Abstract
Evaluating different swabbing materials for spore recovery efficiency (RE) from steel surfaces, we recorded the maximum RE (71%) of 107 Bacillus subtilis spores with Tulips cotton buds, followed by Johnson's cotton buds and standard Hi-Media cotton, polyester, nylon, and foam (23%) swabs. Among cotton swabs, instant water-absorbing capacity or the hydrophilicity index appeared to be the major indicator of RE, as determined by testing three more brands. Tulips swabs worked efficiently across diverse nonporous surfaces and on different Bacillus spp., registering 65 to 77% RE.
TEXT
Proper sampling and retrieval methods are important for the surveillance of spores of hazardous pathogens, like Bacillus anthracis, and for monitoring microbial populations in space research in addition to traditional applications in food, clinical, and general microbiology (1–3). While wipes and vacuum suction are considered ideal for large-area sampling (1), swabs are preferred for small-area monitoring (4–6). Our prime interest in monitoring spore load on nonporous surfaces was directed at increasing our preparedness to address accidental surface contamination from different Bacillus spp. (7) in order to ensure a clean working environment.
The spore recovery efficiency (RE) in past studies employing swabs varied depending on the surface sampled and the swabbing material, with most of the studies generally reporting <50% RE (2–5). In addition, little information has appeared to be available from developing parts of the world with regard to effective spore surveillance, the input of which would become valuable in the event of an unprecedented public health hazard arising from the dreaded B. anthracis. Although different compositions of swabs, such as cotton, foam, polyester, rayon, sponge, and blends, are available commercially, none has been found to be universally acceptable (1, 3–6). Cotton swabs are easily available worldwide and have registered higher RE than synthetic swabs in some studies (6). Further, we also felt it prudent to try the universally available cotton buds (also called ear buds or Q-tips) for spore surveillance. This study was undertaken to develop an efficient spore surveillance methodology applicable across different surfaces and organisms.
B. subtilis (ATCC 6051) was used as the primary test organism. A spore suspension prepared from 7- to 10-day-old nutrient agar (NA) cultures (30°C) in sterile distilled water (DW) after 70°C heat treatment (10 min) was dispersed in 50% ethanol, and the optical density at 600 nm (OD) was adjusted to 2.0. The spore suspension showed an initial CFU of 1.26 × 109 to 1.43 × 109 ml−1, which after overnight storage dropped to 1.02 ± 0.189 × 109 ml−1 but thereafter remained consistent with 4°C storage over 8 weeks of monitoring.
Different standard swab materials from Hi-Media (HM) Biosciences (Mumbai, India), designated HM-foam, HM-nylon, HM-polyester, and HM-cotton, and two brands of cotton buds, namely Johnson's (Johnson & Johnson, manufactured at Mumbai, India) and Tulips (M/s Janes and Jones Pvt. Ltd., New Delhi, India) (see Fig. S1 in the supplemental material), were employed for spore recovery. A preliminary assessment of the extraction efficiency of directly applied spores from different swab materials indicated their ranking in increasing order as HM-cotton, Johnson's cotton, HM-nylon, Tulips cotton, HM-foam, and HM-polyester in the 55 to 95% range (Table 1).
Table 1.
Swab extraction and recovery efficiency in the swabbing trialsa
| Type of swabd | Swab description | Swab extraction efficiencyg |
Recovery efficiency per sampling spotg |
||
|---|---|---|---|---|---|
| CFU (106) | %b | CFU (106) | %c | ||
| HM-foame | 20-mm rough bud; thin solid plastic handle | 9.5 ± 1.48A | 86.2 ± 13.38A | 2.33 ± 0.39D | 22.8 ± 3.9D |
| HM-nylone | 20-mm medium soft bud; thin solid plastic handle | 8.9 ± 7.66B | 80.5 ± 6.94B | 4.53 ± 0.93C | 45.0 ± 9.3C |
| HM-polyestere | 15-mm medium-soft bud; tubular PP handle | 10.5 ± 1.16A | 94.7 ± 10.56A | 5.06 ± 0.91B | 50.2 ± 9.1B |
| HM-cottone | 12-mm firm cotton bud; solid wooden handle | 6.1 ± 0.80C | 54.9 ± 7.23C | 5.70 ± 0.96B | 56.6 ± 9.6B |
| Johnson's budf | 15-mm medium-soft cotton bud; tubular PP handle | 8.1 ± 1.21B | 73.9 ± 10.94B | 6.96 ± 1.29A | 64.1 ± 12.9A |
| Tulips budf | 15-mm soft cotton bud; tubular PP handle | 9.1 ± 0.71A,B | 82.8 ± 6.45A | 7.06 ± 0.76A | 70.6 ± 7.6A |
Different swabs used in the swabbing trials and the assessment of extraction efficiency of directly applied spores on the swab head and the recovery efficiency of Bacillus subtilis spores from inoculum-seeded 5-cm-diameter spots on a stainless steel laminar airflow workbench.
Based on a seeded inoculum of 11.05 ± 0.54 × 106 CFU per swab; six replications.
Based on a seeded inoculum of 10.07 ± 1.57 × 106 CFU per spot; six replications.
HM, Hi-Media source.
Presterilized and individually packed.
To be autoclaved.
Significant at P = 0.001. Values followed by the same letter are not significantly different.
To assess the spore RE with different swabs, spores were seeded by applying 10 μl of a 2.0 OD suspension inside marked 5-cm-diameter sampling spots (approximately 20 cm2) on the steel workbench of a laminar airflow system (LAF), followed by 15 min air drying. The seeded spores was assessed in each trial by adding a 10-μl suspension to 10 ml phosphate-buffered saline (PBS)–0.2% Tween 20 (PBST) in 15-ml polypropylene (PP) tubes (Axygen Scientific Pvt. Ltd., New Delhi, India), followed by CFU assessment. After application of 100 μl PBST as 8 to 12 microdrops, the spore-affixed spots (1.0 × 107 to 1.2 × 107 CFU) were swabbed by repeated gentle circular strokes and turning of the swab head, and the swab head was vortex extracted (4) at top speed for 2 min in 10 ml PBST in PP tubes.
For CFU estimations, a spotting-and-tilt-spreading (SATS) approach was adopted wherein 100 μl of a serial dilution level (103) that yielded 30 to 300 colonies per plate was spotted on plates with 20 ml fresh NA and the inoculum was spread by slightly tilting the plate, followed by surface drying for 4 to 6 min (8). Colony counts were made after 1 to 2 days of incubation at 30°C, and the RE was assessed relative to the seeded CFU (2). The experiments were set up in a completely randomized design with six replicate sampling spots and collection tubes per treatment.
RE, which involved a combination of retrieval efficacy from the surface and extraction efficiency from the swab head, was at its maximum with Tulips cotton, followed by Johnson's cotton, HM-cotton, HM-polyester, HM-nylon, and HM-foam in the 23 to 71% range (P < 0.01; Table 1). As a plausible indicator of RE, different quality parameters of swab materials were assessed, which included the net weight and instant-dip and saturation-dip water-holding capacity. The hydrophilicity index (H-index) of different swabs was defined as the ratio of the amount of water held after a flash-dip of the swab head to the net weight of the swab material (mg/mg), and the gross water-holding capacity index (GWH-index) was defined as the ratio of water held at saturation after 10 min soaking to its net weight.
Considerable variations in the weight of the swab material or the amounts of water absorbed after a flash-dip or with saturation soaking were observed between different swabs (see Table S1 in the supplemental material). Nylon appeared to be the most hydrophilic material (H-index of 16.6), but it had the lowest net swab weight. Tulips cotton, Johnson's cotton, HM-polyester, HM-foam, and HM-cotton ranked for H-index in that order (4.9, 2.1, 2.1, 1.7, and 0.9, respectively). In the case of cotton swabs, a higher H-index appeared to contribute to a higher RE. Cotton swabs also proved very cost-effective compared with synthetic swabs (see Table S1).
An evaluation of the above three brands of cotton swabs on glass and granite surfaces showed 52, 65, and 77% RE for HM, Johnson's and Tulips cotton, respectively, for glass (P = 0.015) and 43, 63, and 76% (P = 0.0247), respectively, for granite. The results indicated that RE for different surfaces varied with the brand of cotton, but there were no significant differences in RE between the two surfaces with the same brand.
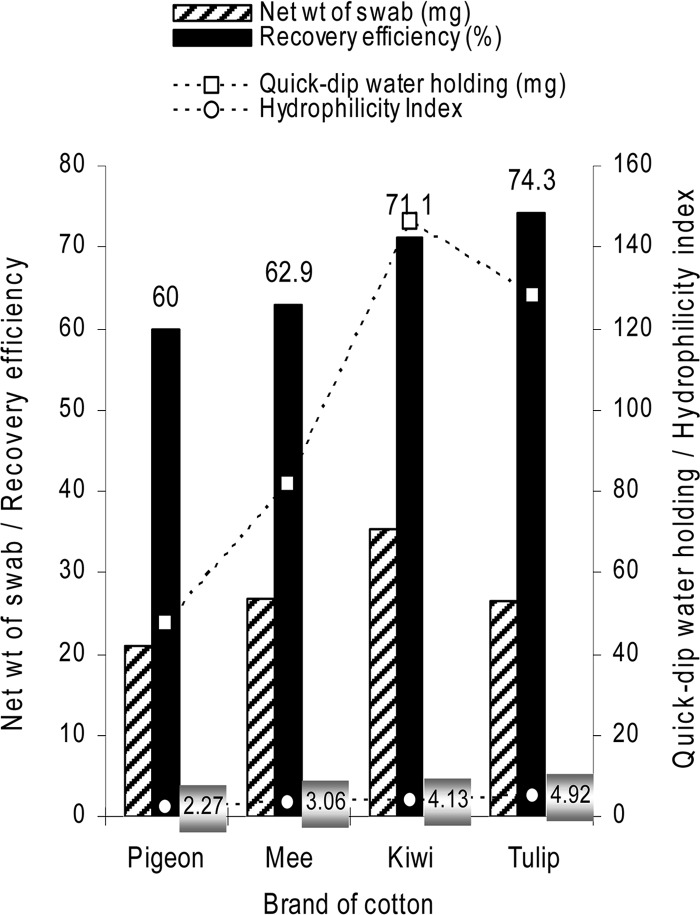
Further, cotton buds of three additional brands, namely Kiwi (Kiwi Cotton Crafts, Himathnagar, Gujarat, India), Mee (ME N′ MOMS Pvt. Ltd., Mumbai, India), and Pigeon (Sanritsu Co. Ltd., Oshima, Philippines), were assessed for H-index and RE on a steel surface. The swabs differed significantly in RE, net weight, and quick-dip and saturation water-holding capacities, but the RE appeared to be governed by the H-index of cotton (Fig. 1).
Fig 1.
Recovery efficiency of Bacillus subtilis spores from a stainless steel surface with different brands of cotton buds in relation to the net weight and water-holding capacity/hydrophilicity index of swab material.
Different nonporous, hydrophobic surfaces were evaluated for RE employing Tulips cotton (30 s swabbing). Glass, laminated plywood, rexine, plastic, granite, vitrified smooth tile, and nonvitrified floor tile showed 75.7, 69.6, 72.9, 70.8, 69.3, 66.6, and 71.7% RE, respectively, in comparison with the 64.5% recorded for steel surfaces (P = 0.724).
An assessment of RE at different spore densities with a Tulips cotton swabbing procedure employing 100 μl PBST and 30 s swabbing was undertaken on LAF by providing higher or lower CFU per spot in comparison with the generally employed 107 spores. The RE amounted to 78.1, 72.2, 66.9, 61.8, and 52.8% at seeding densities of 108, 107, 106, 105, and 104, respectively, per sampling spot (P < 0.01). A significant correlation was observed between seeding density and RE (r = 0.556; degrees of freedom, 29). A further assessment of RE at higher and lower spore densities (108 and 104 spores per spot) with different swab materials indicated a reduction in RE at lower seeding densities for all four swabs (HM-foam, 28.3 and 18.8%; HM-polyester, 53.8 and 24.4%; HM-nylon, 58.0 and 51.8%; Tulips cotton, 74.0 and 51.2%).
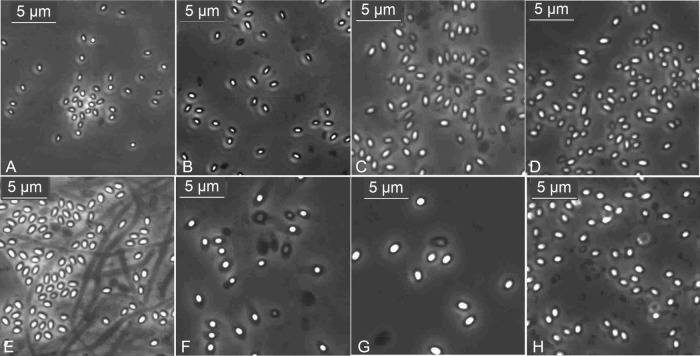
The Tulips cotton swabbing procedure worked with similar RE for different Bacillus spp. (B. pumilus, B. cereus, B. thuringiensis, B. subtilis) and other sporeformers, namely, a Brevibacillus sp., a Lysinibacillus sp., and a Paenibacillus sp., registering 70.1, 76.4, 76.2, 71.5, 72.1, 71.3, and 74.4% RE, respectively, at 107 spores seeded per spot on steel (see Table S2 in the supplemental material). The organisms showed variations in sizes and shapes of spores, with B. cereus, B. thuringiensis, and Brevibacillus sp. almost identical in size to the spores of B. anthracis Sterne (Fig. 2).
Fig 2.
Spores of different organisms under 100× phase objective: Bacillus subtilis (A), B. pumilus (B), B. cereus (C), B. thuringiensis (D), Brevibacillus sp. (E), Lysinibacillus sp. (F), Paenibacillus sp. (G), and formalin-killed spores of B. anthracis Sterne (H).
In the present study, foam, nylon, and polyester swabs registered relatively low RE (23 to 50%) compared with cotton swabs (57 to 71%), and the RE recorded with the chosen cotton swabs (Tulips) was notably higher than what is generally attained by employing synthetic swabs for B. anthracis spore surveillance (2–5). The RE of cotton swabs appeared to be linked to the quick water-absorbing capacity, leading to the identification of H-index as a major criterion for selecting efficient cotton swabs. In different reports, varying RE have been reported (1–6). Such studies have often taken into account various swab types in a study but not the same material from different sources. The variations in the processing of cotton, the wax content, and the bud manufacturing procedure could contribute to the alterations in bud attributes.
It is considered to be advantageous to employ a swab material that works across diverse surfaces (1). In this respect, Tulips cotton proved quite efficient. Tulips swabs also proved effective across different sporeformers. This information assumes significance in view of the reports on varying RE for different organisms (3) and the efforts made to identify proper surrogates for B. anthracis, for which B. thuringiensis and B. cereus are now recommended based on similar spore size (9, 10). All the swab items showed reductions in RE at lower spore densities, as reported previously (2, 3, 6), but Tulips cotton proved more efficient at various spore levels.
The global availability, low cost, feasibility of autoclaving, and ease of handling, together with the high RE, offer the scope for selecting the ideal type of cotton swab for spore monitoring. It is proposed to select swabs with a net weight of ≥25 mg and an H-index of >4.0, while buds with a 2.0 to 4.0 H-index could be used in preference to synthetic swabs. The conclusions from this study will facilitate the monitoring of diverse surfaces for Bacillus spores and help strengthen the preparedness to undertake monitoring for biohazardous agents across developed, developing, and underdeveloped parts of the world.
Supplementary Material
ACKNOWLEDGMENTS
This study was taken as part of the Institute Project 9.3.1, “Tissue culture systems in horticultural crops with reference to management and exploitation of endophytes,” and the ICAR-AMAAS project “Basic and applied investigations on endophytic microorganisms in horticultural crops.”
Listing of vendors of various swabs is for information only and does not imply endorsement of any vendor or product by the authors or the organization.
Footnotes
Published ahead of print 19 October 2012
This article is IIHR contribution no. 65-2011.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02626-12.
REFERENCES
- 1. Edmonds JM. 2009. Efficient methods for large area surface sampling of sites contaminated with pathogenic microorganisms and other hazardous agents: current state, needs and perspectives. Appl. Microbiol. Biotechnol. 84:811–816 [DOI] [PubMed] [Google Scholar]
- 2. Hodges LR, Rose LJ, O'Connell H, Arduino MJ. 2010. National validation study of a swab protocol for the recovery of Bacillus anthracis spores from surfaces. J. Microbiol. Methods 81:141–146 [DOI] [PubMed] [Google Scholar]
- 3. Probst A, Facius R, Wirth R, Wolf M, Moissl-Eichinger C. 2011. Recovery of Bacillus spore contaminants from rough surfaces: a challenge to space mission cleanliness control. Appl. Environ. Microbiol. 77:1628–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rose L, Jensen B, Peterson A, Banerjee SN, Arduino MJ. 2004. Swab materials and Bacillus anthracis spore recovery from non-porous surfaces. Emerg. Infect. Dis. 10:1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodges LR, Rose LJ, Peterson A, Noble-Wang J, Arduino MJ. 2006. Evaluation of a macrofoam swab protocol for the recovery of Bacillus anthracis spores from a steel surface. Appl. Environ. Microbiol. 72:4429–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edmonds JM, Collett PJ, Valdes ER, Skowronski EW, Pellar GJ, Emanuel PA. 2009. Surface sampling of spores in dry deposition aerosols. Appl. Environ. Microbiol. 75:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas P. 2012. Long-term survival of Bacillus spores in alcohol and identification of 90% ethanol as relatively more spori/bactericidal. Curr. Microbiol. 64:130–139 [DOI] [PubMed] [Google Scholar]
- 8. Thomas P, Sekhar AC, Mujawar MM. 2012. Non-recovery of varying proportions of viable bacteria during spread-plating governed by the extent of spreader usage and proposal for an alternate spotting-spreading approach to maximize the CFU. J. Appl. Microbiol. 113:339–350 [DOI] [PubMed] [Google Scholar]
- 9. Carrera M, Zandomeni RO, Fitzgibbon J, Sagripanti J-L. 2007. Difference between the spore sizes of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 102:303–312 [DOI] [PubMed] [Google Scholar]
- 10. Greenberg DL, Busch JD, Keim P, Wagner DM. 2010. Identifying experimental surrogates for Bacillus anthracis spores: a review. Invest. Genet. 1:4 http://www.investigativegenetics.com/content/1/1/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.