Abstract
Calcium carbonate increases growth, substrate utilization, and acetone-butanol-ethanol (ABE) fermentation by Clostridium beijerinckii NCIMB 8052. Toward an understanding of the basis for these pleiotropic effects, we profiled changes in the C. beijerinckii NCIMB 8052 proteome that occur in response to the addition of CaCO3. We observed increases in the levels of different heat shock proteins (GrpE and DnaK), sugar transporters, and proteins involved in DNA synthesis, repair, recombination, and replication. We also noted significant decreases in the levels of proteins involved in metabolism, nucleic acid stabilization, sporulation, oxidative and antibiotic stress responses, and signal transduction. We determined that CaCO3 enhances ABE fermentation due to both its buffering effects and its ability to influence key cellular processes, such as sugar transport, butanol tolerance, and solventogenesis. Moreover, activity assays in vitro for select solventogenic enzymes revealed that part of the underpinning for the CaCO3-mediated increase in the level of ABE fermentation stems from the enhanced activity of these catalysts in the presence of Ca2+. Collectively, these proteomic and biochemical studies provide new insights into the multifactorial basis for the stimulation of ABE fermentation and butanol tolerance in the presence of CaCO3.
INTRODUCTION
Growing concerns over increased emissions of greenhouse gases from the combustion of fossil fuels and the global energy crisis have recently spawned extensive research into renewable energy. As a result, there is a resurgent interest in butanol as an alternative fuel, due mainly to its higher energy content than ethanol and its compatibility with gasoline, with the latter trait making it more compatible with existing pipelines for distribution (1, 2). However, the cost of butanol production, which currently relies on petroleum feedstock, is not favorable compared to gasoline (3). Although acetone-butanol-ethanol (ABE) fermentation with solventogenic Clostridium species holds promise as a potentially cheaper means of butanol production, low yields and productivity due to butanol toxicity to the fermenting cells have hampered the commercialization of biobutanol (4, 5).
To increase yield and productivity, fermentation broth additives such as acetate (6, 7) and calcium carbonate (3, 8, 9) have been successfully utilized. During ABE fermentation by solventogenic Clostridium species, CaCO3 has been shown to stimulate sugar utilization, butanol production, and butanol tolerance (3, 8, 9). For example, during Clostridium acetobutylicum fermentation, the addition of 8 g/liter butanol (to mimic solvent intolerance) limited xylose utilization to 30 g/liter (from a starting concentration of 60 g/liter); however, upon the addition of CaCO3 (10 g/liter), xylose utilization increased to 43 g/liter (8). Similarly, when ABE fermentation was conducted in an iron-deficient medium, which modifies carbon and electron flow to favor early butanol accumulation, the xylose utilization by C. acetobutylicum was drastically inhibited; this effect was significantly reversed by the addition of 10 g of CaCO3/liter (8). In both cases, better sugar utilization resulted in increased butanol production.
To engender these physiological changes, Ca2+ must exert a wide range of effects on the cellular machinery of solventogenic bacteria. Toward uncovering such mechanisms, we first undertook a characterization of the proteome of Clostridium beijerinckii NCIMB 8052 (referred to here as C. beijerinckii) without and with exogenous CaCO3. We used two-dimensional (2-D) gel electrophoresis and mass spectrometry to profile and identify differentially expressed proteins; as support for the proteomic data, changes in the mRNA levels of some of these candidates were validated by real-time quantitative PCR (qRT-PCR). We discuss changes in levels of proteins that could potentially impact the biosynthetic machinery, butanol stress tolerance, cell division, and glucose utilization. Second, to assess the extent to which the buffering capacity of CaCO3 contributes to its stimulatory effects on ABE fermentation, we evaluated the effects of a range of carbonates on butanol production and sugar utilization by C. beijerinckii. Our results indicate that the pronounced pleiotropic effects of CaCO3 exceed its buffering capacity and likely reflect its ability to mediate cellular signaling events. Finally, by measuring the activity of key solventogenic pathway enzymes in the absence and presence of Ca2+, we found that Ca2+ also modestly enhances their activities in vitro, and this might provide an unexpected indirect mechanism in vivo to alter the pH of the medium. We integrate these results to highlight the multifactorial basis for the Ca2+-induced increase in ABE fermentation.
MATERIALS AND METHODS
Fermentation and culture conditions.
C. beijerinckii NCIMB 8052 (ATCC 51743) was obtained from the American Type Culture Collection, Manassas, VA. Laboratory stocks were routinely maintained as spore suspensions in sterile, double-distilled water at 4°C. Spores (200 μl) were heat shocked for 10 min at 75°C and cooled on ice prior to inoculation into 10 ml of anoxic presterilized tryptone-glucose-yeast extract (TGY) medium. To create anaerobic conditions and generate anoxic TGY medium, loosely capped bottles with sterilized TGY medium were kept for 24 h in an anaerobic chamber (Coy Laboratory Products Inc., Ann Arbor, MI) with a modified atmosphere of 82% N2, 15% CO2, and 3% H2. Cultures were incubated for 12 to 14 h at 35°C ± 1°C under anaerobic conditions for inoculum buildup, as described elsewhere previously (4, 5). This was followed by the transfer of 8 ml of the actively growing inoculum into 92 ml anoxic presterilized TGY medium. The culture was incubated until it reached an optical density at 600 nm (OD600) of 0.9 to 1.1 (4 to 5 h).
To determine the optimal CaCO3 concentration for ABE production and for subsequent proteomic studies, fermentations were conducted for 72 h in semidefined P2 medium (4, 5) supplemented with 2, 4, 6, 8, or 10 g/liter of CaCO3. Precultures grown in TGY medium (6%) were transferred into loosely capped 250-ml Pyrex medium bottles containing P2 medium plus CaCO3 (2, 4, 6, 8, or 10 g/liter). P2 medium without CaCO3 was used as the negative control. Unless otherwise stated, all fermentations were conducted in triplicate, and the temperature was maintained at 35°C ± 1°C, without agitation or pH control. The pH profile was monitored with a Beckman Φ500 pH meter (Beckman Coulter Inc., Brea, CA). The growth of C. beijerinckii was estimated by using a DU800 spectrophotometer (Beckman Coulter Inc., Brea, CA) to measure the OD600, which was converted to cell dry weight by using a predetermined correlation (4). The concentration of glucose was analyzed by using a hexokinase- and glucose-6-phosphate dehydrogenase-coupled enzymatic assay, as described previously (5). The concentrations of the fermentation products acetate, butyrate, acetone, butanol, and ethanol were measured by using an Agilent Technologies 7890A gas chromatograph (Agilent Technologies Inc., Wilmington, DE) equipped with a flame ionization detector (FID) and a 30 μm (length) by 320 μm (internal diameter) by 0.50 μm (HP-Innowax film) J x W 19091N-213 capillary column, as described previously (10).
Protein extraction.
To investigate the effect of CaCO3 on the proteome of C. beijerinckii, precultures grown in TGY medium (6%) were transferred, as described above, into CaCO3-supplemented (4 g/liter) (treatment) and unsupplemented P2 medium in triplicate. Samples for protein extraction were taken from the control (P2 medium) and treatment (P2 medium plus 4 g/liter CaCO3) cultures in the early solventogenic phase, when cultures were producing the same amount of butanol (∼5.5 g/liter for 12 h and 24 h for treatment and control cultures, respectively). Sampling at this concentration ensured that cells for protein extraction were actively growing and in the solventogenic phase (when solventogenic enzymes are fully expressed). Cells were harvested from 300-ml cultures by centrifugation at 10,000 × g at 4°C for 15 min, washed twice with sterile distilled water, and stored overnight at −70°C. Cell pellets were then sent to Kendricks Laboratory Inc., Madison, WI, where two-dimensional (2-D) polyacrylamide gel electrophoresis, image analysis, and mass spectrometry were conducted.
Cells were disrupted by vigorous vortexing with intermittent freezing in 600 μl of osmotic lysis buffer (9.5 M urea, 2% [wt/vol] nonyl phenoxypolyethoxylethanol [NP-40], 2% ampholines [pH 4 to 9], 5% [vol/vol] β-mercaptoethanol) containing 1% (vol/vol) protease inhibitor cocktail {20 mM AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride], 1 mg/ml leupeptin, 0.36 mg/ml E-64 cysteine protease inhibitor, 500 mM EDTA, and 5.6 mg/ml benzamidine}, nucleases to final concentrations of 50 μg/ml (RNase) and 100 μg/ml (DNase) in 10 mM Tris-HCl (pH 7) and 5 mM MgCl2, a phosphatase inhibitor (EMD Biosciences, Darmstadt, Germany), and 100 mg of washed glass beads (with a mesh size of 425 to 6,000 μm; catalog number G9268; Sigma). This was followed by the addition of 300 μl of sodium dodecyl sulfate (SDS) boiling buffer (62.5 mM Tris [pH 6.8], 10% [vol/vol] glycerol, 2.3% [wt/vol] SDS, 50 mM dithiothreitol). The samples were then heated in a boiling water bath for 5 min before the protein concentration was determined by using a bicinchoninic acid (BCA) assay kit (Pierce Chemical Co., Rockford, IL) (11). The samples were then diluted to 6 mg/ml in a 1:1 solution of SDS boiling buffer-lysis (sample) buffer, both of which are described above.
Proteomics.
Six hundred micrograms of each crude protein extract was resolved in the first dimension (isoelectric focusing [IEF]) on a thin-tube gel with an inner diameter of 3.3 mm containing 2% ampholines with a pH range of 4 to 9 (Serva, Heidelberg, Germany) at 1,000 V for 20 h, according to methods described previously by O'Farrell (12) and Burgess-Cassler et al. (13). In the second dimension, samples were separated and visualized on 18.5-cm-wide dried-slab gels by using Coomassie blue R-350. Tropomyosin (1 μg) was added to each sample as an IEF internal standard prior to loading. Duplicate gels were run and analyzed for each protein sample from the treated and control cultures. Gel images were captured with a quantitative densitometer (GE Imagescanner III; GE Healthcare, Piscataway, NJ) and analyzed by using Progenesis Same Spots software (version 4.0; Nonlinear Dynamics, Durham, NC) and Progenesis PG240 software (version 2006; Nonlinear Dynamics, Durham, NC), such that all spots of interest and all varying spots were outlined, quantified, and matched on all the gels. The densitometer was tested for linearity prior to scanning with a calibrated neutral-density filter set (Melles Griot, Irvine, CA). The relative amount of each spot was computed by the software as a function of the intensity of each individual spot in comparison to the intensity of the entire gel. Spots of interest were sequenced by matrix-assisted laser desorption ionization–time of flight tandem mass spectrometry (MALDI-TOF-MS/MS). Jvirgel 2.0 (14) (http://www.jvirgel.de/) was used to identify the pI of proteins, followed by validation with the EXPASY Bioinformatics Resource Portal (http://expasy.org/).
qRT-PCR.
Following protein profiling, four proteins (Cbei_0830, Cbei_1049, Cbei_0829, and Cbei_2831) that showed increased levels and five proteins (Cbei_2110, Cbei_1273, Cbei_0476, Cbei_0422, and Cbei_2146) that were detected in smaller amounts in response to CaCO3 treatment were selected for validation by qRT-PCR. Most of these proteins were selected largely due to previous reports on their putative involvement in solventogenesis and/or the response to butanol stress; however, we stress that their role in Ca2+-mediated signaling has not been explored. Culture samples for RNA isolation were taken as described above for protein extraction. RNA was isolated by using the Qiagen RNA isolation kit (Qiagen Inc., Valencia, CA) according to the manufacturer's protocol. The RNA content was determined spectrophotometrically by the use of a NanoDrop 3300 spectrophotometer (Thermo Scientific, Wilmington, DE). For qRT-PCR, primers specific for the Cbei_0830, Cbei_1049, Cbei_0829, Cbei_2831, Cbei_2110, Cbei_1273, Cbei_0476, Cbei_0422, Cbei_2146, and 16S rRNA genes were designed (Table 1). These gene-specific primers were synthesized by Eurofins MWG Operon (Huntsville, AL).
Table 1.
Sequences of oligonucleotides used for amplification of target genes
| Gene (protein)a | Primer (sequence) |
|---|---|
| Cbei_R0001 (16S rRNA) | 16S-F (5′-GAAGAATACCAGTGGCGAAGGC-3′) |
| 16S-R (5′-ATTCATCGTTTACGGCGTGGAC-3′) | |
| Cbei_2110 (ArsA) | ArsA-F (5′-GGGCTTGAAAACGTAAGAGC-3′) |
| ArsA-R (5′-CTCCGCCTTTACCCATTGTA-3′) | |
| Cbei_1273 (hypothetical protein) | hp1-F (5′-ATTAGAGCAGGCGTTTTGGA-3′) |
| hp2-R (5′-AACCTTCTTTCAGCCGCATA-3′) | |
| Cbei_0476 (hypothetical protein) | hp2-F (5′-GCAAGCAGGCTTCTAGCATT-3′) |
| hp2-R (5′-CAGAAATGCAAATTGCTCCA-3′) | |
| Cbei_0422 (SpoIID) | spoIID-F (5′-TGATACAATTACTGCCAAG-3′) |
| spoIID-R (5′-GTTTTTCCAGAGCTGGTTGC-3′) | |
| Cbei_2146 (PdaA) | pdaA-F (5′-CATGAGAAGGATTGGCATCA-3′) |
| pdaA-R (5′-TGGTAAAGCCTTCGCTGTTT-3′) | |
| Cbei_0830 (DnaK) | dnak-F (5′-ACTAAGGATGCAGGTAAG-3′) |
| dnaK-R (5′-CACCACCTAAGTCATAAA-3′) | |
| Cbei_1049 (ESBP) | espb-F (5′-TTGGGAGTAAGCTTGGTTGC-3′) |
| esbp-R (5′-TGGAACTATTTCCCCGTTCTC-3′) | |
| Cbei_0829 (GrpE) | grpE-F (5′-GCTGTTGCAGCTGATGGTAG-3′) |
| grpE-R (5′-CCCTTTTGGAAAACTTCTGC-3′) | |
| Cbei_2831 (CBP) | cbp-F (5′-GCAGCAACAACAAAAGCAGA-3′) |
| cbp-R (5′-GGGTTATATGGTGCTGTTCC-3′) |
ESBP, extracellular solute-binding protein.
The stable 16S rRNA gene was used as a reference for comparing transcript levels of C. beijerinckii grown in the presence (treatment) and the absence (control) of CaCO3 at similar phases in their growth. We are aware that there might be variations in 16S rRNA levels at different stages in a bacterial growth curve, although this phenomenon did not occur in our case. The 16S rRNA qRT-PCRs were performed as internal controls to normalize the quantity of RNA inputs. Total RNAs (2 μg) were reverse transcribed to cDNA with SuperScript III reverse transcriptase (Invitrogen Corporation, Carlsbad, CA), according to the supplier's protocol. The resulting cDNA was used in qRT-PCRs using the DyNAmo HS SYBR green quantitative PCR (qPCR) kit (New England BioLabs Inc., Ipswich, MA). Quantitative real-time PCR (RT-PCR) analysis was conducted in triplicate by using the Bio-Rad iCycler continuous fluorescence detection system (Bio-Rad, Hercules, CA). The RT-PCR conditions were as follows: 15 min at 95°C and then 40 cycles of 10 s at 95°C and 30 s at 55°C, followed by heating from 55°C to 95°C with a ramp speed of 1°C per 10 s. This resulted in melting curves. The expression levels of all the tested genes were standardized against the expression level of the internal control gene (16S rRNA) (10).
Effect of carbonates on C. beijerinckii ABE production.
To evaluate effects of different carbonates on ABE production by C. beijerinckii, batch fermentations were conducted in 250-ml Pyrex screw-cap medium bottles, as previously described (4, 5, 15), with minor modifications. Pyrex screw-cap medium bottles containing 150 ml P2 medium were supplemented with 2 g/liter CaCO3, (NH4)2CO3, NH4HCO3, K2CO3, NaHCO3, and Na2CO3. These carbonates were added to a final concentration of 2 g/liter, because some of the tested compounds were toxic above this concentration. Unless otherwise stated, all fermentations were conducted in triplicate or more, the temperature was maintained at 35°C ± 1°C, and no agitation or pH control was used. Samples (5 ml) were withdrawn at 12-h intervals to measure the cell concentration, pH, glucose concentration, and ABE concentration.
Effect of Ca2+ on C. beijerinckii NCIMB 8052 growth and ABE production.
To test whether the stimulatory effects associated with CaCO3 treatment could in part be ascribed to Ca2+, ABE production by C. beijerinckii was measured in cultures supplemented with CaCO3 and CaCl2 (0.5 g/liter each) in 250-ml Pyrex screw-cap bottles. Fermentation was carried out as described above. Samples (5 ml) were collected at 12-h intervals to measure the cell concentration, ABE concentration, glucose concentration, pH, and acid levels. The growth of C. beijerinckii was estimated by using a DU800 spectrophotometer (Beckman Coulter Inc., Brea, CA) to measure the OD600, which was converted to cell dry weight by using a predetermined correlation (4). The ABE and acid (acetic and butyric) concentrations were measured by using an Agilent Technologies 7890A gas chromatograph, as previously described (10). The yield was defined as total grams of ABE produced per total grams of glucose utilized.
Effect of calcium on EDTA-treated C. beijerinckii fermentations.
To further assess the potency of calcium in enhancing ABE fermentation in C. beijerinckii, a TGY-grown preculture was subcultured in 1 mM EDTA-containing P2 medium. After 4 h of incubation, the following additions were made in triplicate: solution X (0.5 g/liter CaCl2), solution Y (0.23 g/liter total of FeSO4-MnSO4-MgSO4 at a ratio of 2:3:40), and solution Z (0.5 g/liter CaCl2 plus 0.23 g/liter total of FeSO4-MnSO4-MgSO4 at a ratio of 2:3:40). Cultures in P2 medium without the addition of EDTA or metal ion and those containing 1 mM EDTA were used as controls. To further probe whether the effects of CaCO3 on ABE fermentation resulted from its buffering capacity, we supplemented EDTA-treated cultures with nonbuffering CaCl2 (in place of CaCO3 as a source of Ca2+).
Determination of intracellular Ca2+ concentrations in C. beijerinckii.
In alkaline buffer, Ca2+ reacts in a concentration-dependent fashion with o-cresolphthalein complexone to form a purple color that absorbs at 575 nm, and this observation has been employed extensively for the quantification of Ca2+ (16, 17). Fifty milliliters of C. beijerinckii was harvested at 12-h intervals during the course of a 36-h batch fermentation in P2 medium supplemented with 0.5 g/liter Ca2+. P2 medium without CaCO3 was used as a control. Cells were collected by centrifugation at 10,000 × g for 5 min, followed by washing twice with double-distilled H2O and drying at 60°C for 2 to 3 days. Dried C. beijerinckii cells (20 to 40 mg) were suspended in 1 ml of 10% (vol/vol) HCl and incubated overnight at 30°C to break the cells. This was followed by centrifugation at 8,000 × g for 15 min at 4°C. The resulting supernatant was used to determine the intracellular Ca2+ concentration in C. beijerinckii cells according to the method of Dorey and Draves (17).
Enzyme activity assays.
Cell extracts of C. beijerinckii were assayed for solventogenic enzyme (coenzyme A transferase [CoAT], acetate kinase [AK], acetoacetate decarboxylase [ACDC], butyraldehyde dehydrogenase [BDDH], and butanol dehydrogenase [BDH]) activities. C. beijerinckii cells were harvested at 24 h of batch fermentation in semidefined P2 medium and centrifuged at 8,000 × g for 5 min at 4°C. These pellets were suspended in their respective buffers [50 mM MOPS (morpholinepropanesulfonic acid) (pH 7), 500 mM (NH4)2SO4, and 20% (vol/vol) glycerol for CoAT; 100 mM Tris-HCl (pH 7.6) for AK, BDDH, and BDH; and 50 mM sodium acetate (pH 5) for ACDC]. Aliquots (∼2 to 3 ml; 1.8 mg [dry weight]) of C. beijerinckii cells were frozen at −70°C and thawed on ice, followed by the addition of 2 mg/ml lysozyme and incubation at 37°C for 1 h. Complete C. beijerinckii lysis was accomplished by passing the resulting suspension through a TissueLyser LT instrument (Qiagen Inc., Valencia, CA) for 8 min at a setting of 5,000. The mixture was clarified by centrifugation at 14,000 × g for 5 min at 4°C. Cell extracts were transferred into fresh sterile tubes and used immediately for enzyme assays. The protein concentration was estimated by using Bradford reagent according to the manufacturer's protocol (Amresco, Solon, OH).
To determine possible effects of Ca2+ on the activities of the selected enzymes, assays were conducted with the addition of CaCl2 to a final concentration of 2 mM to the assay mixture. The CoAT assay was carried out according to previously described methods based on the extinction coefficient of acetoacetyl-CoA (8 mM−1 cm−1) at 310 nm (6, 18). One unit of CoAT activity was defined as the amount required for the disappearance of 1 μmol acetoacetyl-CoA per min per mg protein. The AK activity assay was conducted by using a combination of the hydroxamate methods described previously by Rose (19) and Chen and Blaschek (6), at an end product molar extinction coefficient of 0.691 mM−1 cm−1 at 540 nm. ACDC activity was determined as described previously (20). One unit of ACDC activity was defined as the amount of CO2 (in microliters) produced per minute per mg protein. BDDH and BDH activities were assayed at 340 nm, as described previously (21, 22). One unit was defined as the amount of enzyme required to oxidize 1 μmol of NADH per min with butyryl-CoA or butyraldehyde as the substrate.
We assayed 6-phospho-β-glucosidase as described previously by Setlow et al. (23). C. beijerinckii cells grown with and without 4 g/liter CaCO3, as described above for the proteomic study, were harvested from 1 ml of culture by centrifugation and resuspended in 200 μl of phosphate-buffered saline. A clear cell lysate was generated by bead beating (100 μl of 0.1-mm zirconia/silica beads; Biospec Products, Bartlesville, OK) and centrifugation at 18,000 × g for 5 min. Glycerol was then added to a final concentration of 10% (vol/vol). The 6-phospho-β-glucosidase in these cell extracts was assayed at 37°C with the substrate 4-methylumbelliferyl-β-d-glucopyranoside-6-phosphate (MUG-P), a generous gift from Barbara and Peter Setlow (University of Connecticut Health Center, Farmington, CT). Time course reactions (50 μl per reaction mixture containing 50 mM potassium phosphate [pH 7.4] and 1 mM MUG-P) were initiated by the addition of crude extracts. Reactions were quenched with 50 μl of 1.4 M K2CO3. The fluorophore 4-methyl-umbelliferone (4-MU) (excitation/emission at 365 nm/455 nm) produced in these assays was measured with an Infinite M1000Pro instrument (Tecan Austria GmbH). The product formed in these assays was calculated by using a 4-MU standard curve as a reference and was used to determine initial velocities. Specific activities were calculated based on the total protein in the crude extract, which was determined by using the Bradford protein assay (24) with bovine serum albumin as the standard.
Statistical analyses.
All fermentation experiments were performed under anaerobic conditions at least in triplicate (n ≥ 3). Multiple one-way analyses of variance (ANOVAs) were conducted to evaluate the effects of different concentrations of CaCO3 (2 to 10 g/liter) (independent variable) in P2 medium on the growth of and sugar utilization, ABE production, acid production, and assimilation (dependent variable) by C. beijerinckii. All statistical analyses were performed by using the general linear model (GLM) procedure of SAS statistical software, version 9.1.3 (SAS Institute Inc., Cary, NC). Differences among means were separated by using the Student-Newman-Keuls test procedure (P ≤ 0.05).
RESULTS
CaCO3-induced modulation of C. beijerinckii ABE fermentation: effects on medium pH, cell density, glucose utilization, and ABE production.
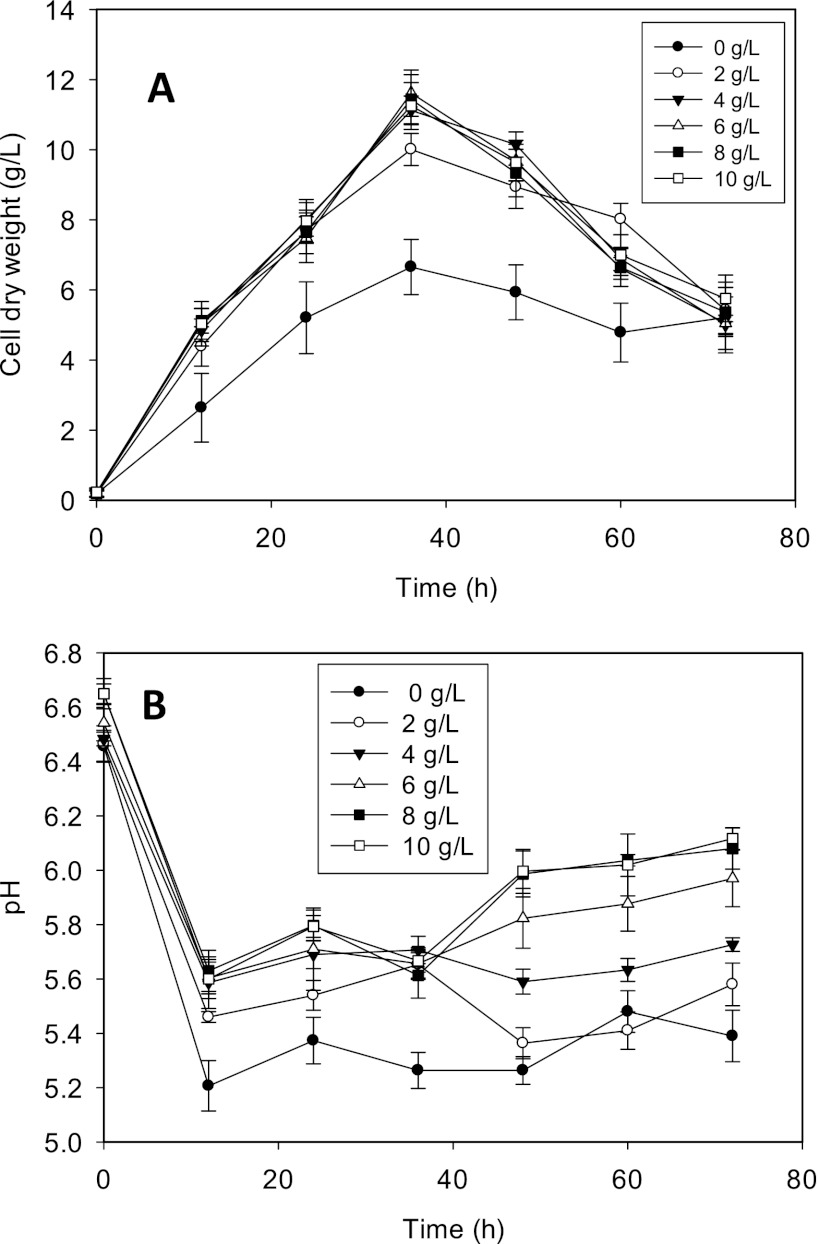
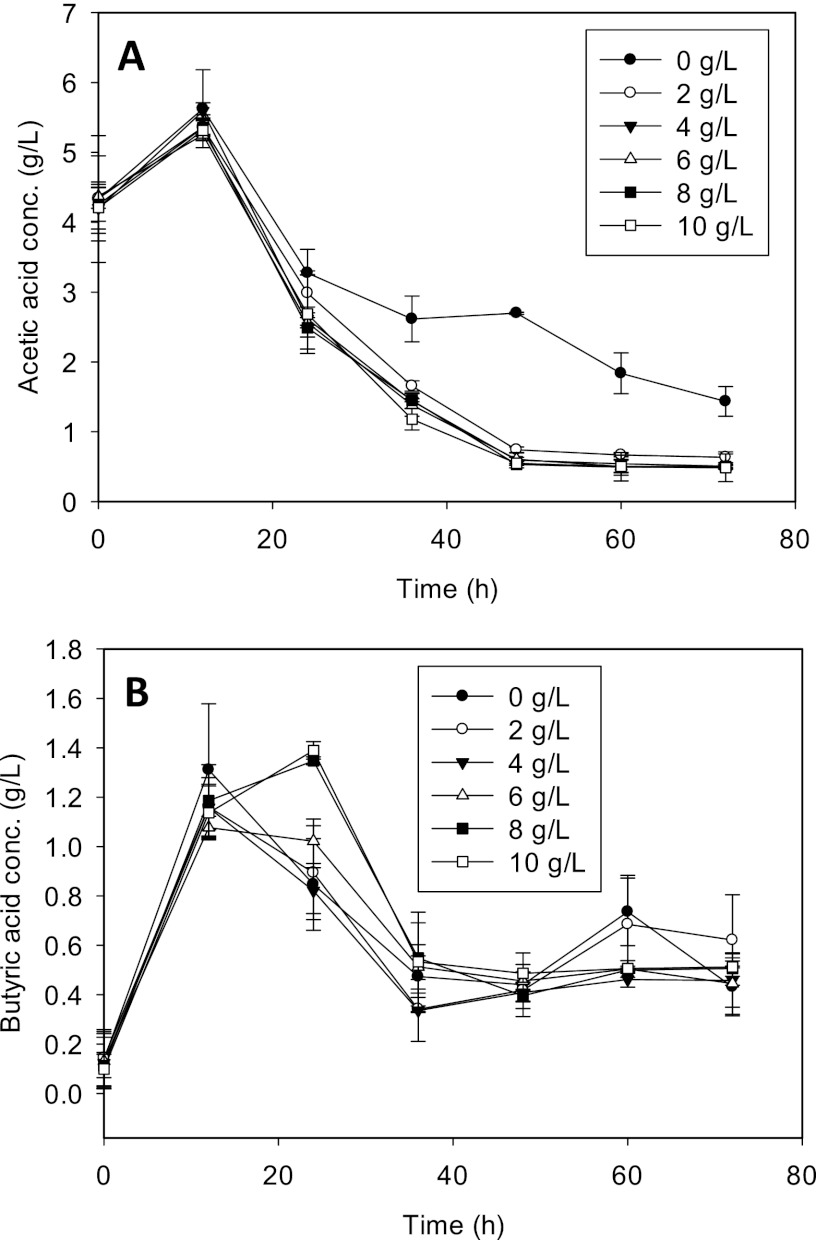
To determine the dose-dependent effects of CaCO3 on C. beijerinckii ABE fermentation and to determine the optimum CaCO3 concentration for proteomic studies, we supplemented P2 medium with 2, 4, 6, 8, or 10 g/liter of CaCO3 and measured changes in pH, cell density, glucose utilization, and ABE production relative to values for cultures grown in P2 medium without CaCO3. The addition of CaCO3 led to the rapid growth of C. beijerinckii cells, resulting in at least a 50% increase in cell dry weight (Fig. 1A). At >2 g/liter CaCO3, the cell densities observed for the treated cultures were nearly indistinguishable. We observed a dose-dependent modulation of the pH of the medium by CaCO3. With the exception of cultures supplemented with 2 g/liter CaCO3, the pH of CaCO3-treated cultures was consistently higher than ∼5.6 for the entire duration of fermentation, whereas the pH of the control cultures ranged from 5.2 to 5.5, following the initial pH decline 12 h after inoculation (Fig. 1B). The total acid (acetic and butyric) levels detected in both treated and untreated cultures were reflective of the respective pH profiles observed. While no significant difference in butyric acid concentrations was observed between treated and untreated cultures, acetic acid levels decreased significantly with treatment (Fig. 2). In fact, the incorporation of CaCO3 led to 2-fold (2 g/liter CaCO3) and 3-fold (4, 6, 8, and 10 g/liter CaCO3) decreases in acetic acid concentrations compared to concentrations in control cultures, thus providing a possible basis for the increased pH which we observed.
Fig 1.
Comparative cell dry weight (A) and pH (B) profiles of C. beijerinckii ABE fermentation in P2 medium supplemented with the indicated concentrations of CaCO3.
Fig 2.
Acetic (A) and butyric (B) acid concentrations in the fermentation medium of C. beijerinckii grown in P2 medium and P2 medium supplemented with the indicated concentrations of CaCO3.
As with cell growth, glucose utilization by C. beijerinckii was rapid during growth in P2 medium supplemented with CaCO3. As a result, the addition of ≥4 g/liter CaCO3 to the fermentation medium resulted in maximum glucose utilization within a shorter period by C. beijerinckii. In effect, glucose was used up by cultures grown in the presence of ≥4 g/liter CaCO3 (4, 6, 8, and 10 g/liter) in 60 h, whereas 17.8 g/liter residual glucose was detected in the control experiments 72 h after inoculation. Additionally, no significant difference in glucose utilization was observed among C. beijerinckii cells grown in P2 medium containing ≥4 g/liter CaCO3 (data not shown). The increased glucose utilization with the addition of CaCO3 translated into higher ABE yields and productivity. A yield of 0.36 g of ABE/g of glucose was determined for the untreated cultures. Conversely, the addition of CaCO3 resulted in ABE yields of 0.43, 0.44, 0.42, 0.41, and 0.41 g/g with 2, 4, 6, 8, and 10 g/liter of CaCO3, respectively. The increased yield was attributable to a relatively shorter fermentation time, thereby minimizing ABE losses and facilitating better acid uptake and conversion to ABE. Similarly, while the ABE productivity level in the control experiments was 0.22 g/liter/h, the average productivity level in cultures grown in the presence of CaCO3 was 0.42 g/liter/h, a 2-fold increase compared with the level in control cultures.
Comparative proteomic analysis of Ca2+-treated C. beijerinckii.
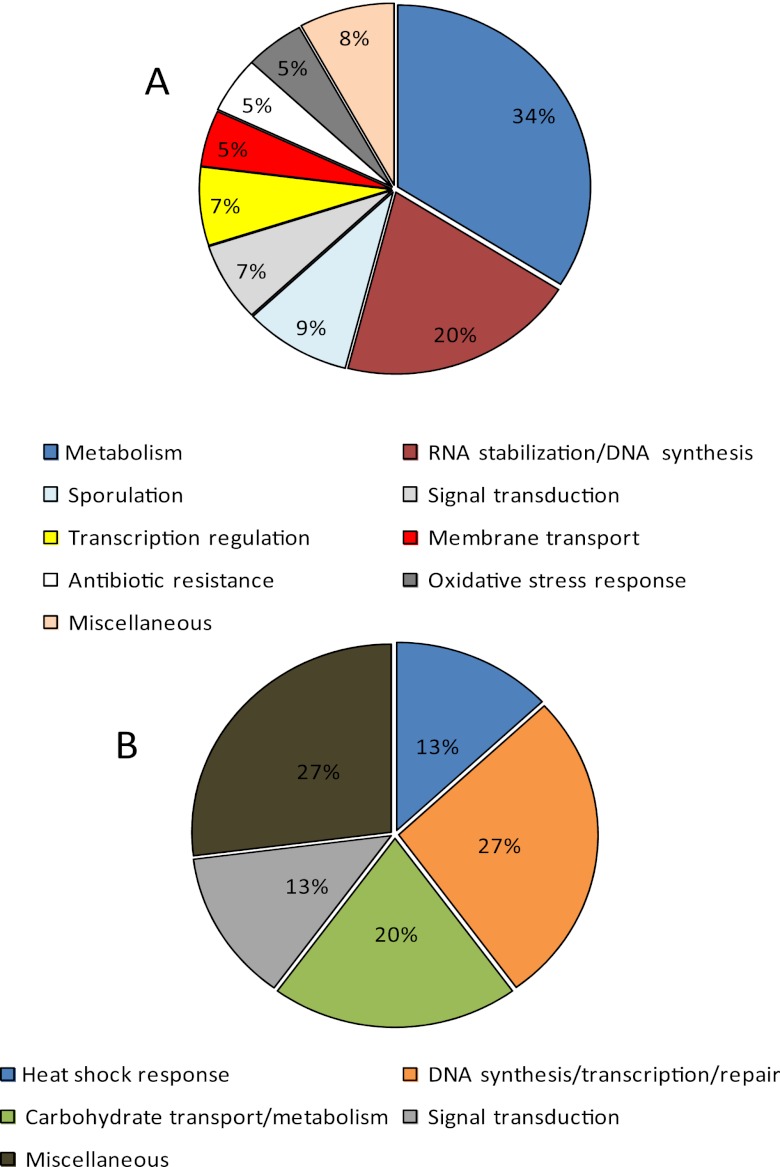
We profiled the global response of C. beijerinckii to CaCO3 (4 g/liter) at the proteome level using 2-D gel electrophoresis. The choice of 4 g/liter was dictated by findings from our dose-dependent studies of CaCO3 establishing the threshold for the maximal increase in cell growth (Fig. 1), superior glucose utilization, enhanced assimilation of acetic acid (Fig. 2), and improved ABE yields and productivity (see Table S1 in the supplemental material). Since 87% of proteins of known function and 93% of solventogenic enzymes in C. beijerinckii NCIMB 8052 have pIs of between 4 and 9 (see Tables S2A and S2B in the supplemental material), we focused on this pH range for isoelectric focusing. A total of 540 protein spots were resolved from pH 4 to 9, of which 96 proteins displayed differences in levels in the Ca2+-treated cultures compared to the controls (fold change ≥1.7; P < 0.05). While one-fifth (of these 96 proteins) showed an increase, the remainder was detected in smaller amounts.
Proteins which showed increased levels are involved predominantly in the heat shock response (13%) (DnaK and GrpE); DNA synthesis, transcription, and repair (27%) (GHKL [gyrase B/topoisomerase VI, Hsp90, histidine kinases, and MutL], nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase, and transketolase); and carbohydrate metabolism/transport (20%) (carbohydrate-binding protein [CBP] with a glycoside hydrolase conserved domain, a glycoside hydrolase, and 6-phospho-β-glucosidase) (Table 2 and Fig. 3B). Proteins with the GHKL (DNA mismatch repair proteins) conserved domain typically comprise ATPases which perform at least one of the highlighted functions.
Table 2.
Complete list of proteins whose levels showed significant increases and partial list of proteins with lower levels when C. beijerinckii cells were grown in P2 medium supplemented with CaCO3a
| Protein | Function or description | Fold change | P value (t test) |
|---|---|---|---|
| YP_001307969.1 | Heat shock protein GrpE | 28.1 | 0.001 |
| YP_001307970.1 | DnaK/molecular chaperone (Hsp) | 11.7 | 0.015 |
| YP_001308102.1 | Sigma 54 factor interaction domain protein | 7.7 | 0.029 |
| YP_001309935.1 | Carbohydrate-binding protein | 7.6 | 0.017 |
| YP_001307580.1 | Integral membrane histidine kinase | 4.4 | 0.004 |
| YP_001307648.1 | Conserved hypothetical protein | 4.2 | 0.006 |
| YP_001308374.1 | Glycoside hydrolase | 3.9 | 0.001 |
| YP_001308398.1 | NNDPb | 2.6 | 0.012 |
| YP_001307436.1 | Hypothetical protein | 2.5 | 0.027 |
| YP_001310550.1 | Histidine kinase/DNA gyrase/Hsp90 | 2.4 | 0.005 |
| YP_001307689.1 | Putative transketolase | 2.2 | 0.020 |
| YP_001308203.1 | Peptidase | 1.9 | 0.006 |
| YP_001311102.1 | 6-Phospho-β-glucosidase | 1.8 | 0.016 |
| YP_001307790.1 | Thiamine biosynthesis protein | 1.8 | 0.003 |
| YP_001310387.1 | Hypothetical protein | −11.5 | 0.034 |
| YP_001307566.1 | Stage II sporulation protein D | −6.7 | 0.001 |
| YP_001311201.1 | Pullulanase/glycoside hydrolase | −6.1 | 0.003 |
| YP_001309441.1 | Rubrerythrin/rubredoxin | −4.9 | 0.007 |
| YP_001311728.1 | Polysaccharide deacetylase | −4.7 | 0.003 |
| YP_001309233.1 | Hypothetical proteinc | −4.3 | 0.032 |
| YP_001309233.1 | Arsenite-activated ATPase | −4.2 | 0.004 |
| YP_001310303.1 | Chloramphenicol O-acetyltransferase | −4.1 | 0.005 |
| YP_001307470.1 | Putative 8-oxoguanine DNA glycosylase | −3.2 | 0.020 |
| YP_001308366.1 | LacI; regulatory protein | −2.6 | 0.034 |
See Table S3 in the supplemental material for a complete list of proteins whose levels decreased when the culture was grown in the presence of CaCO3.
NNDP, nicotinate-nucleotide-dimethylbenzimidazole phosphoribosyltransferase.
Nucleoside triphosphate hydrolase conserved domain.
Fig 3.
Functional classification of proteins whose levels decreased (A) or increased (B) in C. beijerinckii NCIMB 8052 cells grown in Ca2+-supplemented P2 medium relative to growth in P2 medium.
Figure 3A shows the functional classification of proteins whose levels decreased with CaCO3 treatment, most of which are involved in carbohydrate metabolism (34%); the disruption of RNA secondary structures (which normally accumulate under stress conditions); DNA repair (20%); sporulation (9%); signal transduction (7%); transcription regulation (7%); antibiotic (5%) and oxidative stress (5%) responses; and membrane transport (5%) (see Table S3 in the supplemental material for a full list).
Independent confirmation of the findings from proteomic studies.
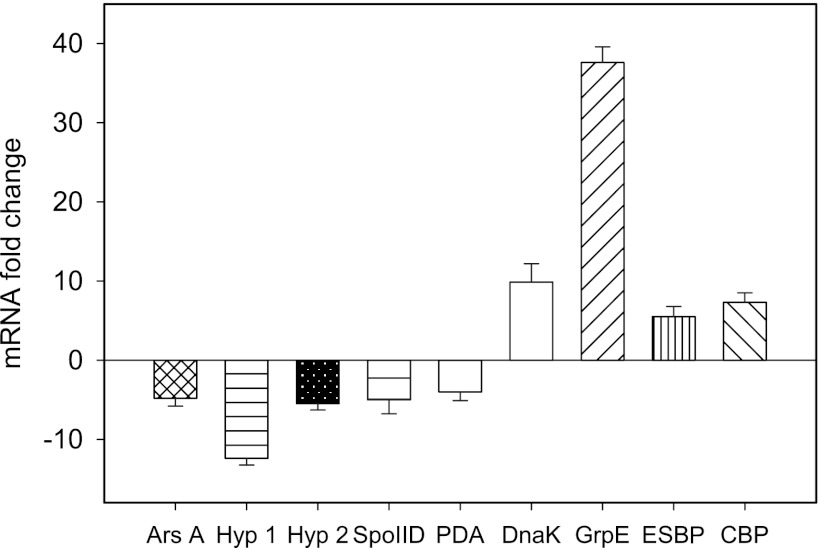
While we realize that posttranscriptional regulation can often complicate correlations between mRNA and protein levels, we undertook quantitative RT-PCR analysis of nine selected genes to determine if we could confirm mRNA level changes for nine of the differentially expressed proteins. Samples for proteomic and mRNA analyses were taken at the same butanol concentration (∼5.5 g/liter), which corresponds to 12 h for the CaCO3 treatment and 24 h for the control. There was a striking agreement between the changes at the mRNA and protein levels, corroborating the patterns of protein expression (Fig. 4 and Table 2). For example, the levels of carbohydrate-binding protein, dnaK, and grpE mRNAs increased 7-, ∼10-, and 38-fold, respectively, in the CaCO3-supplemented cultures relative to the controls (Fig. 4); this mirrors the ∼8-, 12-, and 28-fold changes in their respective protein amounts. Additionally, the mRNA levels of polysaccharide deacetylase and stage II sporulation protein D decreased by 4- and 5-fold, respectively, upon CaCO3 treatment; these changes are consistent with the 5- and 7-fold decreases established for the respective proteins in our proteomic studies.
Fig 4.
qRT-PCR analysis to illustrate changes in the expression levels of selected genes in Ca2+-treated C. beijerinckii NCIMB 8052 cells at 12 h of fermentation relative to levels in untreated cells at 24 h. Relative expression levels confirmed the downregulation of genes encoding an arsenite-activated ATPase (ArsA), two hypothetical proteins (Hyp 1 and Hyp 2), stage II sporulation protein D (SpoIID), and polysaccharide deacetylase (PDA). The genes coding for DnaK, GrpE, an extracellular solute-binding protein (ESBP), and a carbohydrate-binding protein (CBP) also mirrored the increases in levels observed for their respective proteins.
To independently verify the proteomic changes using an enzyme assay, we focused on the 1.8-fold change in the 6-phospho-β-d-glucosidase level (Table 2). In many bacteria (e.g., Bacillus subtilis), cellobiose and aryl-β-d-glucosides are taken up by the phosphotransferase systems, with the subsequent intracellular accumulation of either cellobiose 6-phosphate or an aryl-phospho-β-d-glucoside (23). For these glucosides to be subjected to glycolysis, these phosphoglucosides need to be hydrolyzed by a phospho-β-d-glucosidase. We observed in our proteomic studies that Ca2+ increased the levels of a phospho-β-glucosidase by 1.8-fold. Our enzyme assays using 4-methylumbelliferyl-β-d-glucopyranoside-6-phosphate as the substrate indeed confirmed that Ca2+ elicits a 1.6-fold increase in the level of phospho-β-glucosidase activity; the specific activities from two independent experiments (each involving initial velocity determinations in triplicate) were 2.15 ± 0.23 and 1.34 ± 0.08 nmol/min/mg with and without Ca2+, respectively.
Delineating the dual contribution of CaCO3 to ABE fermentation by C. beijerinckii.
Recognizing that the effects of CaCO3 on ABE fermentation could be due to its buffering capacity and/or other cellular effects mediated by Ca2+ signaling, we sought to delineate this dual contribution even though we realized that this objective is complicated by the peculiarity of solventogenic clostridia (see below). To hone in on only the contribution of buffering, we first compared the effects of different carbonates. Subsequently, we compared CaCO3 and CaCl2, with the expectation that CaCl2, which is incapable of buffering, would only be able to mimic the signaling effects mediated by Ca2+.
To determine whether the effects of CaCO3 on ABE fermentation were due solely to its buffering capacity, C. beijerinckii cells were grown for 72 h individually with each carbonate [(NH4)2CO3, NH4HCO3, K2CO3, NaHCO3, Na2CO3, and CaCO3]. While total ABE levels increased by 1.6-fold with CaCO3, the other carbonates enhanced ABE levels by only 1.3-fold (Table 3); in fact, CaCO3 elicited a 2.5-fold increase in the acetone level, while the highest change elicited by any other carbonate was 1.6-fold (NaHCO3). These results attest to the favorable effect on ABE conferred by the buffering capacities of different carbonates.
Table 3.
List of carbonates tested on C. beijerinckii and their corresponding effects on ABE production
| Treatment (2 g/liter) | Mean product concn (g/liter) ± SDa |
|||
|---|---|---|---|---|
| Acetone | Ethanol | Butanol | Total ABE | |
| Control | 3.01A ± 0.92 | 0.12A ± 0.08 | 11.47A ± 0.96 | 14.6A ± 1.1 |
| Na2CO3 | 3.91AB ± 0.28 | 0.16A ± 0.05 | 12.03A ± 0.38 | 16.1B ± 0.49 |
| K2CO3 | 3.4A ± 0.19 | 0.18A ± 0.03 | 12.48AB ± 0.09 | 16.06B ± 0.3 |
| (NH4)2CO3 | 4.75B ± 0.22 | 0.31B ± 0.11 | 13.43BC ± 0.26 | 18.49C ± 0.52 |
| NaHCO3 | 4.76B ± 0.38 | 0.29B ± 0.01 | 13.8C ± 0.22 | 18.85C ± 0.61 |
| NH4HCO3 | 4.58B ± 0.31 | 0.29B ± 0.09 | 13.34C ± 0.37 | 18.21C ± 0.66 |
| CaCO3 | 7.66C ± 0.81 | 0.33B ± 0.07 | 15.5D ± 0.83 | 23.49D ± 1.2 |
Means with same superscript letters are not significantly different.
The addition of CaCl2 to the medium used for C. beijerinckii ABE fermentation clearly demonstrated that the effects of CaCO3 do not stem solely from its buffering effect. In fact, the addition of 0.5 g/liter CaCl2 to the medium also resulted in increased total ABE production (approximately 17 g/liter) (see Fig. S2 in the supplemental material). Similarly, the total solvent in the cultures treated with 0.5 g/liter CaCO3 was approximately 15 g/liter, whereas the unsupplemented cultures produced less than 14 g/liter total ABE (see Fig S2 in the supplemental material). The moderately more favorable effects on ABE generation by 0.5 g/liter CaCl2 than by CaCO3 are due largely to the former's higher cellular uptake (see below and Fig. 6). However, when the CaCO3 concentration was increased to 4 g/liter, 25.5 g/liter total ABE was produced (see Table S1 in the supplemental material). We could not test the effect of 4 g/liter CaCl2 on ABE fermentation because concentrations above 0.5 g/liter were found to be toxic to C. beijerinckii NCIMB 8052 due to its bacteriostatic and bactericidal effects on the microorganism (data not shown).
Fig 6.
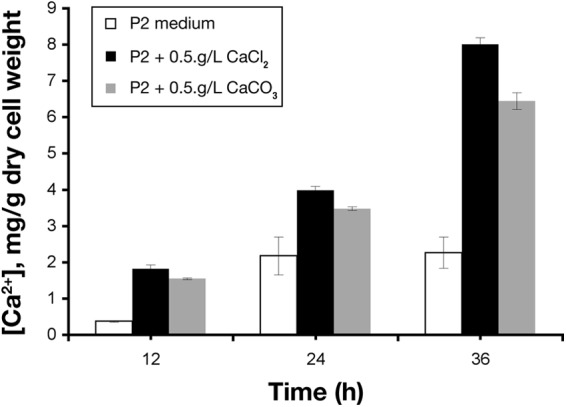
Intracellular Ca2+ concentrations in C. beijerinckii cells during ABE fermentation in P2 medium without or with supplementation of either CaCl2 or CaCO3.
Calcium-mediated recovery of fermentation in EDTA-treated C. beijerinckii.
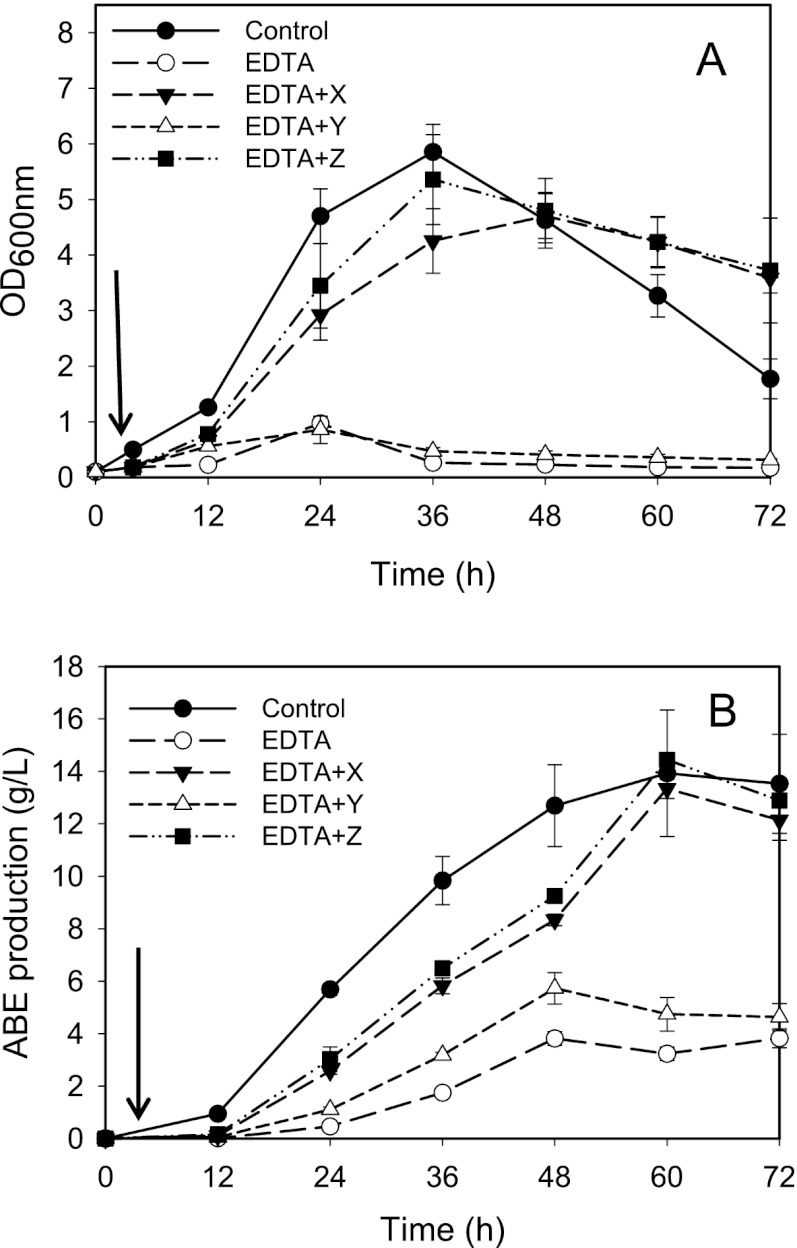
The assumption that Ca2+ elicits key mechanisms which favorably affect ABE fermentation in C. beijerinckii was demonstrated by the patterns of growth and solvent production in EDTA-treated cultures to which either Ca2+; Fe2+, Mg2+, and Mn2+; or both were subsequently added, relative to those of the untreated cultures. Fe2+, Mn2+, and Mg2+ are known to be involved in glycolysis and ABE synthesis. EDTA (1 mM) inhibited C. beijerinckii growth and solventogenesis (Fig. 5A). The addition of Fe2+, Mg2+, and Mn2+ to a total concentration of 0.23 g/liter was unable to alleviate this EDTA-induced inhibition; higher concentrations of Fe2+, Mg2+, and Mn2+ are toxic. However, the addition of 0.5 g/liter Ca2+ increased both cell growth and solventogenesis to the levels observed for the untreated cultures (approximately 14 g/liter ABE) (Fig. 5B). Of further interest is the observation that the addition of Ca2+ alone, i.e., without Fe2+, Mg2+, and Mn2+, abolished (within 1 h) the EDTA-induced inhibition of growth and solventogenesis, suggesting that recovery following the addition of both Ca2+and Fe2+, Mg2+, and Mn2+ is mediated largely by Ca2+.
Fig 5.
Biomass (A) and total ABE (B) profiles of C. beijerinckii fermentation in EDTA-supplemented P2 medium following the addition of solutions X (0.5 g/liter Ca2+), Y (0.23 g/liter total Fe2+, Mn2+, and Mg2+ at a ratio of 2:3:40), and Z (0.5 g/liter Ca2+ plus 0.23 g/liter total Fe2+, Mn2+, and Mg2+ at a ratio of 2:3:40). Arrows indicate the addition of solutions X, Y, and Z at 4 h of fermentation.
Calcium uptake by C. beijerinckii.
Using a fluorescence assay, we investigated if there is an increase in intracellular Ca2+ levels in C. beijerinckii when it is grown in Ca2+-supplemented medium compared to regular P2 medium. Indeed, Ca2+-treated cells had a 3-fold-higher intracellular Ca2+ concentration than the untreated cells at 36 h of fermentation (6.5 mg versus 2 mg Ca2+/g cell dry weight) (Fig. 6). The much higher intracellular Ca2+ concentrations in the CaCl2- and CaCO3-treated cells strongly suggest that Ca2+ plays specific physiological/cellular roles during ABE fermentation, which may account for the stimulatory effects on solventogenesis that were previously ascribed to the buffering capacity of CaCO3.
Ca2+ increases activities of selected solventogenic enzymes of C. beijerinckii.
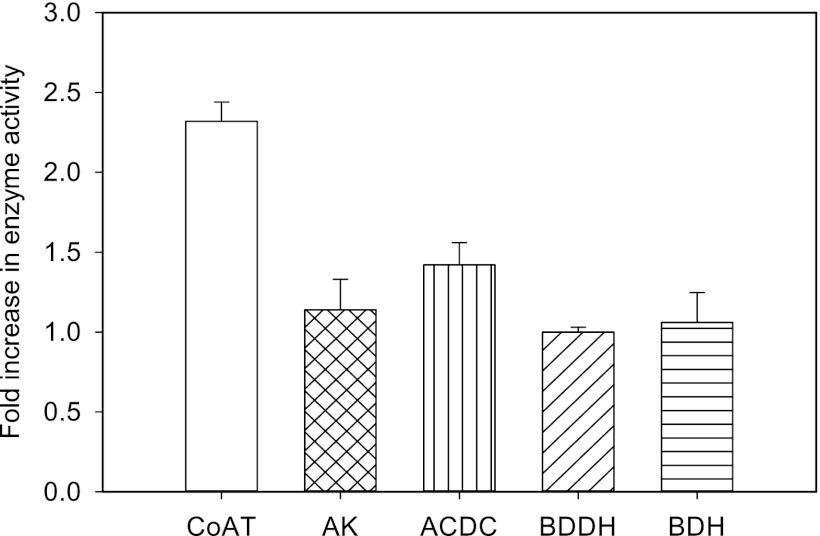
To test the hypothesis that Ca2+ enhances the activities of important enzymes involved in ABE production, aliquots of cell extracts of C. beijerinckii were assayed for CoAT, AK, ACDC, BDDH, and BDH activities with or without 2 mM Ca2+. We observed 2.3-, 1.2-, and 1.4-fold increases in activity for CoAT, AK, and ACDC, respectively, when 2 mM Ca2+ was included in the assay mixtures. A negligible difference in activity was observed for BDH and BDDH upon Ca2+ supplementation in their respective assay mixtures (Fig. 7).
Fig 7.
Ca2+-induced increases in the activities of coenzyme A transferase (CoAT), acetate kinase (AK), and acetoacetate decarboxylase (ACDC) in cell extracts of C. beijerinckii. No changes in activity were observed for butyraldehyde dehydrogenase (BDDH) or butanol dehydrogenase (BDH). Increases in activity are expressed as fold differences between the Ca2+-supplemented cell extracts and the unsupplemented extracts. The basal activities measured in the unsupplemented cell extracts (without Ca2+) were 0.22 (CoAT), 0.07 (AK), 4.56 (ACDC), 0.01 (BDDH), and 0.91 (BDH) U/mg protein/min.
DISCUSSION
While the role of Ca2+ as a universal messenger involved in signaling and regulating a range of cellular processes in eukaryotes is well appreciated (25–27), the emerging picture in bacteria reveals some similarities but also unanticipated roles (28–33). There is a growing body of evidence implicating Ca2+ in cell division, sporulation, the maintenance of cell structure, chemotaxis, and DNA replication in bacteria (29, 31, 34). In this study, we investigated how CaCO3 increases butanol production, sugar utilization, and butanol tolerance by solventogenic Clostridium species (3, 8, 9). We examined whether these multifaceted effects of Ca2+ on solventogenesis are mediated through changes in gene expression levels and/or by other means (e.g., direct changes in the activities of enzymes). The findings from the present study are grouped under different attributes.
Solventogenesis.
Given the favorable effects of CaCO3 on ABE fermentation, it was hypothesized that Ca2+ induced the expressions of key enzymes in the clostridial solventogenic pathway. Although this premise is not supported by our proteomic data, we uncovered, somewhat unexpectedly, that Ca2+ increases the activities of CoAT, AK, and ACDC by 2.3-, 1.2-, and 1.4-fold, respectively (Fig. 7). Since CoAT is central to the uptake of acetic and butyric acids (which in turn is needed for subsequent conversion to butanol), and it is also the rate-limiting enzyme for acetone production (35), we have now identified a novel basis for the favorable solventogenesis upon the uptake of Ca2+ present in the medium. The increase in the CoAT activity is consistent with the marked decrease in acetic acid (and increased pH) levels in the Ca2+-treated cultures (Fig. 2A). We postulate that the activities of a broader range of enzymes/proteins (other than those involved in solventogenesis) are also likely to be positively influenced by Ca2+ in vivo.
Variations in pH exert a major effect on the outcome of ABE fermentation (1, 36, 37). This is ascribed primarily to the severe toxicity of acids produced during acidogenesis (38); as a result, during the exponential phase, it is critical to maintain cell growth within a favorable pH range to achieve significant cell density and considerable acid accumulation for optimal solvent production in the latter stages (36, 37). By using a two-stage controlled-pH strategy, where fermentation was maintained at pH 5.5 during acidogenesis, Guo et al. (37) previously achieved a ∼40% (20.3 g/liter) increase in ABE production when using C. acetobutylicum XY16. Likewise, Bryant and Blaschek (36) previously reported significant increases in cell growth and ABE production (10 g/liter) by incorporating acetate-, carbonate-, citrate-, and phosphate-based buffers in P2 medium when using C. acetobutylicum.
In the present study, the addition of ≥4 g/liter CaCO3 to the fermentation medium resulted in pH values typically >pH 5.6 (Fig. 1B). In parallel, we observed significant increases in cell growth (Fig. 1A), glucose utilization, and ABE production (25.5 g/liter) (see Table S1 in the supplemental material). We infer that the pH profile resulting from treatment with ≥4 g/liter CaCO3 (pH-buffering effect), which may have been influenced in part by the interplay between Ca2+ and CoAT activities, leading to increased acetic acid assimilation (and, consequently, increases in pH) and contributing to the positive effects of CaCO3. This observation echoes previous reports which documented how the efficient buffering of ABE fermentation modulates the equilibrium between dissociated and undissociated acids by favoring the accumulation of the former, which are less toxic to solventogenic clostridia and are more readily utilized for solvent production (1, 36).
Moreover, CaCO3 dissolves sparingly in water and in a pH-dependent manner (39), an attribute that likely favors ABE fermentation. For instance, at pH 6, the solubility of CaCO3 in water is roughly 2 g/liter; however, a decrease in pH promotes the dissolution of CaCO3 with concomitant proton consumption and, thus, alkalinization, a process thought to be critical for natural buffering in coral reefs (39). Hence, the addition of CaCO3 in excess of 2 g/liter can serve as a buffer reservoir, which responds to the characteristic decreases in pH during ABE fermentation. This is underscored by the slightly reduced cell density (an average of ∼11% in the earlier stages of growth) (Fig. 1A), decreased assimilation of acetic acid (∼20% on average) (Fig. 2A), and reduced pH-buffering capacity (Fig. 1B) observed with 2 g/liter CaCO3 relative to ≥4 g/liter CaCO3, where the observed positive effects on C. beijerinckii ABE fermentation appeared to plateau above 4 g/liter.
Although we chose to compare CaCO3 and CaCl2 under the assumption that the latter lacks the natural buffering capacity of the former, we believe that this is an oversimplification, since the biological effects of Ca2+-mediated signaling also encompass pH changes in the medium. Calcium chloride (like CaCO3) will stimulate CoAT activity; in fact, this is likely to be partly responsible for the increased ABE fermentation supported by 0.5 g/liter CaCl2. As a consequence, there will be increased acetic acid assimilation, which in turn will increase the pH of the medium. Taking into consideration the idiosyncratic switch of clostridial growth from acidogenic to solventogenic phases, our findings suggest that explaining pH changes purely on the merits of the additive's buffering capacity alone might underestimate ripple effects from changes in the activities of solventogenic enzymes, which in turn have implications for the pH of the medium.
Butanol toxicity and glucose utilization.
The chaotropic effect of butanol on the cell membrane (and the attendant effects on the pH gradient) is largely the underlying basis for its toxicity. Previous studies, which showed that Ca2+ promotes glucose/xylose utilization while lowering butanol toxicity, speculated that Ca2+ might have stabilizing effects on membrane proteins, particularly xylose and glucose permeases, which are inhibited by 8 and 12 g/liter butanol, respectively (8, 40). Although this is certainly a possibility, we stress that the P2 medium used for ABE fermentation contains Fe2+, Mn2+, and Mg2+, and these cations are unable to confer any Ca2+-like protective traits during solventogenesis (Fig. 5). Either Fe2+, Mn2+, and Mg2+ are unable to mimic Ca2+ in exerting a stabilizing effect on the membrane/membrane proteins, or, in addition to its stabilizing effect on membranes and/or membrane proteins, the ability of Ca2+ to trigger a network of cellular events is the underpinning for its distinctive ability to alleviate butanol toxicity. Although we favor the latter idea, we first consider the former.
The binding of Ca2+ and Mg2+ to the negatively charged surface lipopolysaccharides (LPS) in Gram-negative bacteria generates ionic cross bridges, which exert a stabilizing effect on the outer cell membrane (41–44). Notwithstanding variations in the cell wall/membrane makeup and structure between Gram-positive and Gram-negative bacteria (45), we considered whether the formation of Ca2+-mediated cross bridges in the Gram-positive bacterium C. beijerinckii might partly contribute to the effects of Ca2+ on ABE fermentation. However, such a premise is not supported by our observation that Fe2+, Mn2+, and Mg2+ are poor mimics of Ca2+ in enhancing ABE fermentation (Fig. 5).
Although C. beijerinckii cells grown in the presence of CaCO3 showed elevated levels of growth and glucose utilization (see Table S1 in the supplemental material), glycolytic proteins were not detected in larger amounts upon Ca2+ treatment, suggesting that the barrier to glucose utilization is not its catabolism and that a roadblock elsewhere is somehow being circumvented in the presence of Ca2+. Most likely, impaired sugar transport is the limiting factor for glucose utilization, especially during growth in the presence of butanol. In this regard, we stress that levels of a carbohydrate-binding protein (YP_001309935.1) with a glycoside hydrolase conserved domain, a glycoside hydrolase (YP_001308374.1), and 6-phospho-β-glucosidase (YP_001311102.1) (Table 2), all involved in carbohydrate metabolism and transport, increased significantly (Table 2; see also Fig. S1 in the supplemental material). In fact, since 6-phospho-β-glucosidase is involved in cellobiose metabolism, and our growth substrate in the medium was glucose, we were surprised by this ∼2-fold increase and thus confirmed the same through enzyme activity assays. These data also furnish the first experimental evidence for the presence of this enzyme in C. beijerinckii NCIMB 8052. Broadly, these results suggest that Ca2+ (preemptively) sets the stage for metabolic fine-tuning that is not necessarily based on the growth substrate; in fact, this inference has already inspired us to examine the likely favorable effects of Ca2+ on cellobiose utilization by C. beijerinckii NCIMB 8052.
Butanol tolerance.
The most important conclusions from the proteomic study are the Ca2+-induced 12- and 28-fold increases in the levels of DnaK and GrpE, respectively (Table 2); while the former is a molecular chaperone and heat shock protein (HSP) (member of the Hsp70 family), the latter is a nucleotide exchange factor which facilitates the efficient release of DnaK-bound nonnative protein substrates and aids in the recycling of DnaK. Modest increases in butanol concentrations induce the heat shock response (46), and Hsp70 chaperones have been shown to participate in the repair of aberrant proteins and the prevention of stress-mediated damage to proteins (47); hence, DnaK and GrpE will be crucial when butanol accumulates to high concentrations in the fermentation broth.
Since samples for protein extraction were taken at the same butanol concentration (∼5.5 g/liter) for both CaCO3-treated and untreated cultures, we contend that the pronounced increases in the amounts of DnaK and GrpE are not due to butanol stress but rather to a Ca2+-mediated response, albeit as a secondary effect. The addition of ≥4 g/liter CaCO3 to the C. beijerinckii fermentation medium evoked rapid growth, leading to at least a 1.7-fold increase in cell density (Fig. 1A) compared to that of the control; in fact, similar effects were reported previously for Ca2+ in Escherichia coli (48, 49) and Rhizobium fredii (50). Increased rates of cell replication are accompanied by increases in levels of DNA and protein synthesis (51), which amplify flux through the biosynthesis machinery, often associated with the upregulation of chaperones to prevent protein misfolding or to enhance the refolding of misfolded proteins, among other functions (51–53). Taken together, we conclude that Ca2+ elicits rapid cell replication in C. beijerinckii, thereby triggering attendant increases in the levels of proteins involved in DNA and protein processing and stabilization. Therefore, the Ca2+-induced increase in DnaK/GrpE levels upon an increase in cell replication and the associated stabilization of the biosynthesis and growth machinery might account for the robust growth of C. beijerinckii NCIMB 8052 in the presence of CaCO3; such an expectation is further supported by the finding that DnaK plays a key role in DNA replication and cell division (54). Additionally, increased levels of HSPs have been shown to improve butanol tolerance and/or production in E. coli (2, 55), Lactobacillus plantarum (56), and C. acetobutylicum (57, 58), often with improved substrate utilization.
In the aerobic Gram-positive bacteria Bacillus subtilis and Streptococcus pneumoniae, Ca2+ in fact represses the expression of the dnaK and groE operons by increasing the levels of the class I HSP repressor hrcA, which in turn binds to the conserved cis-active CIRCE (controlling inverted repeat of expression) element required for the expression of class I HSPs (59, 60). Although hrcA lies upstream of the hsp70 gene cluster on the C. beijerinckii genome (hrcA-grpE-dnaK-dnaJ), a search of the promoter regions of the upregulated grpE and dnaK genes with the CIRCE element sequence (TTAGCACTC-N9-GAGTGCTAA) (57) did not produce a significant match. Most likely, the expression of the class I HSPs in C. beijerinckii follows a different regulatory path in comparison to its aerobic relatives. An in-depth understanding of the molecular mechanisms involved in the increased gene expression levels of dnaK and grpE in Ca2+-treated C. beijerinckii NCIMB 8052 cells will prove instructive. Also of further interest is the increased expression level of an ATPase (histidine kinase/DNA gyrase/Hsp90) with the GHKL conserved domain (Table 2), found in DNA gyrase B, topoisomerase VI, Hsp90, histidine kinases, and MutL (61). Proteins carrying the GHKL conserved domain, which have also been associated with increased rates of cell replication (62), have been implicated in the refolding of damaged polypeptides; signal transduction; DNA replication, repair, transcription, and recombination; and genome stabilization (61, 63, 64), which would also be crucial during growth under conditions of butanol stress.
Several other findings from our proteomic study suggest that Ca2+ mitigates stress through unknown mechanisms. In the presence of Ca2+, we found decreases in the levels of DNA/RNA helicases (2-fold for YP_001307495.1 and 3-fold for YP_001309141.1) (see Table S3 in the supplemental material) and rubrerythrin/rubredoxin (5-fold for YP_001309441.1) (see Fig. S1 in the supplemental material), which is involved in the anaerobic oxidative stress response. Under conditions of abiotic stress, helicases function as nucleic acid chaperones by disrupting stable misfolded RNA structures, thereby restoring their normal function (65, 66). They are also central to the DNA repair machinery, especially during heat shock or oxidative stresses (66, 67), which often phenocopy butanol stress (2). Similarly, rubrerythrin has been shown to participate in the detoxification of reactive oxygen species in anaerobic bacteria (68) and exhibits superoxide dismutase activity in vitro (69). In E. coli (2) and C. acetobutylicum (70), the upregulation of the oxidative stress response machinery is among the strongest effects during butanol exposure, perhaps because both oxidative and alcohol stresses primarily cause the membrane potential to collapse (2, 57, 71).
Additional lines of evidence support our premise that Ca2+ alleviates stress caused by solventogenesis. First, the observed protein profile of C. beijerinckii in the presence of Ca2+ agrees with the transcription profile of C. acetobutylicum overexpressing GroESL (a heat shock protein), wherein stress- and metabolism-related genes were downregulated, with concomitant increases in butanol production, sugar utilization, and cell growth (57). Also, a recent study which compared the proteomes of wild-type C. acetobutylicum and an Rh8 mutant strain which has higher butanol tolerance and yields showed that chaperones and solventogenesis enzymes were upregulated in the mutant compared to the wild type (72). While the nature of the mutation in strain Rh8 is unknown, we highlight a thematic parallel with our Ca2+-induced changes and the shared outcomes. Second, sporulation is an independent stress indicator during the onset of solventogenesis and the subsequent accumulation of butanol in C. beijerinckii and C. acetobutylicum; sporulation is initiated upon the expression/activation of a transcription factor encoded by the spo0A gene (73–75). We observed decreases in levels of sporulation-related proteins (YP_001307345.1, YP_001307566.1, and YP_001307539.1) (Table 2; see also Fig. S1 and Table S3 in the supplemental material) in C. beijerinckii NCIMB 8052 cells grown in the presence of CaCO3, even though samples for protein extraction were taken at the same butanol concentration for both treated and control cultures. Finally, while several genetic and biochemical factors dictate biofilm formation, solvent stress is a positive effector (76); in this regard, it is notable that C. beijerinckii cells grown in the presence of CaCO3 showed 5- and 2-fold decreases in levels of polysaccharide deacetylase and diguanylate cyclase, respectively (see Fig. S1 and Table S3 in the supplemental material), two enzymes that facilitate biofilm formation due to their roles in exopolysaccharide deacetylation and the generation of the bis-(3′-5′)-cyclic dimeric GMP (c-di-GMP) biofilm-signaling molecule, respectively (77). Collectively, these data, which indicate the absence of stress-related sequelae, support the idea that Ca2+ treatment eases the stress on C. beijerinckii during solventogenesis.
In conclusion, while pH-buffering effects may contribute to the stimulatory effects of CaCO3 on ABE fermentation, Ca2+ has broader effects at the cellular and protein levels, which directly (through the enhancement of CoAT activity) and indirectly (signaling) amplify the growth of C. beijerinckii and promote acid assimilation and ABE production. Collectively, the stabilizing mechanisms of HSPs, increased DNA synthesis and replication, and the direct enhancement of the activities of key solventogenic enzymes in the presence of Ca2+ contribute to robust cell replication and solventogenesis during ABE fermentation. However, specific analyses of the various aspects of this complex global response to Ca2+ are required to facilitate a rational reconstruction of C. beijerinckii ABE fermentation for improved butanol production.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Barbara and Peter Setlow (University of Connecticut, Farmington, CT) for their generous gift of 4-methylumbelliferyl-β-d-glucopyranoside-6-phosphate. We thank Dmitri Kudryashov (OSU) for his kind and generous assistance in the use of the Tecan Infinite M1000Pro system.
This research was supported in part by a Hatch grant (project no. OHO01222), the U.S. Department of Transportation (grant DTOS59-07-G-00052), Northeast Sun grant initiative award/agreement no. 52110-9615 (to T.C.E. and V.G.), and a seed grant from the Ohio Agricultural Research and Development Center, Wooster, OH (to T.C.E.).
Footnotes
Published ahead of print 26 October 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02969-12.
REFERENCES
- 1. Ezeji TC, Milne C, Price ND, Blaschek HP. 2010. Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol producing microorganisms. Appl. Microbiol. Biotechnol. 85:1697–1712 [DOI] [PubMed] [Google Scholar]
- 2. Rutherford JB, Dahl RH, Price RE, Smidtz HL, Benke PI, Mukhopadhyay A, Keasling JD. 2010. Functional genomic study of exogenous n-butanol stress in Escherichia coli. Appl. Environ. Microbiol. 76:1935–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richmond C, Han B, Ezeji TC. 2011. Stimulatory effects of calcium carbonate on butanol production by solventogenic Clostridium species. Cont. J. Microbiol. 5:18–28 [Google Scholar]
- 4. Ezeji TC, Groberg M, Qureshi N, Blaschek HP. 2003. Continuous production of butanol from starch-based packing peanuts. Appl. Biochem. Biotechnol. 1051–108:375–382 [DOI] [PubMed] [Google Scholar]
- 5. Ezeji TC, Qureshi N, Blaschek HP. 2004. Acetone butanol ethanol (ABE) production from concentrated substrate: reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping. Appl. Microbiol. Biotechnol. 63:653–658 [DOI] [PubMed] [Google Scholar]
- 6. Chen CK, Blaschek HP. 1999. Effect of acetate on molecular and physiological aspects of Clostridium beijerinckii NCIMB 8052 solvent production and strain degeneration. Appl. Environ. Microbiol. 65:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gu Y, Hu S, Chen J, Shao L, He H, Yang Y, Yang S, Jiang W. 2009. Ammonium acetate enhances solvent production by Clostridium acetobutylicum EA 2018 using cassava as fermentation medium. J. Ind. Microbiol. Biotechnol. 36:1225–1232 [DOI] [PubMed] [Google Scholar]
- 8. El Kanouni A, Zerdani I, Zaafa S, Znassni M, Loufti M, Boudouma M. 1998. The improvement of glucose/xylose fermentation by Clostridium acetobutylicum using calcium carbonate. World J. Microbiol. Biotechnol. 14:431–435 [Google Scholar]
- 9. Ren C, Gu Y, Hu S, Wu Y, Wang P, Yang Y, Yang C, Yang S, Jiang W. 2010. Identification and inactivation of pleiotropic regulator CcpA to eliminate glucose repression of xylose utilization in Clostridium acetobutylicum. Metab. Eng. 12:446–454 [DOI] [PubMed] [Google Scholar]
- 10. Han B, Gopalan V, Ezeji TC. 2011. Acetone production in solventogenic Clostridium species: new insights from non-enzymatic decarboxylation of acetoacetate. Appl. Microbiol. Biotechnol. 91:565–576 [DOI] [PubMed] [Google Scholar]
- 11. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Frovenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85 [DOI] [PubMed] [Google Scholar]
- 12. O'Farrell PH. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007–4021 [PMC free article] [PubMed] [Google Scholar]
- 13. Burgess-Cassler A, Johansen JJ, Santek DA, Ide JA, Kendrick NC. 1989. Computerized computative analysis of Coomassie-blue-stained serum proteins separated by two-dimensional electrophoresis. Clin. Chem. 35:2297–2304 [PubMed] [Google Scholar]
- 14. Hiller K, Grote A, Maneck M, Munch R, Jahn D. 2006. JVirGel 2.0: computational prediction of proteomes separated via two-dimensional gel electrophoresis under consideration of membrane and secreted proteins. Bioinformatics 22:2441–2443 [DOI] [PubMed] [Google Scholar]
- 15. Ezeji T, Blaschek HP. 2008. Fermentation of dried distillers' grains and soluble (DDGS) hydrolysates to solvents and value-added products by solventogenic clostridia. Bioresour. Technol. 99:5232–5242 [DOI] [PubMed] [Google Scholar]
- 16. Cohen SA, Sideman L. 1979. Modification of the o-cresolphthalein complexone method for determining calcium. Clin. Chem. 25:1519–1520 [PubMed] [Google Scholar]
- 17. Dorey CR, Draves JA. 1998. Quantitative analysis laboratory: a new approach. Laboratory manual. Department of Chemistry, University of Central Arkansas, Conway, AR [Google Scholar]
- 18. Clark SW, Bennett GN, Rudolph FB. 1989. Isolation and characterization of mutants of Clostridium acetobutylicum ATCC 824 deficient in acetoacetyl-coenzyme A:acetate/butyrate:coenzyme A-transferase (EC 2.8.3.9) and in other solvent pathway enzymes. Appl. Environ. Microbiol. 55:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rose IA. 1955. Acetate kinase of bacteria (acetokinase). Methods Enzymol. 1:591–593 [Google Scholar]
- 20. Gerischer U, Dürre P. 1990. Cloning, sequencing, and molecular analysis of the acetoacetate decarboxylase gene region from Clostridium acetobutylicum. J. Bacteriol. 172:6907–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toth J, Ismaiel AA, Chen JS. 1999. The ald gene, encoding a coenzyme A-acylating aldehyde dehydrogenase, distinguishes Clostridium beijerinckii and two other solvent-producing clostridia from Clostridium acetobutylicum. Appl. Environ. Microbiol. 65:4973–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan RT, Chen JS. 1990. Coenzyme A-acylating aldehyde dehydrogenase from Clostridium beijerinckii NRRL B592. Appl. Environ. Microbiol. 56:2591–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Setlow B, Cabrera-Hernandez A, Cabrera-Martinez RM, Setlow P. 2004. Identification of aryl-phospho-β-D-glucosidases in Bacillus subtilis. Arch. Microbiol. 181:60–67 [DOI] [PubMed] [Google Scholar]
- 24. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 25. Dominguez DC. 2004. Calcium signalling in bacteria. Mol. Microbiol. 54:291–297 [DOI] [PubMed] [Google Scholar]
- 26. Ikura M, Osawa M, Ames JB. 2002. The role of calcium binding proteins in the control of transcription structure and function. Bioessays 24:625–636 [DOI] [PubMed] [Google Scholar]
- 27. Mekalanos JJ. 1992. Environment signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gangola P, Rosen BP. 1987. Maintenance of intracellular calcium in Escherichia coli. J. Biol. Chem. 262:12570–12574 [PubMed] [Google Scholar]
- 29. Norris V, Grant S, Freestone P, Canvin J, Sheikh FN, Toth I, Trinei M, Modha K, Norman RI. 1996. Calcium signalling in bacteria. J. Bacteriol. 178:3677–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paulsen IT, Nguyen L, Sliwinski MK, Rabus R, Saier MH., Jr 2000. Microbial genome analysis: comparative transport capabilities in eighteen prokaryotes. J. Mol. Biol. 301:75–100 [DOI] [PubMed] [Google Scholar]
- 31. Smith RJ. 1995. Calcium and bacteria. Adv. Microb. Physiol. 37:83–133 [DOI] [PubMed] [Google Scholar]
- 32. Torrecilla I, Leganes F, Bonilla I, Fernandez-Pinas F. 2000. Use of recombinant aequorin to study calcium homeostasis and monitor calcium transients in response to heat and cold shock in cyanobacteria. Plant Physiol. 123:161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waditee R, Hossain GS, Tanaka Y, Nakamura T, Shikata M, Takano J, Takabe T, Takabe T. 2004. Isolation and functional characterization of Ca2+/H+ antiporters from cyanobacteria. J. Biol. Chem. 279:4330–4338 [DOI] [PubMed] [Google Scholar]
- 34. Holland IB, Jones HE, Campbell AK, Jacq A. 1999. An assessment of the role of intracellular free Ca2+ in E. coli. Biochimie 81:901–907 [DOI] [PubMed] [Google Scholar]
- 35. Tummala SB, Welker NE, Papoutsakis ET. 2003. Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum. J. Bacteriol. 185:1923–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bryant DL, Blaschek HP. 1988. Buffering as a means for increasing the growth and butanol production of Clostridium acetobutylicum. J. Ind. Microbiol. 3:49–55 [Google Scholar]
- 37. Guo T, Sun B, Jiang M, Wu H, Du T, Tang Y, Wei P, Ouyang P. 2012. Enhancement of butanol production and reducing power using a two-stage controlled-pH strategy in batch culture of Clostridium acetobutylicum XY16. World J. Microbiol. Biotechnol. 28:2551–2558 [DOI] [PubMed] [Google Scholar]
- 38. Costa JM, Moreira AR. 1983. Growth inhibition kinetics for the acetone-butanol-butanol fermentation, p 501–502 In Blanch HW, Papoutsakis ET, Stephanopoulos G. (ed), Foundations of biochemical engineering. American Chemical Society, Washington, DC [Google Scholar]
- 39. Ichikawa K. 2007. Buffering dissociation/formation reaction of biogenic calcium carbonate. Chem. Eur. J. 13:10176–10181 [DOI] [PubMed] [Google Scholar]
- 40. Ounine K, Petitdemange H, Reval G, Gay R. 1985. Regulation and butanol inhibition of d-xylose and d-glucose uptake in Clostridium acetobutylicum. Appl. Environ. Microbiol. 49:874–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hansen LT, Austin JW, Gill TA. 2001. Antibacterial effect of protamine in combination with EDTA and refrigeration. Int. J. Food Microbiol. 66:149–161 [DOI] [PubMed] [Google Scholar]
- 42. Kotra LP, Golemi D, Amro NA, Liu GY, Mobashery S. 1999. Dynamics of the lipopolysaccharides assembly on the surface of Escherichia coli. J. Am. Chem. Soc. 121:8707–8711 [Google Scholar]
- 43. Patrauchan MA, Sarkisova S, Sauer K, Franklin MJ. 2005. Calcium influenced cellular and extracellular product formation during biofilm-associated growth of a marine Pseudoalteromonas sp. Microbiology 151:2885–2897 [DOI] [PubMed] [Google Scholar]
- 44. Schneck E, Schubert T, Konovalov OV, Quinn BE, Gutsman T, Brandenburg K, Oliviera RG, Pink DA, Tanaka M. 2010. Quantitative determination of ion distributions in bacterial lipopolysaccharides membranes by grazing-incidence X-ray fluorescence. Proc. Natl. Acad. Sci. U. S. A. 107:9147–9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farca AM, Nebbia P, Re G. 1994. Potentiation of antibiotic activity by EDTA-tromethamine against three clinically isolated Gram-positive resistant bacteria. An in vitro investigation. Vet. Res. Commun. 18:1–6 [DOI] [PubMed] [Google Scholar]
- 46. Terracciano JS, Kashket ER. 1986. Intracellular conditions required for the initiation of solvent production by Clostridium acetobutylicum. Appl. Environ. Microbiol. 52:86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schröder H, Langer T, Hartl F, Bukau B. 1993. DnaK, DnaJ and GrpE from a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 12:4137–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Campbell AK, Naseem R, Holland IB, Matthews SB, Wann KT. 2007. Fermentation product butane 2,3-diol induces Ca2+ transients in E. coli through activation of lanthanum-sensitive Ca2+ channels. Cell Calcium 41:97–106 [DOI] [PubMed] [Google Scholar]
- 49. Campbell AK, Naseem R, Holland IB, Matthews SB, Wann KT. 2007. Methylglyoxal and other carbohydrate metabolites induce lanthanum-sensitive Ca2+ transients and inhibit growth in E. coli. Arch. Biochem. Biophys. 468:107–113 [DOI] [PubMed] [Google Scholar]
- 50. Balatti PA, Karishnan HB, Puepke SG. 1991. Calcium regulates growth of Rhizobium fredii and its ability to nodulate soybean cv. Peking. Can. J. Microbiol. 37:542–548 [Google Scholar]
- 51. Georgiou G, Valax P. 1996. Expression of correctly folded proteins in Escherichia coli. Curr. Opin. Biotechnol. 7:190–197 [DOI] [PubMed] [Google Scholar]
- 52. Hayes SA, Dice JF. 1996. Roles of molecular chaperones in protein degradation. J. Cell Biol. 132:255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rothman JE. 1989. Chain binding proteins: catalysts of protein folding and related processes in cells. Cell 59:591–601 [DOI] [PubMed] [Google Scholar]
- 54. Zylicz M, Ang D, Liberek K, Georgopoulos C. 1989. Initiation of λDNA replication with purified host- and bacteriophage-encoded proteins: the role of the dnaK, dnaJ and grpE heat shock proteins. EMBO J. 8:1601–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Minty JJ, Lesnefsky AA, Lin F, Chen Y, Zaroff TA, Veloso AB, Xie B, McConnell CA, Ward RJ, Schwartz DR, Rouillard JM, Gao Y, Gulari E, Lin XN. 2011. Evolution combined with genomic study elucidates genetic bases of isobutanol tolerance in Escherichia coli. Microb. Cell Fact. 10:18–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fiocco D, Capozzi V, Goffin P, Hols P, Spano G. 2007. Improved adaptation to heat, cold, and solvent tolerance in Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 77:909–915 [DOI] [PubMed] [Google Scholar]
- 57. Tomas CA, Welker NE, Papoutsakis ET. 2003. Overexpression of groESL in Clostridium acetobutylicum results in increased solvent production and tolerance, prolonged metabolism, and changes in the cell's transcription program. Appl. Environ. Microbiol. 69:4951–4965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tomas CA, Beamish J, Papoutsakis ET. 2004. Transcriptional analysis of butanol stress tolerance in Clostridium acetobutylicum. J. Bacteriol. 186:2006–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim SN, Bae YG, Rhee DK. 2008. Dual regulation of dnaK and groE operons by HrcA and Ca++ in Streptococcus pneumoniae. Arch. Pharm. Res. 31:462–467 [DOI] [PubMed] [Google Scholar]
- 60. Kwon HY, Kim SN, Pyo SN, Rhee DK. 2005. Ca2+-dependent expression of the CIRCE regulon in Streptococcus pneumoniae. Mol. Microbiol. 55:456–468 [DOI] [PubMed] [Google Scholar]
- 61. Dutta R, Inuoye M. 2000. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci. 25:24–28 [DOI] [PubMed] [Google Scholar]
- 62. Rovinskiy N, Agbleke AA, Chesnokova O, Pang Z, Higgins NP. 2012. Rates of gyrase supercoiling and transcription elongation control supercoil density in bacterial chromosome. PLoS Genet. 8:e1002845 doi:10.1371/journal.pgen.1002845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tse-Dinh YG. 2009. Bacterial topoisomerase I as a target for discovery of antibacterial compounds. Nucleic Acids Res. 37:731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang JC. 1996. DNA topoisomerases. Annu. Rev. Biochem. 65:635–692 [DOI] [PubMed] [Google Scholar]
- 65. Lorsch JR. 2002. RNA chaperones exist and DEAD box proteins get a life. Cell 109:797–800 [DOI] [PubMed] [Google Scholar]
- 66. Vashisht AA, Tuteja N. 2006. Stress responsive DEAD-box helicases: a new pathway to engineer plant stress tolerance. J. Photochem. Photobiol. B 84:150–160 [DOI] [PubMed] [Google Scholar]
- 67. Gong Z, Dong C, Lee H, Zhu J, Xiong L, Gong D, Stevenson B, Zhu J. 2005. A DEAD box RNA helicase is essential for mRNA export and import for development and stress response in Arabidopsis. Plant Cell 17:256–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weinberg MV, Jenney FE, Jr, Cui X, Adams MWW. 2004. Rubrerythrin from the hyperthermophilic archaeon Pyrococcus furiosus is a rubredoxin-dependent, iron-containing peroxidase. J. Bacteriol. 186:7888–7895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lehmann Y, Meile L, Teuber M. 1996. Rubrerythrin from Clostridium perfringens: cloning of the gene, purification of the protein, and characterization of its superoxide dismutase function. J. Bacteriol. 178:7152–7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alsaker KV, Paredes C, Papoutsakis ET. 2010. Metabolite stress and tolerance in the production of biofuels and chemicals: gene-expression-based systems analysis of butanol, butyrate, and acetate stresses in the anaerobe Clostridium acetobutylicum. Biotechnol. Bioeng. 105:1131–1147 [DOI] [PubMed] [Google Scholar]
- 71. Russell AD. 2003. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 52:750–763 [DOI] [PubMed] [Google Scholar]
- 72. Mao S, Luo Y, Zhang T, Li J, Bao G, Zhu Y, Chen Z, Zhang Y, Li Y, Ma Y. 2010. Proteome reference map and comparative proteomic analysis between a wild type Clostridium acetobutylicum DSM 1731 and its mutant with enhanced butanol tolerance and butanol yield. J. Proteome Res. 9:3046–3061 [DOI] [PubMed] [Google Scholar]
- 73. Harris LM, Welker NE, Papoutsakis ET. 2002. Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J. Bacteriol. 184:3586–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ravagnani A, Jennert KC, Steiner E, Grunberg R, Jefferies JR, Wilkinson SR, Young DI, Tidswell EC, Brown DP, Youngman P, Morris JG, Young M. 2000. Spo0A directly controls the switch from acid to solvent production in solvent-forming clostridia. Mol. Microbiol. 37:1172–1185 [DOI] [PubMed] [Google Scholar]
- 75. Wilkinson SR, Young DI, Morris JG, Young M. 1995. Molecular genetics and the initiation of solventogenesis in Clostridium beijerinckii (formerly Clostridium acetobutylicum) NCIMB 8052. FEMS Microbiol. Rev. 17:275–285 [DOI] [PubMed] [Google Scholar]
- 76. Baumgarten T, Sperling S, Seifert J, von Bergen M, Steiniger F, Wick LY, Heipieper HJ. 2012. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl. Environ. Microbiol. 78:6217–6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Waters CM. 2012. The meteoric rise of the signaling molecule cyclic di-GMP. Microbe 7:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.