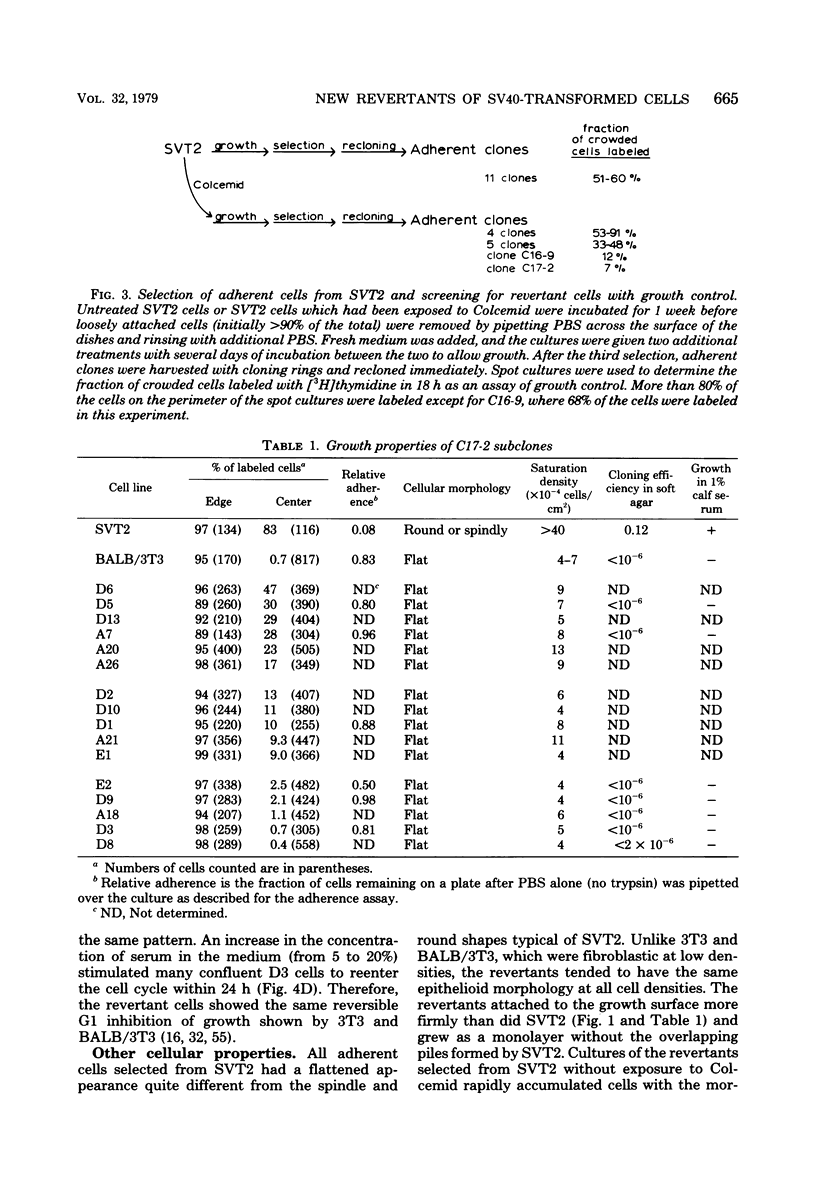
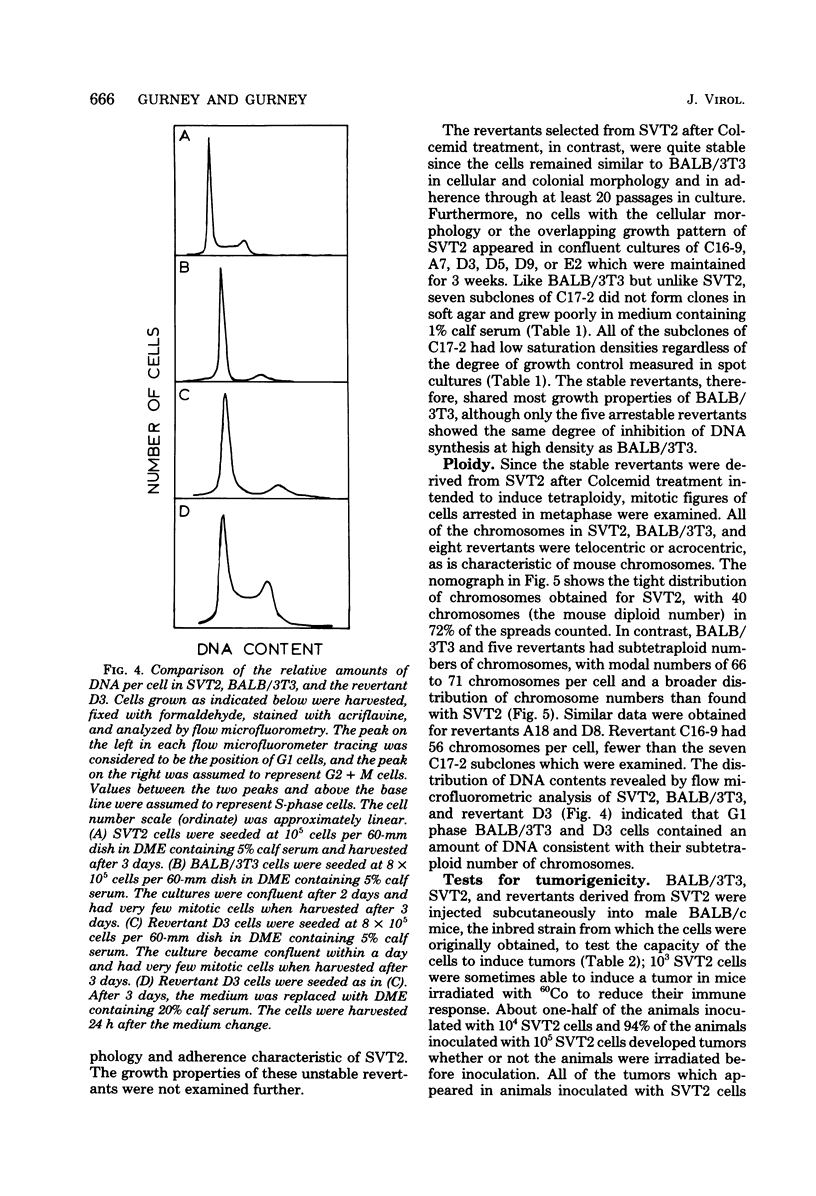
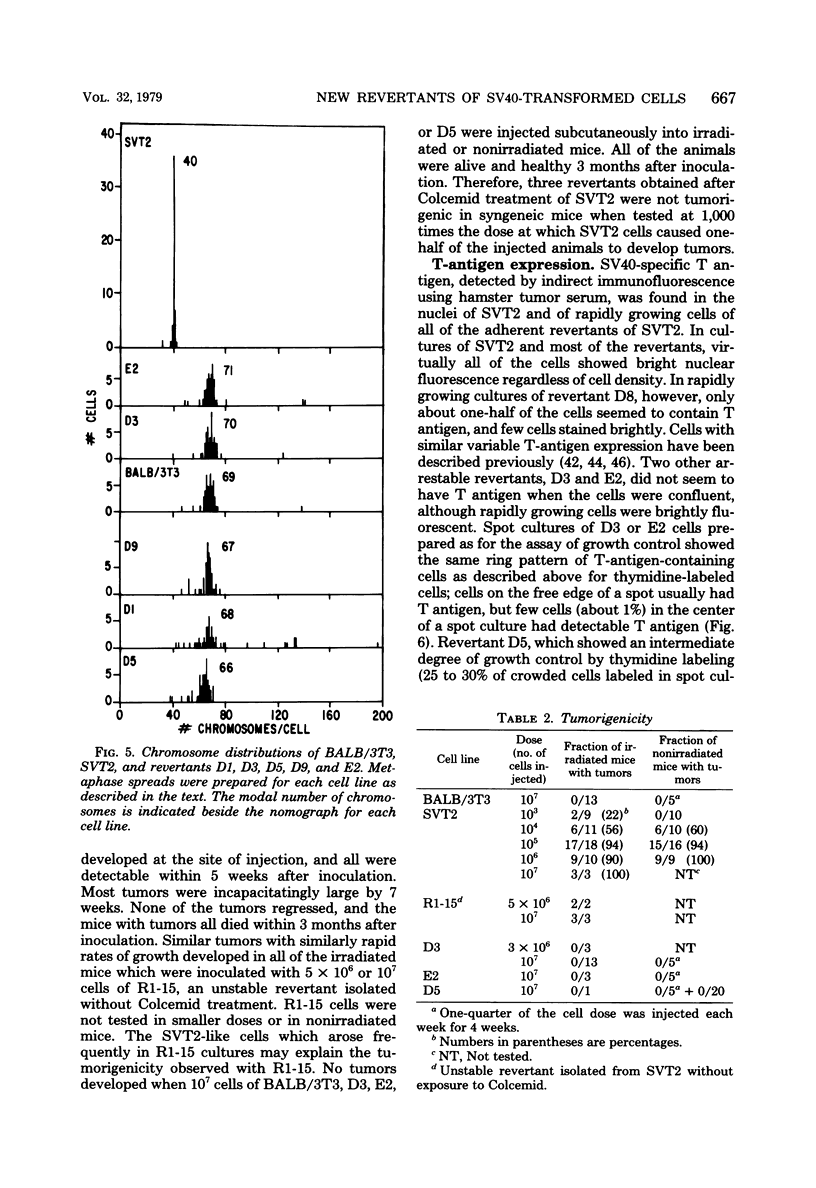
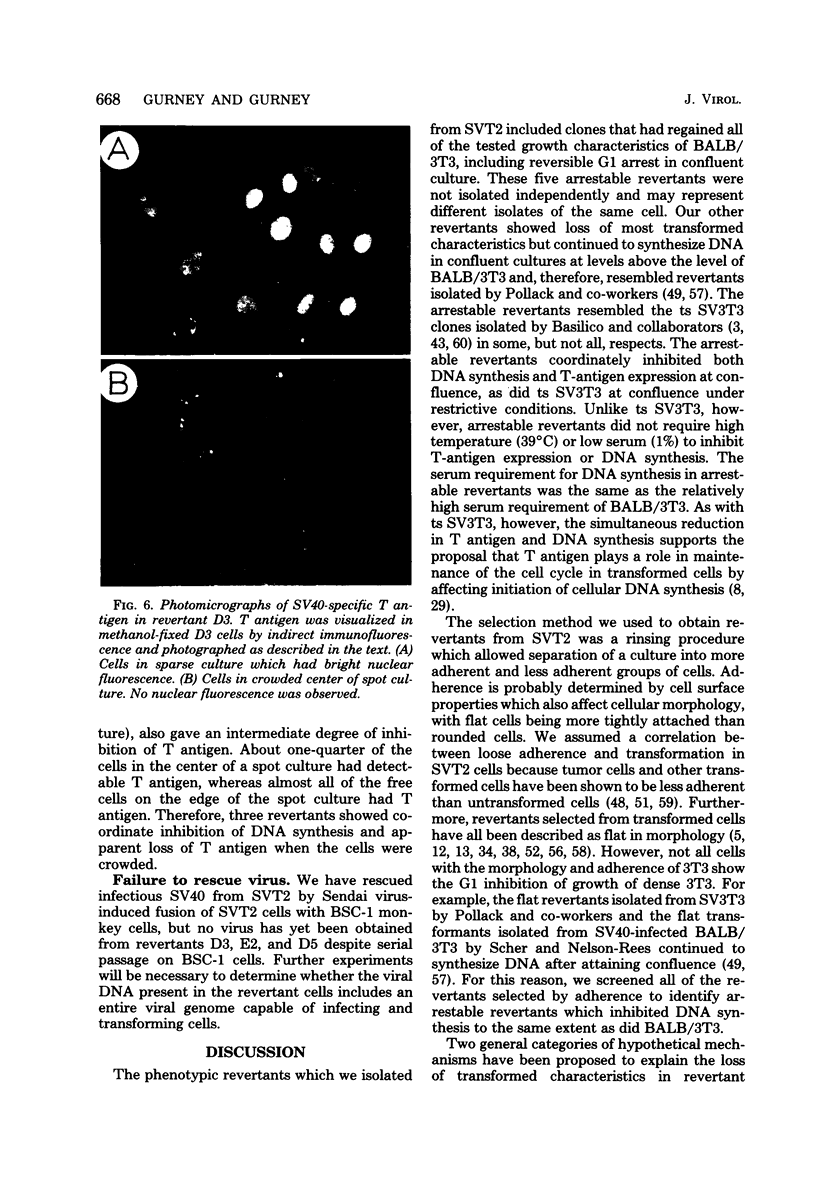
Abstract
Phenotypic revertants were isolated from simian virus 40-transformed cells in order to examine the relationship between simian virus 40 T-antigen expression and G1 arrest of growth. Revertant clones with increased adherence were selected from cultures of SVT2, a simian virus 40-transformed BALB/c mouse cell line, and screened to find arrestable revertant clones which inhibited DNA synthesis when crowded. The clones selected from untreated SVT2 were unstable and showed little or no inhibition of DNA synthesis when crowded. Stable revertants were found after treatment of SVT2 with Colcemid to increase ploidy. The stable revertants all lost most transformed growth properties tested, including tumorigenicity, but only a few showed the same degree of inhibition of DNA synthesis at high cell density as BALB/3T3. All revertant clones expressed T antigen at low cell density. Three revertants showed coordinate inhibition of DNA synthesis and apparent loss of T antigen at high cell density. We suggest that changes in gene dosage rather than mutations caused the altered properties of the new revertants and that continued DNA synthesis in confluent cultures may be the transformed phenotype that requires the least simian virus 40 T antigen.
Full text
PDF

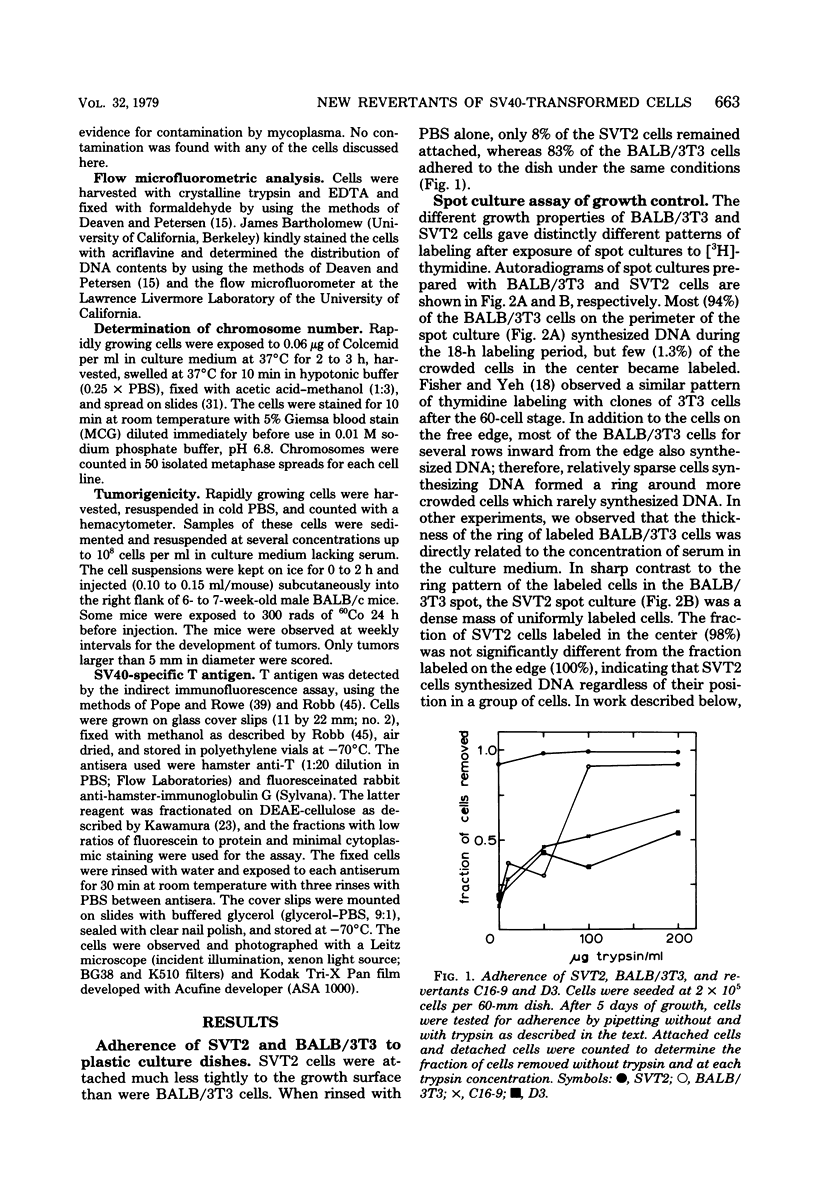
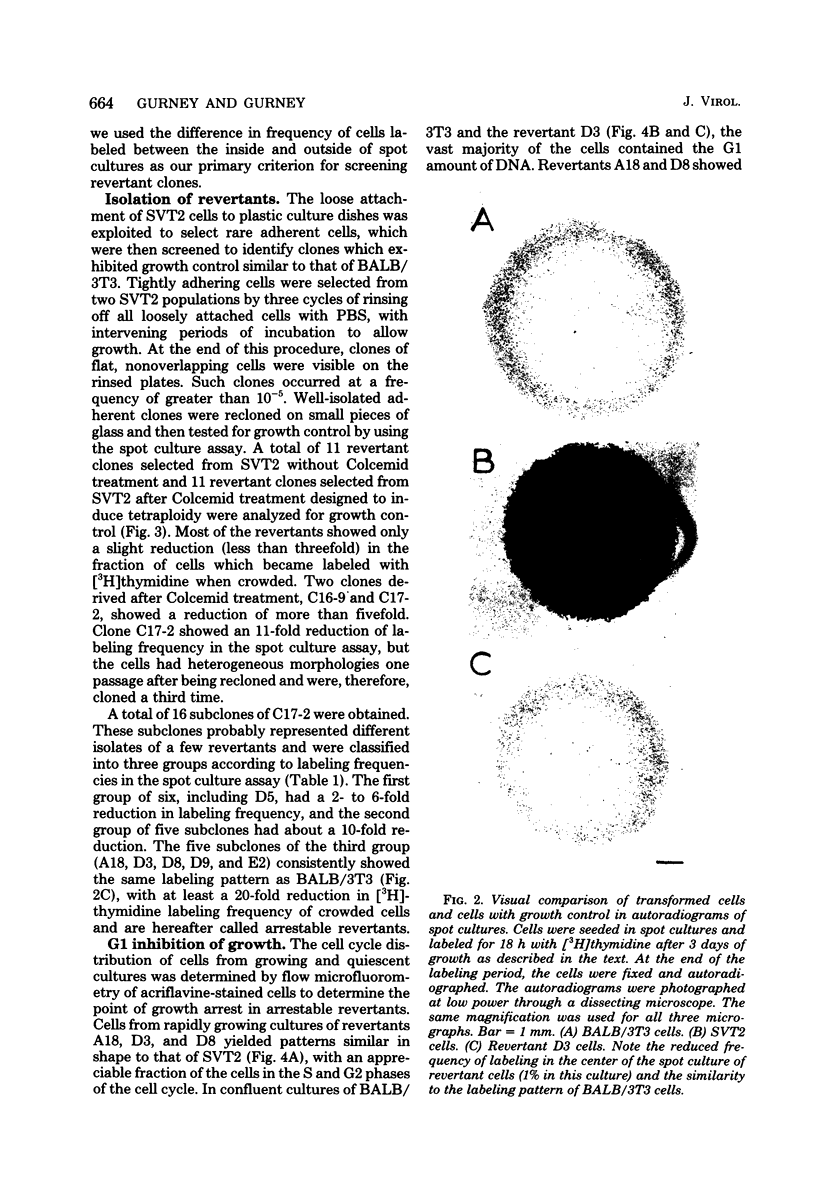



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Basilico C., Zouzias D. Regulation of viral transciption and tumor antigen expression in cells transformed by simian virus 40. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1931–1935. doi: 10.1073/pnas.73.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Ozanne B., Sugden B., Sharp P. A., Sambrook J. Viral DNA in transformed cells. III. The amounts of different regions of the SV40 genome present in a line of transformed mouse cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4183–4187. doi: 10.1073/pnas.71.10.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley W. E., Culp L. A. Contact-inhibited revertant cell lines isolated from simian virus 40-transformed cells. VII. Serum detachment-resistant revertant cells. J Virol. 1977 Mar;21(3):1228–1231. doi: 10.1128/jvi.21.3.1228-1231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Brugge J. S., Noonan C. A. Transformation of primate and rodent cells by temperature-sensitive mutants of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):25–36. doi: 10.1101/sqb.1974.039.01.006. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Soule H. R. Role of the simian virus 40 gene A product in regulation of DNA synthesis in transformed cells. J Virol. 1978 Jun;26(3):584–594. doi: 10.1128/jvi.26.3.584-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin L. A. The effect of ploidy on chemical mutagenesis in cultured Chinese hamster cells. J Cell Physiol. 1973 Oct;82(2):299–307. doi: 10.1002/jcp.1040820218. [DOI] [PubMed] [Google Scholar]
- Cox D. M., Puck T. T. Chromosomal nondisjunction: the action of colcemid on Chinese hamster cells in vitro. Cytogenetics. 1969;8(2):158–169. doi: 10.1159/000130032. [DOI] [PubMed] [Google Scholar]
- Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from simian virus 40-transformed cells. 3. Concanavalin A-selected revertant cells. J Virol. 1972 Apr;9(4):611–620. doi: 10.1128/jvi.9.4.611-620.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp L. A., Grimes W. J., Black P. H. Contact-inhibited revertant cell lines isolated from SV40-transformed cells. I. Biologic, virologic, and chemical properties. J Cell Biol. 1971 Sep;50(3):682–690. doi: 10.1083/jcb.50.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F. M., Adelberg E. A. Use of somatic cell hybrids for analysis of the differentiated state. Bacteriol Rev. 1973 Jun;37(2):197–214. doi: 10.1128/br.37.2.197-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. Measurements of mammalian cellular DNA and its localization in chromosomes. Methods Cell Biol. 1974;8(0):179–204. [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Fisher H. W., Yeh J. Contact inhibition in colony formation. Science. 1967 Feb 3;155(3762):581–582. doi: 10.1126/science.155.3762.581. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Rassoulzadegan M., Cuzin F. Expression of simian virus 40 early genes in transformed rat cells is correlated with maintenance of the transformed phenotype. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4987–4991. doi: 10.1073/pnas.75.10.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney T., Jr Local stimulation of growth in primary cultures of chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1969 Mar;62(3):906–911. doi: 10.1073/pnas.62.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. Polyploid series of mammalian cells. Exp Cell Res. 1971 Jun;66(2):329–336. doi: 10.1016/0014-4827(71)90685-9. [DOI] [PubMed] [Google Scholar]
- Kelly F. Chromosome analysis of a simian virus 40-transformed mouse cell line and two variant sublines that are resistant to cytochalasin B1. Cancer Res. 1975 May;35(5):1210–1213. [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino T., Yamaguchi N. Characterization of T antigen in cells infected with a temperature-sensitive mutant of simian virus 40. J Virol. 1975 Jun;15(6):1302–1307. doi: 10.1128/jvi.15.6.1302-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORHEAD P. S., NOWELL P. C., MELLMAN W. J., BATTIPS D. M., HUNGERFORD D. A. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960 Sep;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Tuan A. A definitive cloning technique for human fibroblast cultures. Proc Soc Exp Biol Med. 1966 Oct;123(1):138–140. doi: 10.3181/00379727-123-31423. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y., Avila J., Saral R. The semiautonomous replicon: a molecular model for the oncogenicity of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):17–24. doi: 10.1101/sqb.1974.039.01.005. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Stein S. Resting state in normal and simian virus 40 transformed Chinese hamster lung cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1655–1659. doi: 10.1073/pnas.73.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilausen K., Green H. Reversible arrest of growth in G1 of an established fibroblast line (3T3). Exp Cell Res. 1965 Oct;40(1):166–168. doi: 10.1016/0014-4827(65)90306-x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B. Variants of simian virus 40-transformed 3T3 cells that are resistant to concanavalin A. J Virol. 1973 Jul;12(1):79–89. doi: 10.1128/jvi.12.1.79-89.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R. E. Cellular and viral contributions to maintenance of the SV40-transformed state. In Vitro. 1970 Jul-Aug;6(1):58–65. doi: 10.1007/BF02616134. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Wolman S., Vogel A. Reversion of virus-transformed cell lines: hyperploidy accompanies retention of viral genes. Nature. 1970 Dec 5;228(5275):938–passim. doi: 10.1038/228938a0. [DOI] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz Z., Sachs L. Control of the reversion of properties in transformed cells. Nature. 1970 Jan 10;225(5228):136–139. doi: 10.1038/225136a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Perbal B., Cuzin F. Growth control in simian virus 40-transformed rat cells: temperature-independent expression of the transformed phenotype in tsA transformants derived by agar selection. J Virol. 1978 Oct;28(1):1–5. doi: 10.1128/jvi.28.1.1-5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C., Basilico C. Mutation causing temperature-sensitive expression of cell transformation by a tumor virus (SV40-3T3 mouse cells-growth control). Proc Natl Acad Sci U S A. 1972 Jan;69(1):109–114. doi: 10.1073/pnas.69.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Robb J. A. Variation in the appearance of SV40 tumor antigen in transformed cells. Exp Cell Res. 1977 May;106(2):441–445. doi: 10.1016/0014-4827(77)90199-9. [DOI] [PubMed] [Google Scholar]
- Robinson C. C., Lehman J. M. Simian virus 40 A gene function: DNA content analysis of Chinese hamster cells transformed by an early temperature-sensitive virus mutant. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4389–4393. doi: 10.1073/pnas.75.9.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Nelson-Rees W. A. Direct isolation and characterization of "flat" SV40-transformed cells. Nat New Biol. 1971 Oct 27;233(43):263–265. doi: 10.1038/newbio233263a0. [DOI] [PubMed] [Google Scholar]
- Sharp J. D., Capecchi N. E., Capecchi M. R. Altered enzymes in drug-resistant variants of mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3145–3149. doi: 10.1073/pnas.70.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R., Pollock K. The adhesion of BHK and PyBHK cells to the substratum. Cell. 1974 Sep;3(1):31–38. doi: 10.1016/0092-8674(74)90034-8. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Pollack R., Topp W., Botchan M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell. 1978 Jan;13(1):19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G., Matsuya Y., Bloom S., Robbins A., Green H. Stimulation of RNA synthesis and cell division in resting cells by a factor present in serum. Wistar Inst Symp Monogr. 1967;7:87–101. [PubMed] [Google Scholar]
- Vogel A., Pollack R. Isolation and characterization of revertant cell lines. IV. Direct selection of serum-revertant sublines of SV40-transformed 3T3 mouse cells. J Cell Physiol. 1973 Oct;82(2):189–198. doi: 10.1002/jcp.1040820207. [DOI] [PubMed] [Google Scholar]
- Vogel A., Pollack R. Isolation and characterization of revertant cell lines. VII. DNA synthesis and mitotic rate of serum-sensitive revertants in non-permissive growth conditions. J Cell Physiol. 1975 Feb;85(1):151–162. doi: 10.1002/jcp.1040850116. [DOI] [PubMed] [Google Scholar]
- Vogel A., Risser R., Pollack R. Isolation and characterization of revertant cell lines. 3. Isolation of density-revertants of SV40-transformed 3T3 cells using colchicine. J Cell Physiol. 1973 Oct;82(2):181–188. doi: 10.1002/jcp.1040820206. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Hale A. H., Losasso L. Decreased adherence to the substrate in Rous sarcoma virus-transformed chicken embryo fibroblasts. Cell. 1977 Jan;10(1):45–51. doi: 10.1016/0092-8674(77)90138-6. [DOI] [PubMed] [Google Scholar]
- Zouzias D., Basilico C. T-antigen expression in proliferating and non-proliferating simian virus 40-transformed mouse cells. J Virol. 1979 Jun;30(3):711–719. doi: 10.1128/jvi.30.3.711-719.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]