |
| 2495 | Blood culture | R. mucilaginosa | JX512698 | R. mucilaginosa | 5461650111 |
| 2552 | Blood culture | R. mucilaginosa | JX512699 | R. mucilaginosa | 5061350113 |
| 2595 | Blood culture | R. mucilaginosa | JX512700 | R. mucilaginosa | 5461750111 |
| 2675 | Blood culture | R. mucilaginosa | JX512701 | R. mucilaginosa | 5461750113 |
| 2708 | Blood culture | R. mucilaginosa | JX512702 | R. mucilaginosa | 5461750113 |
| 2795 | Blood culture | R. minuta | JX512703 | R. minuta | 4513314331 |
| 2843A | Blood culture | R. mucilaginosa | JX512704 | R. mucilaginosa | 7461360113 |
| 2945* | Blood culture | R. dairenensis | JX512705 | R. mucilaginosa | 5421710013 |
| 2984 | Blood culture | R. mucilaginosa | JX512706 | R. mucilaginosa | 5461750111 |
| 2988 | Blood culture | R. mucilaginosa | JX512707 | R. mucilaginosa | 5461750113 |
| 2991B | Blood culture | R. mucilaginosa | JX512708 | R. mucilaginosa | 5461750113 |
| 3020 | Blood culture | R. mucilaginosa | JX512709 | R. mucilaginosa | 5461750113 |
| 3051 | Blood culture | R. mucilaginosa | JX512710 | R. mucilaginosa | 5021610003 |
| 3166 | Blood culture | R. mucilaginosa | JX512711 | R. mucilaginosa | 5461750113 |
| 3201 | Blood culture | R. mucilaginosa | JX512712 | R. mucilaginosa | 5461750113 |
| 3225 | Blood culture | R. mucilaginosa | JX512713 | R. mucilaginosa | 5461750113 |
| 6190 | Blood culture | R. mucilaginosa | JX512714 | R. mucilaginosa | 5461750113 |
| 6437 | Blood culture | R. mucilaginosa | JX512715 | R. mucilaginosa | 5461750113 |
| 04B | Secretion from a fistula hand | R. mucilaginosa | JX499188 | R. mucilaginosa | 5061750113 |
| 130 | Bronchoalveolar lavage | R. mucilaginosa | JX512668 | R. mucilaginosa | 5461750113 |
| 131 | Bronchoalveolar lavage | R. mucilaginosa | JX512669 | R. mucilaginosa | 5461750111 |
| 132A | Bronchoalveolar lavage | R. mucilaginosa | JX512670 | R. mucilaginosa | 5461750113 |
| 150 | Scalp | R. glutinis | JX512671 | R. glutinis | 7067750113 |
| 157 | Sole of the foot | R. glutinis | JX512672 | R. glutinis | 7067750113 |
| 214 | Nasal mucus | R. mucilaginosa | JX512673 | R. mucilaginosa | 5441750113 |
| 215 | Pleural fluid | R. mucilaginosa | JX512674 | R. mucilaginosa | 5461750113 |
| 216 | Pleural fluid | R. mucilaginosa | JX512675 | R. mucilaginosa | 5461750113 |
| 221 | Pleural fluid | R. mucilaginosa | JX512676 | R. mucilaginosa | 5461750113 |
| 258 | Skin biopsy | R. mucilaginosa | JX512678 | R. mucilaginosa | 5061750113 |
| 279 | Catheter | R. mucilaginosa | JX512679 | R. mucilaginosa | 5461640101 |
| 9amb | Env. | R. mucilaginosa | JX272795 | R. mucilaginosa | 5461750113 |
| 10amb | Env. | R. mucilaginosa | JX272796 | R. mucilaginosa | 5461750111 |
| 11amb | Env. | R. mucilaginosa | JX494370 | R. mucilaginosa | 5461750113 |
| 14amb | Env. | R. mucilaginosa | JX494371 | R. mucilaginosa | 5061750113 |
| 3560 | Env. | R. mucilaginosa | JX494373 | R. mucilaginosa | 5461750113 |
| 3630* | Env. | R. slooffiae | JX494375 | R. minuta | 4513214311 |
| 3988A | Env. | R. mucilaginosa | JX494374 | R. mucilaginosa | 7061750113 |
| 15amb | Env. | R. mucilaginosa | JX494372 | R. mucilaginosa | 5461750113 |
| CBS 20 | CBS | R. glutinis | R. glutinis | 5061310013 | |
| CBS 320* | CBS | R. pallida | Rhodotorula sp. | 7067350113 | |
| CBS 329 | CBS | R. mucilaginosa | R. mucilaginosa | 5661750311 | |
Isolates that showed differences between the phenotypic and genotypic identifications are marked with an asterisk. The identification codes in bold represent the two most common biochemical profiles among all R. mucilaginosa isolates tested. Env., air from hospital environment; CBS, reference strains.
The ID32C system reported the final yeast species identification of 9 isolates without requesting the complementary nitrate assimilation test. These results were obtained with 1 R. minuta isolate and 2 R. glutinis isolates that were correctly identified and 6 other isolates that were misidentified, including 4 R. mucilaginosa isolates that were misidentified as R. glutinis, 1 R. slooffiae isolate that was misidentified as R. minuta, and 1 Rhodosporifium fluviale isolate that was misidentified as R. glutinis. For these isolates, after running the ID32C system combined with the nitrate assimilation test, all 4 R. mucilaginosa isolates originally misidentified as R. glutinis had a final identification corrected to R. mucilaginosa.
Of note, 37 out of the 52 (71.2%) R. mucilaginosa isolates identified by the ID32C system along with the nitrate assimilation test generated the codes 5461750113 and 5461750111. Apparently, both carbohydrate assimilation profiles are reliable in the identification of R. mucilaginosa isolates.
A single yeast clinical isolate molecularly identified as Rhodosporidium fluviale was excluded from further experiments.
Biofilm formation by Rhodotorula species isolates.
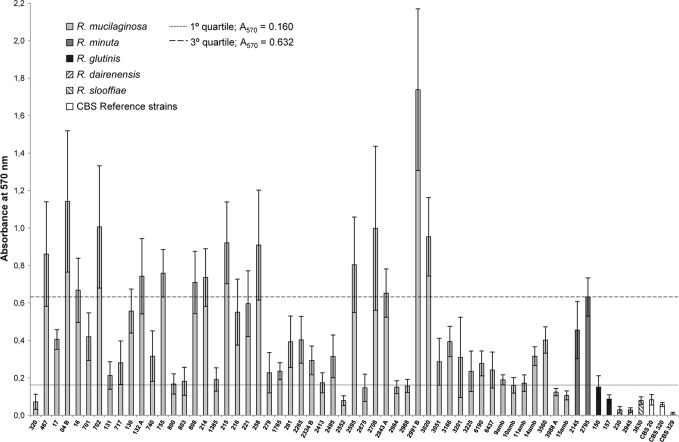
The CV staining assay for the quantification of biofilm formation of the remaining 61 Rhodotorula species strains tested, including the reference strains, had absorbance values at 570 nm (A570), ranging from 0.007 to 1.739. To better illustrate the differences in biofilm formation capability among isolates of Rhodotorula species, we arbitrarily established three categories for the quantification of biofilm production: low (A570, <0.160), medium (A570, 0.160 to 0.632), and high (A570, >0.632). These categories were defined based on the quartile values of the full range of Rhodotorula species A570 values.
The rank scale for Rhodotorula species biofilm formation determined by CV staining was as follows: R. minuta > R. mucilaginosa > R. glutinis > R. slooffiae > R. pallida > R. dairenensis. According to the interpretive criteria adopted in this study, R. minuta and R. mucilaginosa were classified as medium biofilm producers, while the isolates from the remaining species were considered low biofilm producers (Fig. 1).
Fig 1.
Biofilm production of 61 Rhodotorula species isolates, including the reference strains of R. mucilaginosa (CBS 329), R. pallida (CBS 320), and R. glutinis (CBS 20). The histograms represent the means ± SD of the absorbance values at 570 nm (A570) obtained from 15 replicate tests for each sample. According to the interpretive criteria adopted in this study, R. mucilaginosa (mean A570, 0.449) and R. minuta (mean A570, 0.543) were classified as medium biofilm producers, whereas the other species of Rhodotorula were considered low biofilm producers (A570, <0.160).
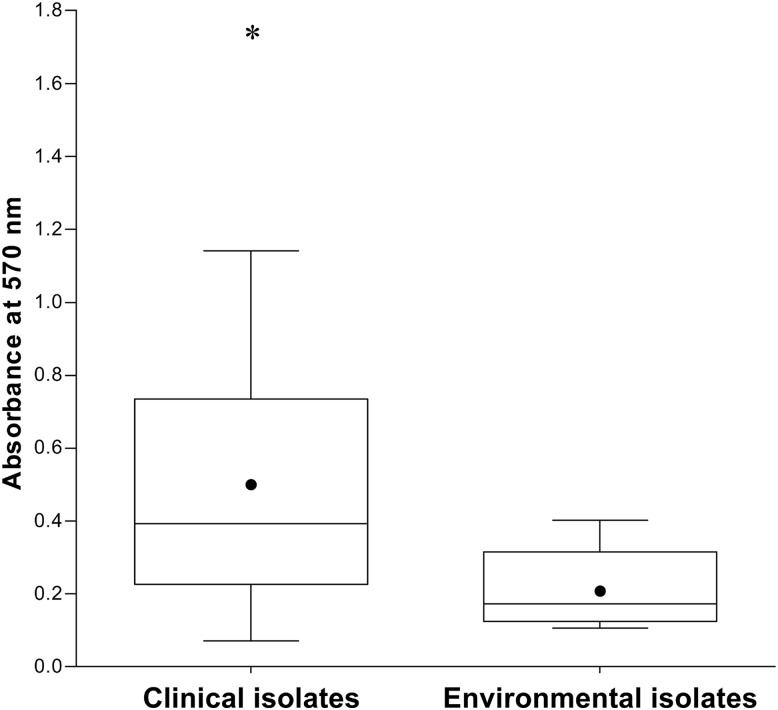
In addition, clinical isolates of R. mucilaginosa showed higher biofilm formation capability than that of environmental isolates (Fig. 2). The clinical isolates exhibited an A570 value of 0.497 ± 0.353 (mean ± SD), and the environmental isolates had an A570 value of 0.210 ± 0.109 (P = 0.04).
Fig 2.
Boxplots of the absorbance values obtained for the clinical and environmental isolates of R. mucilaginosa in the biofilm formation assays. The clinical isolates presented a greater ability to form biofilms than did the environmental isolates (P = 0.04). The solid circle indicates the mean absorbance obtained for the group of clinical (A570, 0.497) and environmental (A570, 0.21) isolates. The asterisk indicates that isolate 2991B showed significantly greater biofilm formation capability (P < 0.05).
Of note, during the period from 1997 to 2010, the storage time at −70°C for all R. mucilaginosa isolates did not affect their biofilm formation capability (data not shown).
Biofilm SEM.
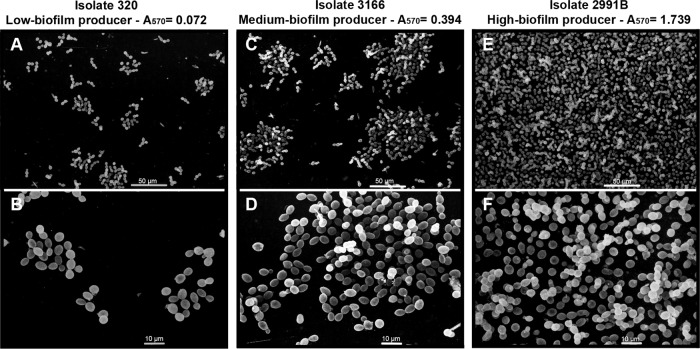
SEM images of the biofilms were used to validate the CV staining interpretive criteria used in our study to quantify the biofilms of Rhodotorula species isolates. For SEM, we used three isolates representing high, medium, and low biofilm producers: isolate 2991B (A570 = 1.739), isolate 3166 (A570 = 0.394), and isolate 320 (A570 = 0.072). It was possible to verify in the SEM images (Fig. 3) that isolate 2991B (Fig. 3E and F) had a higher level of biofilm formation than isolate 3166 (Fig. 3C and D). The isolate 320 presented only microcolonies that did not have the multilayered architecture of a mature biofilm (Fig. 3A and B). An examination of the SEM images revealed that the degree of biofilm formation by high, medium, and low producers was highly correlated to the results of the CV assay.
Fig 3.
Scanning electron microscopy (SEM) images of 3 R. mucilaginosa isolates after the induction of biofilm formation on a PVC strip. Isolate 320, low biofilm producer (A and B); isolate 3166, medium biofilm producer (C and D); and isolate 2991B, high biofilm producer (E and F). Absorbance values at 570 nm obtained for each isolate subjected to the crystal violet staining assay are provided at the top of micrographics. Micrographics B, D, and F are higher magnifications of A, C, and E, respectively. SEM images confirmed the CV staining interpretive criteria to indirectly quantify bulk biofilm production by Rhodotorula species isolates.
Susceptibilities of clinical isolates of Rhodotorula species to 5 antifungal agents.
Table 3 summarizes the MIC50, MIC90, and MIC ranges (μg/ml) obtained for the Rhodotorula species isolates for the five antifungal agents after 48 and 72 h of incubation.
Table 3.
In vitro activity of five antifungal agents against 50 clinical isolates of Rhodotorula species using the CLSI broth microdilution assay
| Rhodotorula species (no. of isolates) | Cell incubation period (h) | MIC (μg/ml) for indicated antifungal agenta |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB |
CAS |
VRC |
PSC |
FLC |
||||||||||||
| MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | Range | ||
| R. mucilaginosa (44) | 48 | 1 | 1 | 0.25–1 | 8 | 8 | 1–16 | 1 | 2 | 0.25–2 | 2 | 2 | 0.5–4 | 64 | >64 | ≥64 |
| 72 | 1 | 1 | 0.5–1 | 8 | 16 | 4–16 | 2 | 4 | 0.5–4 | 2 | 4 | 1–8 | 64 | >64 | ≥64 | |
| R. glutinis (2) | 48 | ND | ND | 0.5 | ND | ND | 4 | ND | ND | 0.25 | ND | ND | 1–2 | ND | ND | 64 |
| 72 | ND | ND | 0.5–1 | ND | ND | 8 | ND | ND | 0.25 | ND | ND | 2 | ND | ND | 64 | |
| R. minuta (2) | 48 | ND | ND | 0.5 | ND | ND | 2 | ND | ND | 4 | ND | ND | 2 | ND | ND | 64 |
| 72 | ND | ND | 1 | ND | ND | 4 | ND | ND | 4 | ND | ND | 2 | ND | ND | 64 | |
| R. dairenensis (2) | 48 | ND | ND | 0.5–1 | ND | ND | 16 | ND | ND | 0.25–0.5 | ND | ND | 2 | ND | ND | 64 |
| 72 | ND | ND | 0.5–1 | ND | ND | >16 | ND | ND | 0.5–1 | ND | ND | 1–2 | ND | ND | 64 | |
AMB, amphotericin B; CAS, caspofungin; VRC, voriconazole; PSC, posaconazole; FLC, fluconazole; ND, not determined.
The MICs of the 50 clinical isolates of Rhodotorula species after 72 h of incubation ranged from 0.5 to 1 μg/ml for AMB, 4 to >16 μg/ml for CAS, 0.25 to 4 μg/ml for VRC, 1 to 8 μg/ml for PSC, and ≥64 μg/ml for FLC.
Of note, all Rhodotorula species isolates tested exhibited AMB MICs of ≤1 μg/ml. The CAS MICs for all isolates were consistently high (MIC ≥ 4 μg/ml). The VRC and PSC MICs were similar (MIC50/MIC90, 2/4 μg/ml). The FLC MICs were very high for all isolates tested (≥64 μg/ml).
DISCUSSION
Rhodotorula species is an emergent pathogen capable of causing invasive infections in humans, particularly in immunocompromised patients (7, 9, 27–29). In spite of the increased number of invasive infections by Rhodotorula species described in the last decades, only a small number of epidemiological studies have used molecular methods for the identification of Rhodotorula species. To our knowledge, this is the first study in which ITS sequencing was used as the gold standard for the species-level identification of a large collection of clinical and environmental Rhodotorula species isolates.
ITS sequencing was a reliable method for the identification of Rhodotorula species, and it was more accurate than conventional phenotypic tests. In our study, the Rhodotorula species distribution among clinical isolates was similar to that reported previously: R. mucilaginosa (n = 44; 88%) was the most prevalent species, followed by R. glutinis (n = 2; 4%), R. minuta (n = 2; 4%), and R. dairenensis (n = 2; 4%) (7, 9). It is worth mentioning that there are no reports in the literature regarding infections caused by the species R. dairenensis and Rhodosporidium fluviale. In addition, R. mucilaginosa was also the most prevalent species found among environmental samples, suggesting that this species is well adapted to both free-living growth and human hosts.
According to the CBS site (http://www.cbs.knaw.nl), Rhodosporidium diobovatum, Rhodosporidium sphaerocarpum, and Rhodosporidium toruloides represent sexual forms of R. glutinis. Indeed, the ITS sequences of our clinical isolates, 150 and 157, matched the sequences of R. glutinis and Rhodosporirium diobovatum when BLAST searches were performed. These BLAST matches showed the same scores for all variables, including the E value, max score, and max identity. Because both taxa appeared to be the same species, we considered the final identification of the isolates 150 and 157 to be R. glutinis.
Currently, yeast identification in clinical laboratories is performed using commercial and automated systems (ID32C and Vitek 2, respectively). These systems show high accuracy for the identification of the most common yeast species causing human infections, such as Candida species. However, they are unable to distinguish genetically similar species such as the Candida parapsilosis species complex and show limited accuracy for identification of emergent pathogens, including Trichosporon species and Rhodotorula species (9, 13–17).
Despite a number of limitations, the ID32C system along with a nitrate assimilation test was able to correctly identify 90.3% of Rhodotorula species isolates. The complementary nitrate assimilation test was crucial to the final species identification, suggesting that this test should be performed even when not requested by the ID32C system report. Inconsistent results in phenotypic identification were mostly related to non-R. mucilaginosa isolates. Indeed, the database of the commercial system ID32C does not include all species in the genera Rhodotorula and Rhodosporidium.
Rhodotorula species infections are frequently associated with the presence of CVCs and other implantable medical devices (9–11, 30). These devices provide the necessary surfaces for biofilm formation and are currently responsible for a significant percentage of human infections. In contrast to the extensive literature dealing with Candida species biofilms (25, 26, 31–33), little attention has been paid to emergent fungal pathogens such as Rhodotorula species.
This is the first study to evaluate the biofilm formation ability of different Rhodotorula species and the putative differences between clinical and environmental isolates. Using CV staining, we were able to demonstrate that clinical isolates of R. mucilaginosa were better at forming biofilms than the environmental isolates. Moreover, we verified that R. mucilaginosa and R. minuta were the best biofilm producers.
The main limitation of our study was the small number of environmental strains and non-R. mucilaginosa species tested. Further studies are needed to confirm if these microorganisms really have a lower capability to produce biofilm than clinical strains of R. mucilaginosa.
Overall, the CV staining was a useful method to indirectly measure bulk biofilm, and the results of biofilm production were confirmed by SEM. It was also observed that the storage time of the samples did not affect the biofilm formation capability of R. mucilaginosa clinical isolates. This finding conflicts with the hypothesis that the expression of virulence factors by fungi may be substantially impaired by long-term storage of strains (34, 35).
The antifungal susceptibility profiles obtained strongly suggest that the genus Rhodotorula is not a target for FLC or CAS. Other azole agents also showed poor activity (≥2 μg/ml) against the majority of the isolates tested. In contrast, AMB showed the best activity in vitro. Overall, the results obtained here are in agreement with previous studies and show that it is more appropriate to read the susceptibility test of Rhodotorula species after 72 h of incubation (4, 7, 9, 12, 29, 36, 37).
In conclusion, we emphasized the importance of molecular methods to correctly identify Rhodotorula species isolates and non-R. mucilaginosa species in particular. R. mucilaginosa was the most prevalent species among the clinical and environmental samples. All Rhodotorula species isolates tested were susceptible to AMB, suggesting that it should be considered the antifungal drug of choice for the treatment of Rhodotorula species invasive infections. Finally, we demonstrated that Rhodotorula species are able to form biofilms, which may play a role in the pathogenesis of infections by this species.
ACKNOWLEDGMENTS
This work was supported in part by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (grant 2007/08575-1), and by the Conselho Nacional de Pesquisas Científicas e Tecnológicas (CNPq), Brazil (grant 308011/2010-4). J.M.N. received a master fellowship from CNPq, Brazil (grant 134544/2009-9). R.C.F. and F.C.B. received postdoctoral fellowships from FAPESP (grants 2009/01230-4 and 2010/17179-5). A.L.C. received grants from FAPESP and CNPq.
We are thankful for Fernando Antonelli's contribution to the statistical analysis.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. Cordeiro RA, Brilhante RSN, Pantoja LDM, Filho M, Vieira RN, Rocha MFG, Sidrim JC. 2010. Isolation of pathogenic yeasts in the air from hospital environments in the city of Fortaleza, northeast Brazil. Braz. J. Infect. Dis. 14:30–34 [DOI] [PubMed] [Google Scholar]
- 2. Galán-Sánchez F, García-Martos P, Rodríguez-Ramos C, Marín-Casanova P, Mira-Gutiérrez J. 1999. Microbiological characteristics and susceptibility patterns of strains of Rhodotorula isolated from clinical samples. Mycopathologia 145:109–112 [DOI] [PubMed] [Google Scholar]
- 3. Silva V, Zepeda G, Eugenia M, Febré N. 2003. Yeast carriage on the hands of medicine students. Rev. Iberoam. Micol. 20:41–45 (In Spanish.) [PubMed] [Google Scholar]
- 4. Diekema DJ, Petroelje B, Messer SA, Hollis RJ, Pfaller MA. 2005. Activities of available and investigational antifungal agents against Rhodotorula species. J. Clin. Microbiol. 43:476–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hazen KC. 1995. New and emerging yeast pathogens. Clin. Microbiol. Rev. 8:462–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miceli MH, Díaz JA, Lee SA. 2011. Emerging opportunistic yeast infections. Lancet Infect. Dis. 11:142–151 [DOI] [PubMed] [Google Scholar]
- 7. Tuon FF, Costa SF. 2008. Rhodotorula infection. A systematic review of 128 cases from literature. Rev. Iberoam. Micol. 25:135–140 [DOI] [PubMed] [Google Scholar]
- 8. Nucci M, Anaissie E. 2006. Emerging fungi. Infect. Dis. Clin. North Am. 20:563–579 [DOI] [PubMed] [Google Scholar]
- 9. De Almeida GMD, Costa SF, Melhem M, Motta AL, Szeszs MW, Miyashita F, Pierrotti LC, Rossi F, Burattini MN. 2008. Rhodotorula spp. isolated from blood cultures: clinical and microbiological aspects. Med. Mycol. 46:547–556 [DOI] [PubMed] [Google Scholar]
- 10. Savini V, Sozio F, Catavitello C, Talia M, Manna A, Febbo F, Balbinot A, Di Bonaventura G, Piccolomini R, Parruti G, D'Antonio D. 2008. Femoral prosthesis infection by Rhodotorula mucilaginosa. J. Clin. Microbiol. 46:3544–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Unal A, Koc AN, Sipahioglu MH, Kavuncuoglu F, Tokgoz B, Buldu HM, Oymak O, Utas C. 2009. CAPD-related peritonitis caused by Rhodotorula mucilaginosa. Perit. Dial. Int. 29:581–582 [PubMed] [Google Scholar]
- 12. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Bijie H, Dzierzanowska D, Klimko NN, Letscher-Bru V, Lisalova M, Muehlethaler K, Rennison C, Zaidi M. 2009. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: 10.5-year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 47:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chagas-Neto TC, Chaves GM, Colombo AL. 2008. Update on the genus Trichosporon. Mycopathologia 166:121–132 [DOI] [PubMed] [Google Scholar]
- 14. Ciardo DE, Schär G, Böttger EC, Altwegg M, Bosshard PP. 2006. Internal transcribed spacer sequencing versus biochemical profiling for identification of medically important yeasts. J. Clin. Microbiol. 44:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. GarcíA-Martos P, GarcíA-Agudo L, Ruiz-Aragón J, Saldarreaga A, Marín P. 2004. Carbohydrate assimilation by clinical and environmental Rhodotorula glutinis strains. Rev. Iberoam. Micol. 21:90–92 (In Spanish.) [PubMed] [Google Scholar]
- 16. Meletiadis J, Arabatzis M, Bompola M, Tsiveriotis K, Hini S, Petinaki E, Velegraki A, Zerva L. 2011. Comparative evaluation of three commercial identification systems using common and rare bloodstream yeast isolates. J. Clin. Microbiol. 49:2722–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silva JO, Candido RC. 2005. Evaluation of the API20C AUX system for the identification of clinically important yeasts. Rev. Soc. Bras. Med. Trop. 38:261–263 (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 18. de Hoog GS, Guarro J, Gené J, Figueras MJ. 2000. Atlas of clinical fungi, 2nd ed Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands [Google Scholar]
- 19. Fell JW, Statzell-Tallman A. 1998. Rhodotorula F. C. Harrison, p 800–827 In Kurtzman P, Fell JW. (ed), The yeasts: a taxonomic study, 4th ed Elsevier, New York, NY [Google Scholar]
- 20. Wach A, Pick H, Philippsen P. 1994. Procedures for isolating yeast DNA for different purposes, p 10–11 In Johnston JR. (ed), Molecular genetics of yeast: a practical approach. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 21. Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74:5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Souza ACR, Ferreira RC, Gonçalves SS, Quindós G, Eraso E, Bizerra FC, Briones MRS, Colombo AL. 2012. Accurate identification of Candida parapsilosis (sensu lato) by use of mitochondrial DNA and real-time PCR. J. Clin. Microbiol. 50:2310–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Isenberg HD. 1998. Nitrate assimilation test for identification of yeasts to the species level, p 311–312 In Isenberg HD. (ed), Essential procedures for clinical microbiology. ASM Press, Washington, DC [Google Scholar]
- 24. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 25. Melo AS, Bizerra FC, Freymüller E, Arthington-Skaggs BA, Colombo AL. 2011. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med. Mycol. 49:253–262 [DOI] [PubMed] [Google Scholar]
- 26. Bizerra FC, Nakamura CV, de Poersch C, Estivalet Svidzinski TI, Borsato Quesada RM, Goldenberg S, Krieger MA, Yamada-Ogatta SF. 2008. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 8:442–450 [DOI] [PubMed] [Google Scholar]
- 27. Pasqualotto GC, Copetti FA, Meneses CF, Machado ARL, Brunetto AL. 2005. Infection by Rhodotorula sp. in children receiving treatment for malignant diseases. J. Pediatr. Hematol. Oncol. 27:232–233 [DOI] [PubMed] [Google Scholar]
- 28. Samonis G, Anatoliotaki M, Apostolakou H, Maraki S, Mavroudis D, Georgoulias V. 2001. Transient fungemia due to Rhodotorula rubra in a cancer patient: case report and review of the literature. Infection 29:173–176 [DOI] [PubMed] [Google Scholar]
- 29. Zaas AK, Boyce M, Schell W, Lodge BA, Miller JL, Perfect JR. 2003. Risk of fungemia due to Rhodotorula and antifungal susceptibility testing of Rhodotorula isolates. J. Clin. Microbiol. 41:5233–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tuon FF, de Almeida GMD, Costa SF. 2007. Central venous catheter-associated fungemia due to Rhodotorula spp.—a systematic review. Med. Mycol. 45:441–447 [DOI] [PubMed] [Google Scholar]
- 31. Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hawser SP, Douglas LJ. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramage G, Martínez JP, López-Ribot JL. 2006. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 6:979–986 [DOI] [PubMed] [Google Scholar]
- 34. Brummer E, Restrepo A, Hanson LH, Stevens DA. 1990. Virulence of Paracoccidiodes brasiliensis: the influence of in vitro passage and storage. Mycopathologia 109:13–17 [DOI] [PubMed] [Google Scholar]
- 35. Svidzinski TI, Miranda Neto MH, Santana RG, Fischman O, Colombo AL. 1999. Paracoccidioides brasiliensis isolates obtained from patients with acute and chronic disease exhibit morphological differences after animal passage. Rev. Inst. Med. Trop. Sao Paulo 41:279–283 [DOI] [PubMed] [Google Scholar]
- 36. Gomez-Lopez A, Mellado E, Rodriguez-Tudela JL, Cuenca-Estrella M. 2005. Susceptibility profile of 29 clinical isolates of Rhodotorula spp. and literature review. J. Antimicrob. Chemother. 55:312–316 [DOI] [PubMed] [Google Scholar]
- 37. Guinea J, Recio S, Escribano P, Peláez T, Gama B, Bouza E. 2010. In vitro antifungal activities of isavuconazole and comparators against rare yeast pathogens. Antimicrob. Agents Chemother. 54:4012–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]