Abstract
Nontypeable Haemophilus influenzae (NTHI) is a commensal inhabitant of the human nasopharynx and a causative agent of otitis media and other diseases of the upper and lower human airway. During colonization within the host, NTHI must acquire essential nutrients and evade immune attack. We previously demonstrated that the NTHI Sap transporter, an inner membrane protein complex, mediates resistance to antimicrobial peptides and is required for heme homeostasis. We hypothesized that Sap transporter functions are critical for NTHI interaction with the host epithelium and establishment of colonization. Thus, we cocultured the parent or the sapA mutant on polarized epithelial cells grown at an air-liquid interface, as a physiological model of NTHI colonization, to determine the contribution of the Sap transporter to bacterium-host cell interactions. Although SapA-deficient NTHI was less adherent to epithelial cells, we observed a significant increase in invasive bacteria compared to the parent strain. Upon internalization, the sapA mutant appeared free in the cytoplasm, whereas the parent strain was primarily found in endosomes, indicating differential subcellular trafficking. Additionally, we observed reduced inflammatory cytokine production by the epithelium in response to the sapA mutant strain compared to the parental strain. Furthermore, chinchilla middle ears challenged with the sapA mutant demonstrated a decrease in disease severity compared to ears challenged with the parental strain. Collectively, our data suggest that NTHI senses host environmental cues via Sap transporter function to mediate interaction with host epithelial cells. Epithelial cell invasion and modulation of host inflammatory cytokine responses may promote NTHI colonization and access to essential nutrients.
INTRODUCTION
Nontypeable Haemophilus influenzae (NTHI) is a commensal inhabitant of the human nasopharynx, yet it can cause opportunistic infections in compromised upper and lower respiratory tracts. As such, NTHI is a leading cause of otitis media, sinusitis, and community-acquired pneumonia and is also commonly associated with exacerbations of chronic obstructive pulmonary disease and cystic fibrosis (1–7). Commensal and opportunistic colonization of the host requires NTHI to overcome a myriad of host defense mechanisms, such as production of bactericidal proteins, physical mucociliary clearance, and sequestration of essential nutrients (8–15). NTHI strains have developed several successful strategies to evade host immunity and equip themselves for survival in vivo, including production of IgA1 protease, expression of aggregative adhesins, formation of biofilms, and modification of surface lipooligosaccharide (LOS) (16–28). Gram-negative outer membrane components such as LOS can be potent stimulators of the host inflammatory response and serve as binding targets for immunoprotective antibodies, complement proteins, and antimicrobial peptides (21, 29, 30). Modification of LOS provides a first line of defense for NTHI and, as such, a mechanism to evade the host innate immune system. For instance, addition of sialic acid to LOS prevents complement deposition, and phosphorylcholine (ChoP) modification inhibits the binding of immunoprotective antibodies, further serving to mask bacterial surface charge and minimize antimicrobial peptide binding (17, 30–32). In addition, LOS modifications induce changes in host epithelial responses. ChoP-decorated LOS facilitates attachment to and invasion of the epithelium and signaling through the platelet-activating factor receptor (PAFr). ChoP activation of the PAFr signaling cascade downregulates host expression of Toll-like receptor 2 (TLR2), TLR4, and TLR9 and promotes NTHI invasion of host epithelial cells (20, 21, 33, 34). Traditionally, NTHI has been categorized as an extracellular pathogen; however, invasion of host epithelial cells can offer a temporary or long-term respite from the host immune response and can counter active nutrient depletion by the host. The process of active nutrient depletion, termed “nutritional immunity,” is a host mechanism to inhibit microbial growth by sequestering essential nutrients (i.e., iron, zinc, and manganese) (9, 11, 12, 14, 15). Recalcitrance to antibiotic therapy, persistence in the presence of protective antibodies, and culture-negative clinical status suggest that biofilm formation and development of bacterial reservoirs within host cells may contribute to the chronic nature of NTHI infections (1, 4, 35–40).
Bacterial mechanisms to acquire essential nutrients and evade innate immune responses are essential for NTHI survival as a commensal in the nasopharynx and as a pathogen at privileged sites in the host. We have previously demonstrated an essential role for the Sap transporter, a multifunctional inner membrane ABC transport complex, in resistance to antimicrobial peptide killing and the transport of the essential heme iron (41–44). Antimicrobial peptides are transported into the bacterial cytoplasm in a Sap-dependent manner and are subsequently degraded by cytoplasmic peptidase activity (45). Similarly, the Sap transporter functions in the uptake of heme iron. First bound by the SapA periplasmic binding protein, heme iron is delivered for transport across the cytoplasmic membrane through the SapBC permease complex (44). In addition to the multifunctional roles of Sap transporter function in innate immune resistance and nutrient acquisition, Sap transporter function influences NTHI biofilm development and architecture. NTHI deficient in the SapF ATPase protein developed a more robust biofilm than that of the parental strain, coincident with morphological plasticity of NTHI, including increased chain length and filament production within the biofilm architecture (46). We have further demonstrated that neutralization of host antimicrobial peptides restores virulence to the sapA mutant in vivo, suggesting an essential role for Sap-dependent antimicrobial peptide resistance during the acute phase of disease in the host (45). Sap transporter function thus serves to maintain NTHI heme iron homeostasis and persistence in the host, providing mechanisms to resist antimicrobial peptides, aid in nutrient acquisition, and influence NTHI biofilm formation.
Due to these essential roles, we investigated the consequence of loss of Sap transporter functions on colonization of epithelial cells. We determined that NTHI deficient in SapA was less adherent to epithelial cells yet was associated with membrane ruffling and epithelial cell destruction. In addition, we observed that SapA-deficient NTHI was more invasive and had a decreased immune-stimulatory effect on host epithelium compared to host responses induced by the parental strain. Collectively, these data support an important role for Sap transporter function in NTHI interaction with host epithelial cells. We propose that NTHI utilizes the Sap transporter to sense microenvironmental cues such as heme iron limitation and antimicrobial peptide production to modulate the host environment, gain access to essential nutrients, and evade the innate immune system. Additionally, these data suggest that NTHI residence in the epithelial cytoplasm may function as a bacterial reservoir during chronic infections.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The parental NTHI strain 86-028NP::rpsLA128G is a streptomycin-resistant strain constructed as previously described (47). Construction of a strain with an unmarked, nonpolar deletion of the sapA gene was performed by the recombineering strategy as previously described (46, 47). Briefly, primers 5′-AAGTGCGATGGTATTTTGACGAA-3′ and 5′-ACGAGTAATATGATCCGCCTTTGT-3′ were used to amplify sapA and 1 kb of the flanking DNA both 5′ and 3′ to sapA. The subsequent amplicon was ligated into the pGEM-T Easy vector (pFR001) and transformed into Escherichia coli strain DY380. In parallel, primers, 5′ TAATATGCCTTACAATTTGACACATAATTTATCACAATGCATTTGTTATG 3′ and 5′ CAGAATATGGCGAAGAACCGACCAGAACATTAGTGTTTCTCCTGAATAAA 3′, each containing 50 bp of DNA homologous to the 5′ and 3′ ends of the sapA gene, were used to amplify the spec-rpsL cassette from pRSM2832 (47). This amplicon was then electroporated into strain E. coli DY380/pFR001 to form strain DY380/pFR002, in which the sapA gene in pFR001 has been replaced by the cassette. The plasmid pFR002 was then used to transform NTHI 86-028NP::rpsLA128G, and transformants were selected by growth on spectinomycin-containing Chocolate II agar plates. To generate a nonpolar deletion mutant, the sapA mutant was transformed with plasmid pRSM2947 and grown at 32°C, and FLP expression was induced using anhydrotetracycline. The cells were cured of the plasmid by growth at 37°C. Green fluorescent protein (GFP)-expressing parent and sapA mutant strains were created by electroporation of pGM1.1 as published previously (43).
Bacterial strains were grown overnight on Chocolate II agar (Becton, Dickinson, Sparks, MD) and then subcultured into prewarmed brain heart infusion broth supplemented with 2 μg heme/ml (Becton, Dickinson, Sparks, MD) and 1 μg NAD/ml (Becton, Dickinson, Sparks, MD) (sBHI). Cultures were normalized to an optical density at 490 nm (OD490) of 0.65, diluted 1:6 in sBHI, and grown for 3 h to logarithmic phase at an OD490 of 0.65. Logarithmic-phase bacteria were inoculated onto epithelial cells at a multiplicity of infection (MOI) of 50.
Epithelial cell adherence assay.
Adherence of the parent strain and the sapA mutant was determined on epithelial cell monolayers in a 96-well plate. Two microliters of logarithmic-phase bacteria (MOI = 50) was inoculated onto confluent monolayers of chinchilla middle ear epithelial (CMEE) cells, A549 human adenocarcinoma epithelial cells (American Type Tissue Collection, Manassas, VA), or normal human bronchial epithelial (NHBE) cells (American Type Tissue Collection, Manassas, VA). After 30, 60, and 90 min, the cell culture medium was removed and the epithelial cell layers were washed three times with 200 μl Dulbecco's phosphate-buffered saline (DPBS) (Mediatech, Manassas, VA), followed by a 3-min incubation with 0.25% trypsin–2.21 mM EDTA in Hanks balanced salt solution (HBSS) (Mediatech, Manassas, VA). Cell suspensions were serially diluted, and the CFU of adherent bacteria was determined by plating on Chocolate II agar. Adherent bacteria were calculated as a percentage of the inoculum. The adherence assay was repeated for a total of three biological replicates on each cell type, and significance for CMEE cells was determined by a two-tailed Student t test and two biological replicates for NHBE cells.
Transwell model of respiratory epithelial cell growth.
Normal human bronchial epithelial cells, chinchilla middle ear epithelial cells, and primary human airway epithelial cells were seeded onto Transwell membranes and grown to confluence. Confluent monolayers were determined by measuring a resistance of greater than 1,000 Ω/4.5 cm2 across the Transwell membrane using an Epithelial Voltohmmeter (World Precision Instruments, Sarasota, FL). After confluence, cell culture medium on the apical surface was removed and epithelial cell growth and differentiation were monitored for 2 weeks prior to inoculation. A 28.3-μl sample (MOI = 50) of logarithmic-phase bacteria was inoculated onto the apical surface of the Transwell-grown epithelial cells in 300 μl DPBS for 1 h, after which nonadherent bacteria were removed and the epithelial cell surface was washed once with 500 μl DPBS. After 24 h, spent medium was collected from the basolateral surface and the apical surface was washed once with DPBS and collected. The Transwell contents were fixed for electron microscopy in 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) in DPBS for scanning electron microscopy (SEM) or in 2.5% glutaraldehyde and 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in DPBS for transmission electron microscopy or were treated with TRIzol (Invitrogen, Carlsbad, CA) for RNA isolation.
Scanning electron microscopy.
After fixation, cells were washed two times in 0.2 M sodium cacodylate buffer, followed by subsequent incubation with 1% osmium tetroxide (Electron Microscopy Sciences, Hatfield, PA) for 2 h, 1% thiocarbohydrazide (Electron Microscopy Sciences, Hatfield, PA) for 30 min, and 1% osmium tetroxide to enhance electron contrast of osmiophilic structures on the cell's surface. Between each step, the samples were washed 5 times with double-distilled water. The samples were then dehydrated in a graded series of ethanol solutions, followed by critical-point dehydration in hexamethyldisilazane (Electron Microscopy Sciences, Hatfield, PA) for 15 and then 10 min. Samples were air dried overnight and adhered to SEM specimen mount stubs with colloidal silver (Electron Microscopy Sciences, Hatfield, PA). Images were obtained on a Hitachi S4800 scanning electron microscope at 3 kV.
Transmission electron microscopy.
For ultrastructural analysis, Transwells were fixed in 2% paraformaldehyde–2.5% glutaraldehyde in PBS for 1 h at room temperature. Samples were washed in phosphate buffer and postfixed in 1% osmium tetroxide (Polysciences Inc., Warrington, PA) for 1 h. Samples were then rinsed extensively in distilled water (dH2O) prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA) for 1 h. Following several rinses in dH2O, samples were dehydrated in a graded series of ethanol solutions and embedded in Eponate 12 resin (Ted Pella Inc.). Ultrathin sections of 90 nm were obtained with a Leica Ultracut UCT ultramicrotome (Leica Microsystems Inc., Bannockburn, IL), stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA Inc., Peabody, MA).
NTHI inoculation onto epithelial cell monolayers for microscopy.
Normal human bronchial epithelial cells and chinchilla middle ear epithelial cells were grown to confluence on a glass coverslip. Epithelial cells were inoculated with 3.3 μl of mid-logarithmic-phase bacteria (MOI, 50) in 100 μl DPBS. One hour after inoculation, the coverslips were flooded with 1 ml DPBS and replaced with cell culture medium. After 24, 72, or 96 h, the cells were fixed in 2.5% glutaraldehyde in DPBS for scanning electron microscopy or in 2% paraformaldehyde in DPBS for fluorescence microscopy. For fluorescence microscopy, epithelial cell membranes were labeled with wheat germ agglutinin (WGA)-Alexafluor 594 (Life Technologies), and DNA was counterstained with Hoechst 34580 (Life Technologies). For immunofluorescence microscopy, NTHI was labeled with rabbit anti-outer membrane protein (OMP) and detected with anti-rabbit GFP.
Cytokine array.
Spent medium was collected from the basolateral surface of the Transwell model at 24 h after inoculation with NTHI. Cytokine secretion into the spent medium was measured using the Proteome Profiler human cytokine array kit (R&D Systems, Minneapolis MN). Briefly, medium samples were incubated with biotinylated detection antibodies. The complex of cytokine and detection antibody was then bound to a cognate antibody immobilized on a nitrocellulose membrane. Relative amounts of the cytokine were detected by measuring streptavidin-horseradish peroxidase (HRP) chemiluminescence, and the fold change in cytokine production was determined by measuring the pixel density at each cognate antibody spot.
Gentamicin protection assay.
Invasion by the parent strain and the sapA mutant was determined on epithelial cell monolayers grown to confluence in a 96-well plate. Two microliters of logarithmic-phase bacteria (MOI = 50) was inoculated onto confluent monolayers of normal human bronchial epithelial cells. After adherence for 1 h, the cells were washed once with DPBS to removed nonadherent bacteria. At 24 h after inoculation, the wells were washed once with DPBS and then treated with 50 μg/ml gentamicin (Sigma-Aldrich) in tissue culture medium for 1 h. Following gentamicin treatment, the epithelial cells were lysed in 0.1% Triton X-100 (Fischer Scientific, Fair Lawn, NJ) in DPBS, and the number of invaded bacteria was determined by protection from gentamicin killing and enumerated by serial dilution and plating. The gentamicin protection assay was repeated for a total of three biological replicates, and significance was determined with a two-tailed Student t test.
Animal studies.
Healthy adult chinchillas (Chinchilla lanigera) purchased from Rauscher's chinchilla ranch (LaRue, OH) were used to assess disease progression after inoculation with either the parent strain or the sapA mutant. Chinchillas were anesthetized with xylazine (2 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and ketamine (10 mg/kg; Phoenix Scientific Inc., St. Joseph, MO), and middle ears were challenged with 2,500 CFU of either the parent strain or the sapA mutant by transbullar inoculation. At 3 days postinoculation, the animals were sacrificed and the middle ears were fixed for histological examination. Middle ear samples were fixed in 4% paraformaldehyde in DPBS, decalcified, paraffin embedded, section, and stained with hematoxylin and eosin (H&E).
RESULTS
Loss of SapA decreases adherence to epithelial cells.
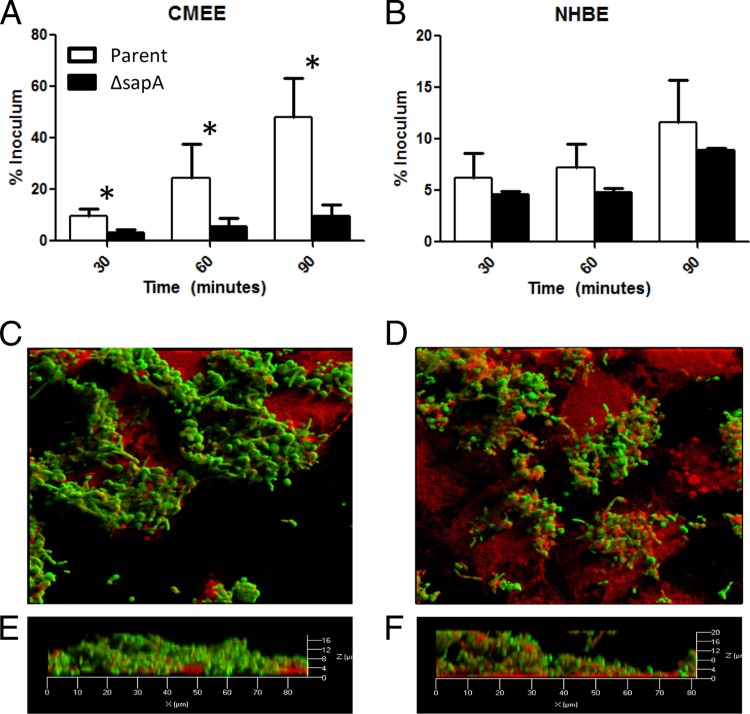
Sap transporter function maintains NTHI heme iron homeostasis and persistence in the host, providing mechanisms to resist antimicrobial peptides, acquire heme, and influence NTHI biofilm formation (41–45). The host microenvironment at the mucosal surface challenges NTHI survival through nutrient (i.e., heme iron) limitation and bactericidal innate immune effectors (48); thus, we hypothesized that Sap transporter functions are critical for NTHI interaction with the host epithelial cells and establishment of colonization. To investigate the influence of Sap transporter function on this critical initial interaction, we monitored NTHI adherence to anatomically variant and species-variant primary epithelial cells: normal human bronchial epithelial (NHBE) cells and chinchilla middle ear epithelium (CMEE). The chinchilla has been used extensively as a model for NTHI-mediated otitis media, and cells established from chinchilla middle ear explants have been used to study NTHI-host epithelial interactions (49, 50). NHBE cells are derived from donor epithelial tissue and are used as a physiological model to study upper respiratory tract infections (48). The parent and sapA mutant strains were evaluated for adherence to epithelial cell monolayers. Independent of epithelial cell source, we observed a reduction in the ability of a sapA mutant to adhere to epithelial cells compared to adherence of the parent strain (Fig. 1). NTHI has been shown to preferentially adhere to nonciliated cells of the respiratory tract and to utilize host structures such as ICAM-1, CEACAM1, sialic acid-containing lactosylceramide, glycoproteins related to heparan sulfate and mucin, and plasma membrane receptors (16, 22, 25–28, 33, 49, 51–56). Therefore, the disparity in NTHI adherence to these different cell types was not unexpected. In fact, the higher efficiency of binding to the CMEE cells may have allowed for better detection of changes in adherence to these cells in the absence of SapA. These results demonstrate that the Sap transporter function can influence adherence of NTHI to epithelial cells.
Fig 1.
Adherence and epithelial cell surface remodeling in the absence of SapA. (A and B) CMEE (A) and NHBE (B) cells were inoculated with either the parent strain (white bars) or the sapA mutant strain (black bars). Numbers of bacteria adherent to the epithelial cell monolayers were determined at each time point and are depicted as the mean adherent bacteria ± standard deviation for triplicate wells from three (B) or two (A) independent experiments. The asterisk depicts a significant change in adherence between the sapA mutant and the parent strain (P < 0.05). (C to F) The parent (C and E) or the sapA mutant (D and F) GFP reporter strains (green) were inoculated onto CMEE cell monolayers (red), incubated for 72 h, and monitored for colonization and epithelial cell surface changes by confocal microscopy. Panels E and F are three-dimensional rendered optical sections of the bacterium-epithelial cell interface.
Loss of SapA perturbs NTHI-epithelial cell interaction.
Since we observed a reduction in the adherence of a mutant deficient in SapA, we sought to determine the effects of this mutation on colonization and biofilm formation on the surface of host epithelial cells. The importance of biofilm formation in the resistance to antimicrobial peptides and antibiotic therapy has been well established, and it contributes to persistence in the host (31, 57, 58). In addition, biofilm formation is influenced by host microenvironments that are limited in available iron, which is particularly important as the host sequesters free iron as a means to limit bacterial growth (59–67). We have demonstrated that transient restriction of heme iron enhances biofilm structural complexity and peak height in wild-type NTHI (B. R. Szelestey and K. M. Mason, unpublished observations). Since microenvironmental cues can influence biofilm formation, we hypothesized that Sap transporter functions contribute to NTHI colonization and biofilm formation on host epithelial cells. NTHI inhabits a number of different types of epithelium in the host, including the oropharynx, middle ear, and lung epithelia. The best representative cultured cell condition to model the privileged middle ear is CMEE cells. Since we observed a more pronounced adherence defect of the sapA mutant on CMEE monolayers (Fig. 1), we examined colonization and biofilm formation by the parental or sapA mutant strain on these cells. To that end, parent or sapA mutant GFP reporter strains were cocultured with CMEE cell monolayers for 72 h, washed to remove nonadherent bacteria, fixed, and monitored for community development and epithelial cell membrane perturbations. Epithelial cell membranes were labeled with wheat germ agglutinin (WGA) and visualized for bacterial interaction by confocal microscopy. We observed that the parental strain was primarily cell surface associated as a biofilm (Fig. 1C). In addition, three-dimensional rendered optical sections of the bacterium-epithelial cell interface revealed little perturbation of the epithelial cell membrane as indicated by green pseudocolor (bacteria) and red pseudocolor (epithelial cell membrane) in different focal planes (Fig. 1E, orthogonal view), supporting surface colonization by the bacteria. In contrast, the sapA mutant remodels the epithelial cell surface, resulting in membrane perturbations that enveloped the bacteria (Fig. 1D). In fact, the sapA mutant was observed in the same focal plane as the epithelial cell membrane, in many cases extending 20 μm above the planer surface, which is suggestive of epithelial cell membrane ruffling and compromise of the epithelial cell membrane integrity (Fig. 1F, orthogonal view). We observed similar results when the parent strain or sapA mutant was cocultured with NHBE cell monolayers (see Fig. S1 in the supplemental material). These observations were thus independent of epithelial cell type and initial levels of adherence (compare Fig. 1A and B and Fig. S1 in the supplemental material).
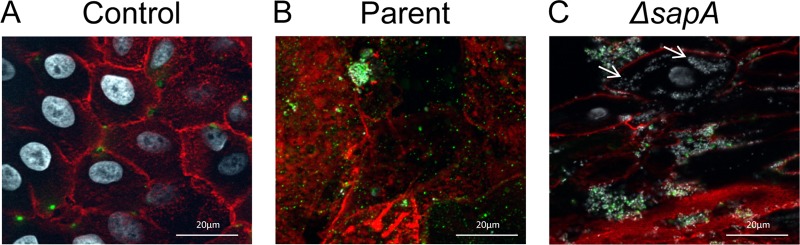
The membrane changes associated with colonization by the sapA mutant suggested that the sapA mutant had penetrated the epithelial cell membrane and colonized the cytoplasm. To more closely examine the cellular localization of NTHI on and within epithelial cells, NTHI was cocultured with NHBE monolayers for 24 h, the monolayers were washed to remove nonadherent bacteria, and surface-exposed NTHI was immunolabeled. Invasive bacteria, which were inaccessible to immunolabeling, were subsequently identified by DNA counterlabeling. Thus, surface-associated bacteria were detected by punctate fluorescence (pseudocolored green), whereas internalized bacteria were visualized as white. Epithelial cell membranes were labeled with WGA and visualized for bacterial localization by immunofluorescence microscopy. We observed the parental strain colonizing the surface of the epithelial cells, predominantly visualized by punctate green fluorescence (Fig. 2B). In contrast, we observed that the sapA mutant localized to both the surface and cytoplasm of the epithelial cells (Fig. 2C). Cytoplasmic localization of the sapA mutant was indicated by white DNA counterlabeling throughout the cytoplasm of the epithelial cells. The fluorescence of the bacteria in the cytoplasm in conjunction with the absence of antibody labeling is highly suggestive of internalization of the sapA mutant. Moreover, the intensity and proximity of the signal are indicative of bacterial microcolonies within the cytoplasm. Collectively, these data suggest that Sap transporter function mediates NTHI adherence, influences colonization of epithelial cells, and maintains epithelial cell membrane homeostasis.
Fig 2.
Epithelial cell invasion in the absence of SapA. NHBE cell monolayers (A) were inoculated with the parent strain (B) or the sapA mutant strain (C), incubated for 24 h, and monitored for colonization by fluorescence microscopy. NTHI cells were labeled with a anti-OMP antibody and detected by anti-rabbit FITC antibody (green). Epithelial cell membranes were stained with wheat germ agglutinin conjugated to Alexafluor 594 (red), and DNA was counterstained with Hoechst 34580 (white). Arrows indicate cytoplasmic NTHI.
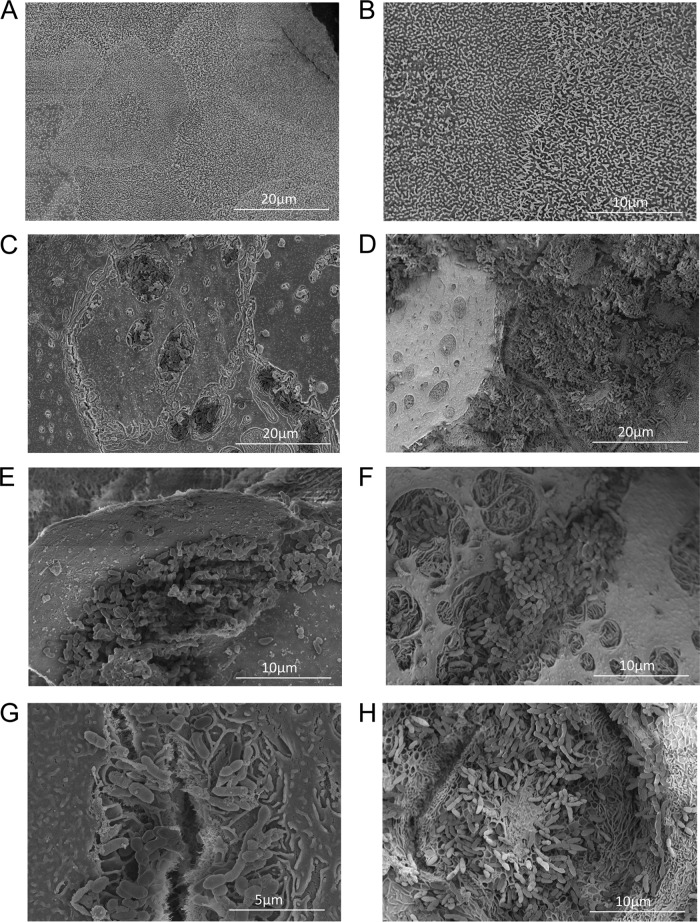
Next, we sought to better define bacterium-host interactions under conditions that more closely mimic bacterial infections of the respiratory tract (48). Polarized, differentiated NHBE cells were grown on semipermeable Transwell membrane supports at an air-liquid interface; similarly to epithelial cells of the respiratory tract, the NHBE cells formed polarized semistratified cell layers that produced mucus and expressed microvilli and cilia. Therefore, bacteria cocultured with the apical surface of the epithelial cells must colonize at the air-exposed surface and acquire nutrients from the epithelial cell, unlike colonization of epithelial cell monolayers which are submerged in nutrient-rich medium (48). Thus, to determine the influence of the Sap transporter on colonizing the nutrient-limited apical surface of epithelial cells, we exposed polarized NHBE cells to either the parental strain or the sapA mutant strain for 24 h and visualized bacterium-host cell interactions by SEM. The parental strain colonized the apical surface by formation of microcolonies or larger biofilm communities (Fig. 3C and E). We typically observed small clusters of the parent strain in patches on the epithelial cell surface; these patches appear to form in minor lesions on the epithelial cell surface. The lesions are absent in the control cells (Fig. 3A and B), suggesting that they are triggered by colonization of the bacteria. Despite the presence of small lesions, there was very little disruption of the epithelial cell layer integrity (i.e., the surface appears relatively smooth). In contrast, although able to colonize the apical surface, the biofilms formed by the sapA mutant were more dense and coincided with evidence of epithelial cell destruction associated with bacterial colonization (Fig. 3D and F). In contrast to the case for parental strain (Fig. 3G), populations of the sapA mutant were often associated with “honeycomb”-like structures which seemed to have been left behind by bacteria that were disassociated during the processing of the samples (Fig. 3H). The “honeycomb”-like structures appeared to be of epithelial cell origin. Consistent with our observations with polarized NHBE cells, we also observed enhanced biofilm formation and paracellular localization by the sapA mutant on CMEE cells (see Fig. S2 in the supplemental material). Thus, our observations of colonization on polarized NHBE cells were similar to our observations of that on epithelial cell monolayers; both strains colonized the surface of the epithelial cells, and the sapA mutant modified the epithelial cell surface and was associated with epithelial cell membrane perturbations. However, we observed small lesions induced by both the parental strain and the sapA mutant in this nutrient-restricted, air-exposed microenvironment. Furthermore, the biofilms formed by the sapA mutant were associated with epithelial cell destruction and invasion by the sapA mutant. Thus, the polarized tissue culture model enabled us to study colonization of NTHI using a more relevant, nutrient-restricted microenvironment.
Fig 3.
SapA mediates epithelial cell surface colonization by NTHI. Polarized epithelial cells grown at an air-liquid interface (A and B) were inoculated with the parent strain (C, E, and G) or the sapA mutant strain (D, F, and H) and incubated for 24 h. NTHI-epithelial cell interactions were monitored by scanning electron microscopy.
Loss of SapA function results in a hyperinvasive phenotype.
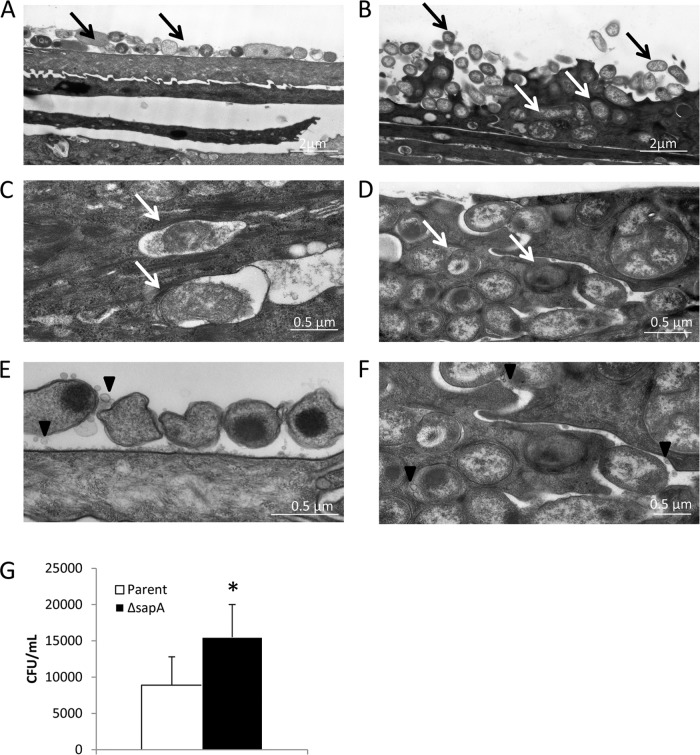
Although it is classically considered an extracellular, opportunistic pathogen, there is increasing evidence of intracellular and intercellular niches for NTHI in vitro (36). Further, the presence of NTHI within adenoids and bronchial epithelium suggests that an invasive phenotype may coincide with the chronic nature associated with NTHI-mediated diseases (68–72). Invasion of epithelial cells could provide NTHI with an environment rich in nutrients and a refuge from immune pressures. Our previous data indicate an increased propensity for the sapA mutant to disrupt the epithelial permeability barrier and invade bronchial epithelial cells (Fig. 2C). Therefore, we sought to further investigate this hyperinvasive phenotype and determine the ultrastructural localization of the parental strain or the sapA mutant following coculture on the apical surface of polarized NHBE cells by examining cross sections of the epithelium by transmission electron microscopy. We observed that the parental strain colonized primarily the apical surface of the epithelial cells, with little evidence of membrane perturbations (Fig. 4A and C), consistent with our previous results (Fig. 1C and 3C, E, and G). This rather benign surface association did not preclude NTHI invasion, as the parent strain was also observed to localize to the cell cytoplasm, typically in membrane-enclosed vacuoles (Fig. 4C). Although invasion appeared to be detrimental as observed by loss of bacterial density and membrane integrity within the presumed phagosomal vacuoles, these data are consistent with previous observations of NTHI intracellular invasion (48). In contrast, although the sapA mutant also colonized the apical surface, we observed enhanced epithelial cell membrane perturbations, disruption of the epithelial membrane barrier, and populations of the sapA mutant in the cytoplasm of epithelial cells (Fig. 4B and D). Interestingly, these invasive bacteria did not appear to be contained within a membranous compartment but appeared to colonize the host cytoplasm, in most cases retaining bacterial density and membrane integrity (Fig. 4B and D). Similarly, we observed invasive phenotypes of the parent and sapA mutant in primary differentiated chinchilla nasopharyngeal epithelial cells (data not shown), suggesting different mechanisms of intracytoplasmic trafficking and survival of the sapA mutant and the parental strain. The hyperinvasive phenotype of the sapA mutant was confirmed by gentamicin protection, demonstrating a significant increase in invasion by the sapA mutant compared to the parental strain (Fig. 4E). In addition, we observed outer membrane vesicle (OMV) production by NTHI at the epithelial cell surface by the parent strain (Fig. 4E) and both within and between NHBE cells by the sapA mutant strain (Fig. 4F). These data are consistent with recent observations of NTHI OMV production following long-term (5-day) colonization of EpiAirway cells, a commercially available cell model system (48). However, our observations indicate that OMVs not only are produced rapidly, 1 day following infection of polarized NHBE cells, but are shed differentially at subcellular locations of NHBE cells. Previously, we demonstrated that NTHI OMVs are internalized by host epithelial cells and trigger host cell signaling and cytokine production (73). These data, in addition to observed hypervesiculation of the SapA-deficient strain compared to the parental strain in vitro (S. W. Sharpe and K. M. Mason, unpublished data), indicate an important role for OMV production in NTHI-host cell interactions, which is under investigation in our laboratory.
Fig 4.
Loss of SapA promotes a hyperinvasive phenotype. (A to D) Polarized epithelial cells grown at an air-liquid interface were inoculated with the parent strain (A and C) or the sapA mutant strain (B and D) and incubated for 24 h. The subcellular ultrastructural localization of the parent strain or the sapA mutant was determined by transmission electron microscopy. Black arrows indicate bacteria that colonized the epithelial cell surface, and white arrows indicate bacteria present in the cytoplasm of the epithelial cells. (E and F) The parent strain produced outer membrane vesicles at the epithelial cell surface (E), and the sapA mutant strain produced outer membrane vesicles both within and between NHBE cells (F). (G) A gentamicin protection assay indicates an ∼2-fold increase in invasion by the sapA mutant compared to the parent strain when comparing the mean number of bacteria protected from gentamicin lethality ± standard deviation for triplicate wells performed in three independent experiments. The asterisk indicates a significant increase in survival of the sapA mutant compared to that of the parent strain (P < 0.05).
Collectively, these data indicate that loss of Sap transporter function promotes a hyperinvasive phenotype of NTHI, and they further suggest a role for the function of the Sap transporter in mediating a homeostatic interaction with host epithelial cells. Invasion into epithelial cells may benefit survival of NTHI in nutrient-restricted microenvironments as a mechanism to gain access to available nutrients in the cytoplasm of the host epithelium. Our observations suggest that invasion of the sapA mutant may compensate for the loss of Sap transporter function to counter nutrient starvation.
Sap transporter function mediates epithelial cell stimulation and cytokine response to colonization.
The epithelium provides the host with a first-line defense to the external environment (74). In addition to providing a selective barrier, the epithelium is capable of mounting an inflammatory response by the secretion of antimicrobial molecules and the production of cytokines after stimulation by conserved microbial structures. Signaling pathways are often manipulated by bacteria to gain access to the cytoplasm in nonphagocytic cells (75). Our data suggest that the sapA mutant compensates for the loss of Sap transporter function via altered host cell membrane interactions and invasion of epithelial cells. The epithelial cell membrane perturbations and hyperinvasion of the epithelial cells by the sapA mutant suggest an alteration in epithelial cell homeostasis. To investigate this change in epithelial cell homeostasis, we monitored cytokine secretion by the epithelial cell in response to colonization by the parent strain or the sapA mutant. Polarized epithelial cells were cocultured with either the parent strain or the sapA mutant strain at the air-exposed apical surface of NHBE cells and assessed for fold change in basolateral cytokine production. We observed a decrease in proinflammatory cytokine and chemokine production by polarized normal human bronchial epithelial cells when exposed to the sapA mutant strain for 24 h compared to that in cells exposed to the parental strain (Table 1). We extended our analysis to other cell types, including CMEE cells and primary human alveolar epithelial (HAE) cells from human lung explants. We again observed a decrease in proinflammatory cytokine and chemokine production on these other primary cell types (Table 1). It is intriguing to note that interleukin-25 (IL-25) was elevated due to exposure of the parental strain in all three cell types tested. IL-25 stimulation of epithelial cells triggers the production of the type 2 cytokine response, characterized by the secretion of IL-4, IL-5, and IL-13, which were also increased in production in response to the parent strain (76). These data indicate that loss of Sap transporter function decreases NTHI stimulation of epithelial cell cytokine production. Further NTHI sensing of host microenvironmental cues (heme iron or antimicrobial peptides) via Sap transporter function may ultimately result in a decrease in epithelial cell cytokine production and thus contribute to the chronicity of NTHI-mediated diseases.
Table 1.
Cytokine production by respiratory epithelial cells stimulated by NTHI
| Cytokinea | Fold increase in productionb by epithelial cells |
||
|---|---|---|---|
| NHBE | CMEE | HAE | |
| G-CSF | 2.21 | ND | ND |
| I-309 | 3.37 | 2.56 | ND |
| IL-23 | 2.99 | 3.87 | ND |
| IL-16 | 1.95 | ND | 3.29 |
| CD40 L | 10.39 | ND | 2.08 |
| IL-25 | 2.59 | 3.31 | 2.88 |
| IL-1a | ND | 2.54 | 4.81 |
| IL-1b | ND | 3.51 | 7.15 |
| IL-2 | ND | 2.52 | 1.97 |
| IFN-γ | ND | 2.52 | 2.00 |
| IL-17 | ND | 2.62 | 10.63 |
| IL-4 | ND | 3.71 | 3.40 |
| IL-13 | ND | ND | 8.09 |
| MCP-1 | ND | ND | 3.68 |
| GM-CSF | ND | 2.38 | ND |
| IL-12p70 | ND | 2.86 | ND |
| IL-5 | ND | 4.85 | ND |
| IL-6 | ND | 2.44 | ND |
| IP-10 | ND | 4.05 | ND |
| MIP-1a | ND | 4.27 | ND |
| sICAM-1 | ND | 5.74 | ND |
G-CSF, granulocyte colony-stimulating factor; IFN-γ, gamma interferon; GM-CSF, granulocyte-macrophage colony-stimulating factor; MCP-1, monocyte chemoattractant protein 1.
Data represents the fold increase in production of the listed cytokines stimulated by the parent strain compared to the mutant strain; cytokines not listed had a fold change of less than 1.5. ND, no difference in cytokine production stimulated by the parent strain and the sapA mutant strain.
Sap transporter function modulates severity of experimental otitis media.
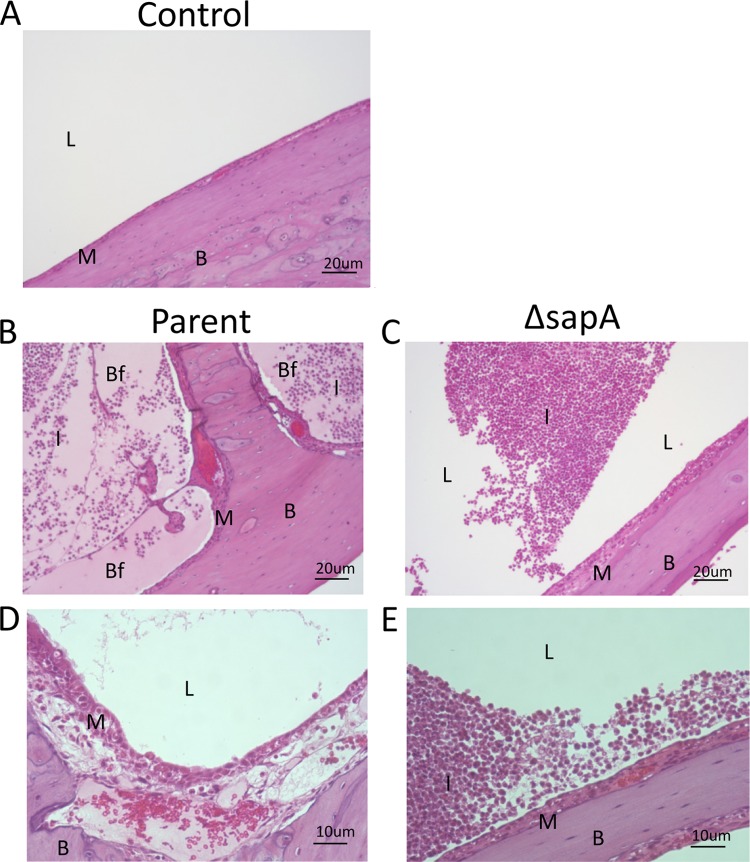
The decrease in the epithelial cytokine response to the sapA mutant suggested that there would be an alteration in disease progression in the middle ear. To characterize middle ear mucosal changes, chinchilla middle ear inferior bullae were examined histologically at 4 days following infection with either the parental strain or the sapA mutant. We observed capillary dilation, erythema, hemorrhagic foci, and host immune cell infiltrate in middle ears inoculated with the parent strain (Fig. 5B) compared to naïve middle ears (Fig. 5A). Upon closer examination of the middle ear mucosa, we observed mucosal epithelial cell thickening and edema (Fig. 5D). Middle ears inoculated with the sapA mutant also presented similar markers of middle ear disease; however, there was a clear decrease in the disease severity compare to that in the middle ears inoculated with the parent strain (Fig. 5C and E) despite no significant difference in bacterial burden compared to that of the parental strain (data not shown). These observations, concurrent with diminished cytokine production, suggest that the sapA mutant is less immunostimulatory than the parent strain, likely compensating for the increased susceptibility to antimicrobial peptides and thus decreasing epithelial cell stimulation.
Fig 5.
Loss of SapA attenuates NTHI-induced disease severity. Naïve chinchilla middle ears (A) were inoculated transbullarly with the parent strain (B and D) or the sapA mutant strain (C and E). Three days after inoculation, the middle ear inferior bullae were excised, embedded, and sectioned. Sections were H&E stained to monitor middle ear mucosal inflammation and NTHI biofilm formation in the middle ear cavity. L, middle ear lumen; I, infiltrating leukocyte; M, mucosa; B, bone; Bf, biofilm.
DISCUSSION
Elucidation of NTHI pathogenic survival strategies will provide the necessary platform for the development of novel treatment modalities that will ultimately reduce the burden of NTHI-mediated diseases. NTHI is exposed to complex host microenvironments that are typically nutrient limited due to host sequestration strategies, such as limitation of available iron, and are replete with bactericidal immune molecules. Our previous work described an essential role for Sap transporter function in the acquisition of heme iron and resistance to host antimicrobial peptides (41–46). Therefore, we exploited the use of NTHI deficient in Sap transporter function as a means to elucidate the consequences of microenvironmental cues for NTHI-host interactions that modulate disease severity. Here, we demonstrated increased ruffling of the epithelial membrane when cells were cocultured with NTHI deficient in SapA, indicating an altered interaction with the host cell surface. The increase in membrane ruffling coincides with an increased propensity of the sapA mutant to invade the apical layers of polarized, differentiated epithelial cells, in contrast to that of the parental isolate, which associated primarily with the cell surface and did not induce membrane ruffling. This hyperinvasive phenotype was coincident with a dysregulation in epithelial cell cytokine production, which was dampened in inflammatory cytokine production in vitro and, further, less inflammatory in vivo, compared to the observed responses to the parental strain. Bacteria invasion has been shown to dysregulate epithelial cytokine production (74, 77–81). Collectively, our data suggest a dynamic interplay between NTHI and the host that is mediated, at least in part, by host microenvironmental cues. Thus, microbial sensing of nutrient availability influences interactions with host cells. It has previously been demonstrated that the Ami oligopeptide ABC transporter in Streptococcus influences interaction with epithelial cells, which is hypothesized to be regulated by oligopeptide uptake and contribution to global metabolic gene regulation and thus dependent upon nutrient availability (82, 83). Our data are consistent with this hypothesis, suggesting that NTHI utilizes the Sap transporter to sense host-derived molecules that can differ in availability in distinct microenvironments of the respiratory tract. Our data further indicate that NTHI invades the epithelium, likely in order to gain access to essential nutrients, evade the innate immune response, and provide a bacterial population that upon reemergence may seed recurrent infections that contribute to chronic otitis media.
NTHI colonization is dependent on bacterial adherence to the mucosal surface. Additionally, epithelial cell invasion has been shown to be mediated by the Hap, protein D, and protein E adhesins (16, 28, 33) and ChoP moieties on NTHI LOS (33, 80). Our observations indicated that loss of SapA reduced the initial adherence of NTHI yet did not affect colonization of the cell surface. In fact, loss of SapA promoted invasion of epithelial cells. We do not observe alterations in ChoP expression in the LOS or changes in the expression of known adhesins in the outer membrane of the sapA mutant (F. K. Raffes and K. M. Mason, unpublished observations). Thus, these data suggest a previously uncharacterized mechanism for NTHI invasion. Further, the hyperinvasive phenotype of the sapA mutant suggests that this process may be regulated by the function of the Sap transporter and therefore be influenced by host microenvironmental cues such as heme limitation and antimicrobial peptide production during pathogenesis. It is interesting to note that although it was predominately surface associated, the parent strain also invaded the epithelial cells. However, the parent strain was observed in membrane-bound vacuoles, in contrast to localization of the sapA mutant in the cytoplasm, suggesting differential trafficking once the bacteria are internalized by the epithelial cells. Additionally, the fate of the internalized bacteria was different for the parent strain and the sapA mutant. The parent strain lost electron density and membrane integrity within the phagosomal vacuoles, consistent with previous observations of NTHI intracellular invasion. In contrast, the sapA mutant invasive bacteria did not appear to be contained within phagosomes but appeared to colonize the host cytoplasm, in most cases retaining bacterial density and membrane integrity. Invasion into epithelial cells provides an additional mechanism for resisting host nutritional and innate immune responses. It has been previously demonstrated that auxotrophic E. coli mutants are unable to survive in the extracellular environment but are able to survive in the cytoplasm of host epithelial cells (84, 85). The survival of auxotrophic E. coli in the cytoplasm suggests increased access to nutrients in the cytoplasm compared to the extracellular environment of the host. The clustering of the cytoplasmic NTHI that we observed is suggestive of intracellular growth (Fig. 2C and 4B and D). These data suggest that exposure of NTHI to host immune pressures such as increased concentrations of antimicrobial peptides or host sequestration of essential nutrients promotes epithelial cell invasion and survival in the cytoplasm (36). Interestingly, NTHI does not have a dedicated invasion or secretion system to initiate epithelial cell receptor independent invasion, which suggests that NTHI must exploit host cell signaling to gain access to the cytoplasm, mechanisms that are under investigation in our laboratory. It is also interesting to note the presence of NTHI membrane vesicles in our polarized NHBE model (Fig. 4). We have previously determined that NTHI vesicles are able to stimulate epithelial cells (73), and we are currently investigating their relevance in NTHI-epithelial cell homeostasis.
We examined the contribution of Sap transporter function to NTHI colonization of polarized, differentiated epithelial cells grown at an air-liquid interface, a physiological model of epithelial cells of the upper respiratory tract. Here, we observed that the sapA mutant formed a dense biofilm on the apical surface and invaded into the apical-most cell layers. Biofilms provide a mechanism for survival in a nutrient-limited environment and resistance to bactericidal molecules (31, 57, 58). Biofilm survival strategies are mediated by the expression of quorum-sensing-regulated genes, an increase in persister cell formation, metabolic heterogeneity, and secretion of extracellular polymeric structures. This suggests that the sapA mutant may compensate for the inability to acquire heme iron and resist antimicrobial peptides by preferentially forming biofilms on the epithelial cell surface to survive in the host epithelial cell microenvironment. Host microenvironmental cues may influence a similar phenotype during NTHI colonization. In fact, we have demonstrated that transient restriction of heme iron mediates NTHI morphological changes that influence biofilm architecture, attenuates the host response, and thus promotes NTHI persistence in the middle ear (Szelestey and Mason, unpublished observations), suggesting that at least the heme iron acquisition function of SapA contributes to the phenotypes described here. Both the increased propensity for biofilm formation and invasion of the epithelial cells could represent survival strategies utilized by NTHI in vivo.
Epithelial cells respond to bacterial colonization and invasion by the secretion of cytokines and chemokines that serve initially to attract innate immune leukocytes and subsequently to attract B cells and T cells to remove the invading pathogens. We determined that epithelial cell colonization by the sapA mutant stimulated decreased production of inflammatory cytokines compared to those produced in response to colonization by the parent strain. In order to determine if this differential host response to colonization would alter disease progression, we monitored the middle ear mucosa of chinchillas inoculated with either the parent strain or the sapA mutant. Histological analysis of middle ears infected with parental strain demonstrated severe edema, capillary dilation, erythema, hemorrhagic foci, host immune cell infiltrate, and mucosal epithelial cell destruction 3 days after inoculation. In contrast, middle ears inoculated with the sapA mutant demonstrated a striking decrease in epithelial inflammation that maintained an intact mucosal surface, despite no significant difference in bacterial burden compared to the parental strain as evidenced by biofilm formation and leukocyte influx in the middle ear. These data suggest that NTHI utilizes the Sap transporter to sense the host microenvironment and mediate interactions with the host mucosal surface. In fact, we have determined that transiently heme iron-restricted NTHI elicited a similar reduction in inflammation and epithelial cell damage that enhanced NTHI persistence in the middle ear (Szelestey and Mason, unpublished observations). Therefore, NTHI will decrease the stimulation of epithelial cells, resulting in a decreased expression and production of cytokines and ultimately altering disease progression.
Our results highlight the delicate balance maintained between the host epithelium and commensal bacteria at the epithelial cell interface. Here we demonstrated that the Sap transporter function influenced NTHI interaction with host epithelium, revealing a mechanism by which NTHI can sense the host epithelial cell microenvironmental cues that impact NTHI behavior. Our data suggest that NTHI colonization of the nasopharynx is dictated by host microenvironmental cues that limit host cell interaction to thus establish a commensal relationship in this environment. However, upon transition to other host sites, NTHI senses changes in nutrient availability and innate immune pressures, which triggers a more pathogenic lifestyle which coincides with enhanced biofilm community development and modulation of epithelial cell responses. The lifestyle changes by NTHI in these environments will promote survival and resistance to clearance mechanisms. The information gathered here provides new avenues of investigation to determine the NTHI and epithelial cell factors that NTHI will use to decrease stimulation of epithelial cells to diminish the immune response and alter disease.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant R21 A1070825 from the NIAID/NIH to K.M.M.
We thank S. S. Justice for assistance with microscopy and critical analysis of the manuscript and M. Peeples and the Nationwide Children's Hospital Cell Line core for the HAE cells.
Footnotes
Published ahead of print 15 October 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00942-12.
REFERENCES
- 1. Groeneveld K, van Alphen L, Eijk PP, Visschers G, Jansen HM, Zanen HC. 1990. Endogenous and exogenous reinfections by Haemophilus influenzae in patients with chronic obstructive pulmonary disease: the effect of antibiotic treatment on persistence. J. Infect. Dis. 161:512–517 [DOI] [PubMed] [Google Scholar]
- 2. Klein JO. 1997. Role of nontypeable Haemophilus influenzae in pediatric respiratory tract infections. Pediatr. Infect. Dis. J. 16:S5–8 [DOI] [PubMed] [Google Scholar]
- 3. Murphy TF. 2003. Respiratory infections caused by non-typeable Haemophilus influenzae. Curr. Opin. Infect. Dis. 16:129–134 [DOI] [PubMed] [Google Scholar]
- 4. Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. 2004. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 170:266–272 [DOI] [PubMed] [Google Scholar]
- 5. Roman F, Canton R, Perez-Vazquez M, Baquero F, Campos J. 2004. Dynamics of long-term colonization of respiratory tract by Haemophilus influenzae in cystic fibrosis patients shows a marked increase in hypermutable strains. J. Clin. Microbiol. 42:1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sethi S, Murphy TF. 2001. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14:336–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. St Geme JW., III 2000. The pathogenesis of nontypable Haemophilus influenzae otitis media. Vaccine 19(Suppl. 1):S41–S50 [DOI] [PubMed] [Google Scholar]
- 8. Bals R, Hiemstra PS. 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23:327–333 [DOI] [PubMed] [Google Scholar]
- 9. Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965 [DOI] [PubMed] [Google Scholar]
- 10. Diamond G, Legarda D, Ryan LK. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27–38 [DOI] [PubMed] [Google Scholar]
- 11. Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. 2005. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 6:57–64 [DOI] [PubMed] [Google Scholar]
- 12. Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10:525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Selsted ME, Ouellette AJ. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551–557 [DOI] [PubMed] [Google Scholar]
- 14. Weinberg ED. 2009. Iron availability and infection. Biochim. Biophys. Acta 1790:600–605 [DOI] [PubMed] [Google Scholar]
- 15. Weinberg ED. 1975. Nutritional immunity. Host's attempt to withold iron from microbial invaders. JAMA 231:39–41 [DOI] [PubMed] [Google Scholar]
- 16. Ahren IL, Janson H, Forsgren A, Riesbeck K. 2001. Protein D expression promotes the adherence and internalization of non-typeable Haemophilus influenzae into human monocytic cells. Microb. Pathog. 31:151–158 [DOI] [PubMed] [Google Scholar]
- 17. Armbruster CE, Hong W, Pang B, Dew KE, Juneau RA, Byrd MS, Love CF, Kock ND, Swords WE. 2009. LuxS promotes biofilm maturation and persistence of nontypeable Haemophilus influenzae in vivo via modulation of lipooligosaccharides on the bacterial surface. Infect. Immun. 77:4081–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hardy GG, Tudor SM, St Geme JW., III 2003. The pathogenesis of disease due to nontypeable Haemophilus influenzae. Methods Mol. Med. 71:1–28 [DOI] [PubMed] [Google Scholar]
- 19. Hong W, Juneau RA, Pang B, Swords WE. 2009. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J. Innate Immun. 1:215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong W, Mason K, Jurcisek J, Novotny L, Bakaletz LO, Swords WE. 2007. Phosphorylcholine decreases early inflammation and promotes the establishment of stable biofilm communities of nontypeable Haemophilus influenzae strain 86-028NP in a chinchilla model of otitis media. Infect. Immun. 75:958–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hong W, Pang B, West-Barnette S, Swords WE. 2007. Phosphorylcholine expression by nontypeable Haemophilus influenzae correlates with maturation of biofilm communities in vitro and in vivo. J. Bacteriol. 189:8300–8307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jurcisek JA, Bookwalter JE, Baker BD, Fernandez S, Novotny LA, Munson RS, Jr., Bakaletz LO. 2007. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol. Microbiol. 65:1288–1299 [DOI] [PubMed] [Google Scholar]
- 23. Miyamoto N, Bakaletz LO. 1996. Selective adherence of non-typeable Haemophilus influenzae (NTHi) to mucus or epithelial cells in the chinchilla eustachian tube and middle ear. Microb. Pathog. 21:343–356 [DOI] [PubMed] [Google Scholar]
- 24. Mulks MH, Kornfeld SJ, Plaut AG. 1980. Specific proteolysis of human IgA by Streptococcus pneumoniae and Haemophilus influenzae. J. Infect. Dis. 141:450–456 [DOI] [PubMed] [Google Scholar]
- 25. Noel GJ, Love DC, Mosser DM. 1994. High-molecular-weight proteins of nontypeable Haemophilus influenzae mediate bacterial adhesion to cellular proteoglycans. Infect. Immun. 62:4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pang B, Winn D, Johnson R, Hong W, West-Barnette S, Kock N, Swords WE. 2008. Lipooligosaccharides containing phosphorylcholine delay pulmonary clearance of nontypeable Haemophilus influenzae. Infect. Immun. 76:2037–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ronander E, Brant M, Janson H, Sheldon J, Forsgren A, Riesbeck K. 2008. Identification of a novel Haemophilus influenzae protein important for adhesion to epithelial cells. Microbes Infect. 10:87–96 [DOI] [PubMed] [Google Scholar]
- 28. St Geme JW., III 1994. The HMW1 adhesin of nontypeable Haemophilus influenzae recognizes sialylated glycoprotein receptors on cultured human epithelial cells. Infect. Immun. 62:3881–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Risberg A, Masoud H, Martin A, Richards JC, Moxon ER, Schweda EK. 1999. Structural analysis of the lipopolysaccharide oligosaccharide epitopes expressed by a capsule-deficient strain of Haemophilus influenzae Rd. Eur. J. Biochem. 261:171–180 [DOI] [PubMed] [Google Scholar]
- 30. Wong SM, St Michael F, Cox A, Ram S, Akerley BJ. 2011. ArcA-regulated glycosyltransferase lic2B promotes complement evasion and pathogenesis of nontypeable Haemophilus influenzae. Infect. Immun. 79:1971–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swords WE, Moore ML, Godzicki L, Bukofzer G, Mitten MJ, VonCannon J. 2004. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect. Immun. 72:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. West-Barnette S, Rockel A, Swords WE. 2006. Biofilm growth increases phosphorylcholine content and decreases potency of nontypeable Haemophilus influenzae endotoxins. Infect. Immun. 74:1828–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swords WE, Buscher BA, Ver Steeg Ii K, Preston A, Nichols WA, Weiser JN, Gibson BW, Apicella MA. 2000. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13–27 [DOI] [PubMed] [Google Scholar]
- 34. Swords WE, Ketterer MR, Shao J, Campbell CA, Weiser JN, Apicella MA. 2001. Binding of the non-typeable Haemophilus influenzae lipooligosaccharide to the PAF receptor initiates host cell signalling. Cell. Microbiol. 3:525–536 [DOI] [PubMed] [Google Scholar]
- 35. Casey JR, Pichichero ME. 2004. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr. Infect. Dis. J. 23:824–828 [DOI] [PubMed] [Google Scholar]
- 36. Clementi CF, Murphy TF. 2011. Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Front. Cell Infect. Microbiol. 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. 2006. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296:202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moller LV, Regelink AG, Grasselier H, Dankert-Roelse JE, Dankert J, van Alphen L. 1995. Multiple Haemophilus influenzae strains and strain variants coexist in the respiratory tract of patients with cystic fibrosis. J. Infect. Dis. 172:1388–1392 [DOI] [PubMed] [Google Scholar]
- 39. Moxon ER, Sweetman WA, Deadman ME, Ferguson DJ, Hood DW. 2008. Haemophilus influenzae biofilms: hypothesis or fact? Trends Microbiol. 16:95–100 [DOI] [PubMed] [Google Scholar]
- 40. Post JC. 2001. Direct evidence of bacterial biofilms in otitis media. Laryngoscope 111:2083–2094 [DOI] [PubMed] [Google Scholar]
- 41. Mason KM, Bruggeman ME, Munson RS, Bakaletz LO. 2006. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol. Microbiol. 62:1357–1372 [DOI] [PubMed] [Google Scholar]
- 42. Mason KM, Munson RS, Jr., Bakaletz LO. 2005. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect. Immun. 73:599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mason KM, Munson RS, Jr., Bakaletz LO. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 71:3454–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mason KM, Raffel FK, Ray WC, Bakaletz LO. 2011. Heme utilization by nontypeable Haemophilus influenzae is essential and dependent on Sap transporter function. J. Bacteriol. 193:2527–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shelton CL, Raffel FK, Beatty WL, Johnson SM, Mason KM. 2011. Sap transporter mediated import and subsequent degradation of antimicrobial peptides in Haemophilus. PLoS Pathog. 7:e1002360 doi:10.1371/journal.ppat.1002360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vogel AR, Szelestey BR, Raffel FK, Sharpe SW, Gearinger RL, Justice SS, Mason KM. 2012. SapF-mediated heme-iron utilization enhances persistence and coordinates biofilm architecture of Haemophilus. Front. Cell Infect. Microbiol. 2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tracy E, Ye F, Baker BD, Munson RS., Jr 2008. Construction of non-polar mutants in Haemophilus influenzae using FLP recombinase technology. BMC Mol. Biol. 9:101 doi:10.1186/1471-2199-9-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ren D, Nelson KL, Uchakin PN, Smith AL, Gu XX, Daines DA. 2012. Characterization of extended co-culture of non-typeable Haemophilus influenzae with primary human respiratory tissues. Exp. Biol. Med. 237:540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bookwalter JE, Jurcisek JA, Gray-Owen SD, Fernandez S, McGillivary G, Bakaletz LO. 2008. A carcinoembryonic antigen-related cell adhesion molecule 1 homologue plays a pivotal role in nontypeable Haemophilus influenzae colonization of the chinchilla nasopharynx via the outer membrane protein P5-homologous adhesin. Infect. Immun. 76:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Novotny LA, Mason KM, Bakaletz LO. 2005. Development of a chinchilla model to allow direct, continuous, biophotonic imaging of bioluminescent nontypeable Haemophilus influenzae during experimental otitis media. Infect. Immun. 73:609–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Avadhanula V, Rodriguez CA, Ulett GC, Bakaletz LO, Adderson EE. 2006. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect. Immun. 74:830–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fink DL, Green BA, St Geme JW., III 2002. The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect. Immun. 70:4902–4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Foxwell AR, Kyd JM, Cripps AW. 1998. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol. Mol. Biol. Rev. 62:294–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krivan HC, Roberts DD, Ginsburg V. 1988. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc. Natl. Acad. Sci. U. S. A. 85:6157–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. St Geme JW., III 2002. Molecular and cellular determinants of non-typeable Haemophilus influenzae adherence and invasion. Cell. Microbiol. 4:191–200 [DOI] [PubMed] [Google Scholar]
- 56. van Alphen L, Geelen-van den Broek L, Blaas L, van Ham M, Dankert J. 1991. Blocking of fimbria-mediated adherence of Haemophilus influenzae by sialyl gangliosides. Infect. Immun. 59:4473–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Byrd MS, Pang B, Hong W, Waligora EA, Juneau RA, Armbruster CE, Weimer KE, Murrah K, Mann EE, Lu H, Sprinkle A, Parsek MR, Kock ND, Wozniak DJ, Swords WE. 2011. Direct evaluation of Pseudomonas aeruginosa biofilm mediators in a chronic infection model. Infect. Immun. 79:3087–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prince AS. 2002. Biofilms, antimicrobial resistance, and airway infection. N. Engl. J. Med. 347:1110–1111 [DOI] [PubMed] [Google Scholar]
- 59. Andreini C, Banci L, Bertini I, Rosato A. 2006. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 5:196–201 [DOI] [PubMed] [Google Scholar]
- 60. Banin E, Vasil ML, Greenberg EP. 2005. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 102:11076–11081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moreau-Marquis S, O'Toole GA, Stanton BA. 2009. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am. J. Respir. Cell Mol. Biol. 41:305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. O'May CY, Sanderson K, Roddam LF, Kirov SM, Reid DW. 2009. Iron-binding compounds impair Pseudomonas aeruginosa biofilm formation, especially under anaerobic conditions. J. Med. Microbiol. 58:765–773 [DOI] [PubMed] [Google Scholar]
- 63. Ojha A, Hatfull GF. 2007. The role of iron in Mycobacterium smegmatis biofilm formation: the exochelin siderophore is essential in limiting iron conditions for biofilm formation but not for planktonic growth. Mol. Microbiol. 66:468–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Papp-Wallace KM, Maguire ME. 2006. Manganese transport and the role of manganese in virulence. Annu. Rev. Microbiol. 60:187–209 [DOI] [PubMed] [Google Scholar]
- 65. Reid DW, O'May C, Roddam LF, Lamont IL. 2009. Chelated iron as an anti-Pseudomonas aeruginosa biofilm therapeutic strategy. J. Appl. Microbiol. 106:1058. [DOI] [PubMed] [Google Scholar]
- 66. Rhodes ER, Shoemaker CJ, Menke SM, Edelmann RE, Actis LA. 2007. Evaluation of different iron sources and their influence in biofilm formation by the dental pathogen Actinobacillus actinomycetemcomitans. J. Med. Microbiol. 56:119–128 [DOI] [PubMed] [Google Scholar]
- 67. Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, Coburn B, Kehres DG, Maguire ME, Fang FC, Finlay BB. 2004. The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect. Immun. 72:5522–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bandi V, Apicella MA, Mason E, Murphy TF, Siddiqi A, Atmar RL, Greenberg SB. 2001. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 164:2114–2119 [DOI] [PubMed] [Google Scholar]
- 69. Forsgren J, Samuelson A, Ahlin A, Jonasson J, Rynnel-Dagoo B, Lindberg A. 1994. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect. Immun. 62:673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hers JF, Mulder J. 1953. The mucosal epithelium of the respiratory tract in muco-purulent bronchitis caused by Haemophilus influenzae. J. Pathol. Bacteriol. 66:103–108 [DOI] [PubMed] [Google Scholar]
- 71. Moller LV, Timens W, van der Bij W, Kooi K, de Wever B, Dankert J, van Alphen L. 1998. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am. J. Respir. Crit. Care Med. 157:950–956 [DOI] [PubMed] [Google Scholar]
- 72. Morey P, Cano V, Marti-Lliteras P, Lopez-Gomez A, Regueiro V, Saus C, Bengoechea JA, Garmendia J. 2011. Evidence for a non-replicative intracellular stage of nontypable Haemophilus influenzae in epithelial cells. Microbiology 157:234–250 [DOI] [PubMed] [Google Scholar]
- 73. Sharpe SW, Kuehn MJ, Mason KM. 2011. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect. Immun. 79:4361–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kagnoff MF, Eckmann L. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Invest. 100:6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Falkow S, Isberg RR, Portnoy DA. 1992. The interaction of bacteria with mammalian cells. Annu. Rev. Cell Biol. 8:333–363 [DOI] [PubMed] [Google Scholar]
- 76. Petersen BC, Budelsky AL, Baptist AP, Schaller MA, Lukacs NW. 2012. Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nat. Med. 18:751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146:3217–3226 [DOI] [PubMed] [Google Scholar]
- 78. Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. 2001. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166:1148–1155 [DOI] [PubMed] [Google Scholar]
- 79. Sugano N, Ikeda K, Oshikawa M, Sawamoto Y, Tanaka H, Ito K. 2004. Differential cytokine induction by two types of Porphyromonas gingivalis. Oral Microbiol. Immunol. 19:121–123 [DOI] [PubMed] [Google Scholar]
- 80. Svanborg C, Godaly G, Hedlund M. 1999. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr. Opin. Microbiol. 2:99–105 [DOI] [PubMed] [Google Scholar]
- 81. Wongprompitak P, Sirisinha S, Chaiyaroj SC. 2009. Differential gene expression profiles of lung epithelial cells exposed to Burkholderia pseudomallei and Burkholderia thailandensis during the initial phase of infection. Asian Pac. J. Allergy Immunol. 27:59–70 [PubMed] [Google Scholar]
- 82. Claverys JP, Grossiord B, Alloing G. 2000. Is the Ami-AliA/B oligopeptide permease of Streptococcus pneumoniae involved in sensing environmental conditions? Res. Microbiol. 151:457–463 [DOI] [PubMed] [Google Scholar]
- 83. Cundell DR, Pearce BJ, Sandros J, Naughton AM, Masure HR. 1995. Peptide permeases from Streptococcus pneumoniae affect adherence to eucaryotic cells. Infect. Immun. 63:2493–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cersini A, Salvia AM, Bernardini ML. 1998. Intracellular multiplication and virulence of Shigella flexneri auxotrophic mutants. Infect. Immun. 66:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Goebel W, Kuhn M. 2000. Bacterial replication in the host cell cytosol. Curr. Opin. Microbiol. 3:49–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.