Abstract
In the United States, the production of the Klebsiella pneumoniae carbapenemase (KPC) is an important mechanism of carbapenem resistance in Gram-negative pathogens. Infections with KPC-producing organisms are associated with increased morbidity and mortality; therefore, the rapid detection of KPC-producing pathogens is critical in patient care and infection control. We developed a real-time PCR assay complemented with traditional high-resolution melting (HRM) analysis, as well as statistically based genotyping, using the Rotor-Gene ScreenClust HRM software to both detect the presence of blaKPC and differentiate between KPC-2-like and KPC-3-like alleles. A total of 166 clinical isolates of Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii with various β-lactamase susceptibility patterns were tested in the validation of this assay; 66 of these organisms were known to produce the KPC β-lactamase. The real-time PCR assay was able to detect the presence of blaKPC in all 66 of these clinical isolates (100% sensitivity and specificity). HRM analysis demonstrated that 26 had KPC-2-like melting peak temperatures, while 40 had KPC-3-like melting peak temperatures. Sequencing of 21 amplified products confirmed the melting peak results, with 9 isolates carrying blaKPC-2 and 12 isolates carrying blaKPC-3. This PCR/HRM assay can identify KPC-producing Gram-negative pathogens in as little as 3 h after isolation of pure colonies and does not require post-PCR sample manipulation for HRM analysis, and ScreenClust analysis easily distinguishes blaKPC-2-like and blaKPC-3-like alleles. Therefore, this assay is a rapid method to identify the presence of blaKPC enzymes in Gram-negative pathogens that can be easily integrated into busy clinical microbiology laboratories.
INTRODUCTION
The increased prevalence in antibiotic resistant Gram-negative pathogens poses a threat to public health. Of particular concern are pathogens that are resistant to the carbapenems, since these β-lactams are often used as the last treatment option for infections with multidrug-resistant organisms. Klebsiella pneumoniae carbapenemase (KPC)-producing organisms have been reported worldwide and are endemic in certain regions, such as New York City, Puerto Rico, Israel, and Greece (1). These enzymes are associated most often with Klebsiella pneumoniae but have been identified in other genera of Enterobacteriaceae, such as Escherichia coli and Enterobacter spp., as well as Pseudomonas aeruginosa and Acinetobacter baumannii (2). Infections with KPC-producing organisms are associated with mortality rates ranging from 27.5 to 57% (3, 4, 17). These high mortality rates may be due in part to inappropriate or inadequate antimicrobial therapy due to the inability to detect carbapenem resistance in these strains using current susceptibility methods.
The use of molecular based assays has become more prevalent in today's clinical microbiology laboratories to decrease turnaround time, as well as increase the sensitivity and specificity of detection for a specific organism and/or resistance mechanism (5). There are molecular assays for the detection of blaKPC available, but these often require specialized equipment and expensive reagents (3, 6–8). Moreover, no existing assay is able to differentiate between blaKPC-2-like (including alleles 2, 4, 5, 6, 11, and 12) and blaKPC-3-like (including alleles 7, 8, 9, 10, and 13) genes without direct sequencing or digestion of the amplified product with restriction enzymes (7). Although the identification of the specific KPC allele will not modify specific treatment options, differentiation can be useful for infection control and epidemiological studies. Incorporating new advances in instrumentation, detection software, and fluorescent dyes, several recent studies have detected and identified bacterial species and antibiotic resistance mechanisms that utilize high-resolution melting (HRM) analysis (9–11). Another advance in HRM analysis is to statistically quantify the differences between amplicons, which we have done using Rotor-Gene ScreenClust HRM software, making interpretation of test results extremely easy and reliable (12). Our objective was to develop a PCR-based assay that would rapidly identify the presence of blaKPC in addition to differentiating between the two most prevalent gene variant groups, blaKPC-2-like and blaKPC-3-like, using HRM analysis in a single closed reaction.
MATERIALS AND METHODS
Bacterial strains.
To develop and validate this assay, 125 previously characterized strains of Gram-negative pathogens that produced various β-lactamases and 14 isolates designated as wild type (either non-β-lactamase-producing strains or strains producing chromosomal β-lactamases but susceptible to oxyimino-cephalosporins and carbapenems) were tested (Table 1). The β-lactamases produced in these organisms included class A carbapenemases (KPC, SME, NMCA, and OXA), metallo-β-lactamases (IMP, VIM, NDM, GIM, and SPM), ESBLs (TEM, SHV, CTX-M-15, and CTX-M-14), and ampC β-lactamases (chromosomal, plasmid encoded CMY-2, DHA, and FOX). The identification of these known β-lactamases was determined using the following techniques: endpoint PCR using gene specific primers, direct sequencing of the β-lactamase gene, isoelectric focusing, and/or phenotypic methods (i.e., disk diffusion assays). During the validation of this assay, 27 additional isolates were submitted to our laboratory for KPC-specific PCR testing. When screened at the referring laboratories, these 27 isolates demonstrated positive or weak positive results with the modified Hodge test (MHT), which is recommended for screening potential KPC-producing isolates by the Centers for Disease Control and Prevention (13). Real-time positive KPC PCR results were confirmed by endpoint KPC-specific PCR as previously described (14).
Table 1.
Clinical isolates used during the validation processa
| Organism | Total no. of strains | No. of known KPC+ strains | No. of known other β-lactamase+ strains | No. of unknown KPC+ strains | No. of unknown KPC− strains | No. of WT strainsb |
|---|---|---|---|---|---|---|
| Klebsiella sp. | 78 | 48 | 15 | 9 | 2 | 3 |
| Escherichia coli | 34 | 2 | 25 | 1 | 5 | 1 |
| Enterobacter sp. | 27 | 9 | 8 | 2 | 6 | 2 |
| Serratia marcescens | 4 | 0 | 3 | 0 | 0 | 1 |
| Citrobacter freundii | 3 | 1 | 0 | 1 | 0 | 1 |
| Salmonella sp. | 4 | 0 | 3 | 0 | 0 | 1 |
| Shigella sp. | 2 | 0 | 0 | 0 | 0 | 2 |
| Pseudomonas aeruginosa | 11 | 5 | 4 | 0 | 1 | 1 |
| Acinetobacter baumannii | 2 | 0 | 0 | 0 | 0 | 2 |
| Proteus mirabilis | 1 | 0 | 1 | 0 | 0 | 0 |
| Total | 166 | 66 | 59 | 13 | 14 | 14 |
Known strains were previously characterized for possessing specific β-lactamases and were used for initial assay validation. Unknown isolates were tested after initial assay validation between May 2011 and February 2012.
Non-β-lactamase-producing strains or strains producing chromosomal β-lactamases but susceptible to oxyimino-cephalosporins and carbapenems.
DNA isolation.
Strains were inoculated on blood agar media and incubated for 18 h at 37°C. DNA was extracted from 2 ml of an overnight bacterial culture grown in Luria-Bertani (LB) broth or a 1.0 McFarland suspension in LB broth made of colonies grown on a blood agar plate using the DNeasy Blood & Tissue kit (Qiagen, Valencia, CA) and eluted with 100 μl of AE buffer according to recommendations of the manufacturer.
Development of real-time PCR assay with HRM analysis.
In order to detect blaKPC and differentiate between various blaKPC alleles, a single set of primers were designed that amplified a 189-bp region that flanked the nucleotide positions 716, 814, and 843 (Table 2). Real-time PCR and HRM analysis were performed in 25-μl reaction volumes using the Rotor-Gene Q (Qiagen). Each reaction volume contained 8 μl of sterile water, 12.5 μl of 2× HRM PCR master mix (Qiagen), 0.7 μM concentrations of the primers KPC F9 (5′-GCAGACTGGGCAGTCGG-3′) and KPC 4R (5′-GACTCGCGGTCGAGGGATTG-3′), and 1 μl of template DNA (∼25 pg). Negative control reactions that lacked DNA template were included in each PCR run. The PCR was performed using the following conditions: initial denaturation at 95°C for 5 min, followed by 30 cycles of denaturation (95°C for 10 s), annealing (55°C for 30 s), and extension (72°C for 10 s). To ensure the accuracy of amplification, all generated amplicons were separated by agarose gel electrophoresis in a 2% (wt/vol) agarose gel, stained with ethidium bromide, and visualized on a UV transilluminator.
Table 2.
In silico and in vitro Tm analysis of the 189-bp amplicon for 12 blaKPC alleles
| KPC | Nucleotide at positiona: |
Tm (°C) |
|||
|---|---|---|---|---|---|
| 716 | 814 | 843 | Predictedb | Actualc | |
| KPC-2 | T | C | A | 87.6 | 87.2 |
| KPC-3 | T | T | A | 87.3 | 86.9 |
| KPC-4 | G | C | A | 87.7 | ND |
| KPC-5 | T | C | A | 87.6 | 87.2 |
| KPC-6 | G | C | A | 87.7 | ND |
| KPC-7 | T | T | A | 87.3 | ND |
| KPC-8 | G | T | A | 87.3 | ND |
| KPC-9 | C | T | A | 87.6 | ND |
| KPC-10 | T | T | A | 87.3 | ND |
| KPC-11 | T | C | A | 87.6 | ND |
| KPC-12 | T | C | A | 87.6 | ND |
| KPC-13 | T | T | G | 87.4 | ND |
The nucleotide position in comparison to GenBank accession number EU176012.
The predicted Tm using a web-based oligonucleotide calculator (http://www.basic.northwestern.edu/biotools/oligocalc.html).
The Tm observed in this assay. ND, not determined.
After amplification, HRM analysis and genotyping were performed by detecting the fluorescent signal during a temperature rise of 0.1°C increments from 84 to 90°C. The Rotor-Gene Q operating software was used to determine the results. After the normalization of the raw data, the fluorescence signal intensity was plotted on the y axis, and the temperature was plotted on the x axis. The melting temperature (Tm) of each sample was the temperature at which 50% of the amplified product was dissociated into single-stranded DNA for that sample, which was visualized as a decrease in fluorescence as the EvaGreen dye was released. HRM genotypes were determined by establishing one previously sequenced blaKPC-2 and one blaKPC-3 sample as the reference genotypes in each run. Their Tms were calculated and used as reference values for determining which blaKPC allele was present in the amplified products of the other samples. These blaKPC-2 or blaKPC-3 reference Tms were then subtracted from the normalized value of each test sample and plotted on the y axis. Samples with a vertical shift in fluorescence intensity within an 80% confidence interval (CI) of either the blaKPC-2 or blaKPC-3 reference sample were genotyped as blaKPC-2-like or blaKPC-3-like, respectively.
ScreenClust HRM analysis.
In order to standardize the interpretation process, the HRM results from each PCR run were analyzed with the ScreenClust HRM software (Qiagen, Hilden, Germany). First, HRM curves were normalized to decrease the differences in raw fluorescence between samples. After normalization, a residual plot was then created by subtracting the differentiated curves from a median of all of the curves. Principal components (PCs) were then able to be determined based on the residual plots. A single PC is the most varied combination of data among the samples in a single run. Second and third PCs were then generated to account for the remaining variability in the data.
The ScreenClust HRM software enables the user to analyze data in either a supervised or unsupervised mode, where the former requires known positive genotype controls, and the latter is meant for de novo single nucleotide polymorphism (SNP) discovery. We validated this assay using known controls and therefore used the supervised mode with a minimum of two blaKPC-2 and blaKPC-3 controls for each PCR run. Once the positive controls are identified, the remaining unknown samples are sorted using linear discriminant analysis, which calculates cluster distribution.
Verification of blaKPC allele by direct sequence analysis.
To verify that the differentiation between blaKPC-2-like and blaKPC-3-like genes using HRM and ScreenClust were true to the specific allele, direct sequencing of the blaKPC was performed on amplified products from a subset of 21 clinical isolates as previously described (14). Sequence analysis was performed using the DNA Baser version 2.75 software program (Heracle Software, Lilienthal, Germany).
RESULTS
Detection of blaKPC by real-time PCR using characterized isolates.
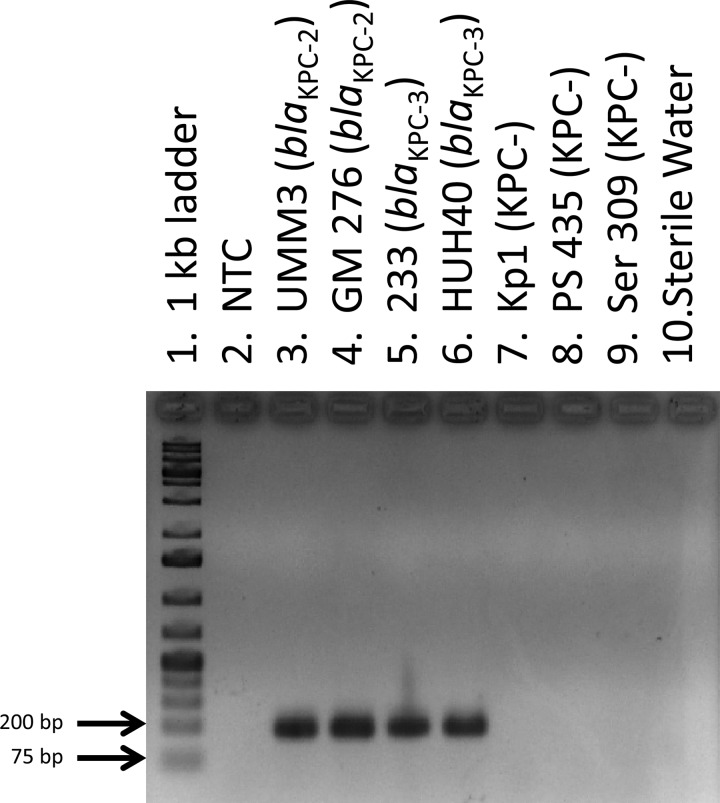
The primary purpose for developing this assay was to detect the presence of blaKPC by real-time PCR. Fluorescence as a result of PCR amplification was visualized using the Rotor-Gene Q Series software (Qiagen) over the course of 30 three-step cycles for each sample, and the cycle threshold (CT) of each sample was determined by establishing an arbitrary threshold of fluorescence detection (between 1.5 and 1.7 normalized fluorescence units). A total of 66 known KPC-producing isolates had CTs between 12 and 22; 73 isolates that were negative for KPC production had CTs of >29 and were thus interpreted as having a negative result for the presence of blaKPC (Table 3). Analysis of the amplified products by agarose gel electrophoresis from all samples correlated with the CT. No amplified products were detected in samples that had CTs of >29, and samples that had CTs between 12 and 22 had bands of the expected amplified product size of 189 bp (Fig. 1).
Table 3.
Sensitivity and specificity of HRM and ScreenClust analyses for the presence of blaKPC and differentiation of blaKPC-2-like and blaKPC-3-like gene variants
| Parameter | Presence of blaKPCa | Variantsb |
|
|---|---|---|---|
| blaKPC-2-like | blaKPC-3-like | ||
| Tm ± SD (°C) | 87 ± 0.17 | 87.2 ± 0.03 | 86.8 ± 0.03 |
| No. of tested strains | 66 | 14 | 17 |
| Sensitivity (%) | 66/66 (100) | 14/14 (100) | 17/17 (100) |
| Specificity (%) | 73/73 (100) | 17/17 (100) | 14/14 (100) |
Compared to previous characterization by endpoint PCR.
Compared to direct sequencing of blaKPC gene in a subset of 31 isolates.
Fig 1.
Agarose gel electrophoresis of amplified products generated using real-time PCR. Lane 1, 1-kb molecular-weight ladder; lane 2, no template control (NTC); lanes 3 to 6, amplified products from KPC-producing test isolates; lanes 7 to 9, amplified products from KPC-negative isolates; lane 10, water-only control.
Detection of blaKPC by real-time PCR using uncharacterized isolates.
Following validation of the KPC HRM assay, an additional 27 clinical isolates that demonstrated positive or weakly positive MHT results were also screened for the presence of blaKPC (Table 1). Of these isolates, 13 were positive for the presence of blaKPC (average CT = 17.8) and 14 were KPC negative (CT > 29).
Differentiation of blaKPC-2-like and blaKPC-3-like gene variants by HRM curve analysis.
In addition to determining the presence of blaKPC, we sought to differentiate between the two most common blaKPC alleles in the United States, blaKPC-2 and blaKPC-3, by using HRM curve analysis of the amplified products. The fluorescence levels for each of the 79 KPC-producing samples were normalized after the removal of background fluorescence, and the decrease was plotted against increasing temperature increments of 0.1°C in a dissociation curve. These results from the first set of PCR samples tested are shown in Fig. 2. A signal/noise ratio difference was then calculated for each sample in comparison to two reference samples, one for blaKPC-2-like producers and the other for blaKPC-3-like producers.
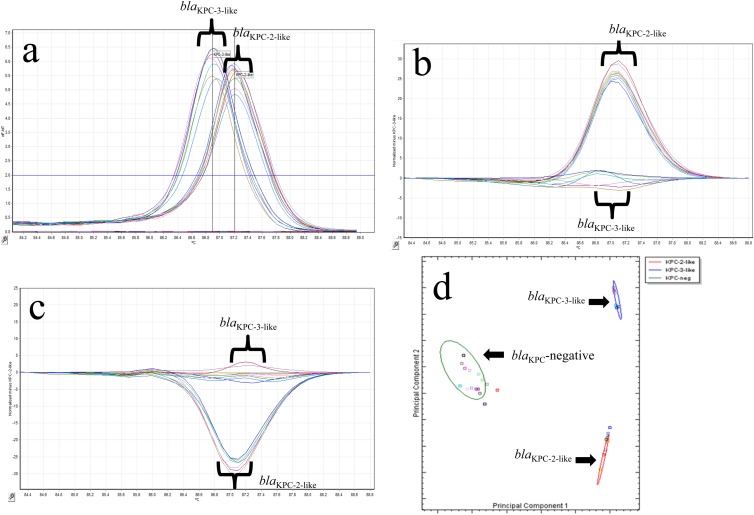
Fig 2.
(a) Representative melting curves for 17 isolates with blaKPC-2-like and blaKPC-3-like genotypes using single-tube analysis of blaKPC real-time PCR with HRM. The −dF/dT (negative first derivative of the change in fluorescence) is shown on the y axis, and the temperature shown on the x axis. (b) Representative difference graph of 10 KPC-2-like-producing isolates compared to 7 KPC-3-like-producing isolates using single-tube analysis of blaKPC real-time PCR with HRM. The relative fluorescence units compared to the genotype control are shown on the y axis; the temperature is shown on the x axis. (c) Representative difference graph of 7 KPC-3-like-producing isolates compared to 10 KPC-2-like-producing isolates using single-tube analysis of blaKPC real-time PCR with HRM. The relative fluorescence units compared to the genotype control are shown on the y axis; the temperature is shown on the x axis. (d) Cluster plot showing the differentiation of 17 isolates positive for KPC-2-like (red oval) or KPC-3-like (blue oval) and 16 KPC-negative samples (green oval) generated by the Rotor-Gene ScreenClust HRM software.
The 66 KPC-producing organisms tested each had a single dominant peak generated by melting curve analysis that fell into one of two distinct melting temperature ranges (Fig. 2a). The presence of a blaKPC-2-like gene was detected in 26 strains with a melting temperature range of 87.08 to 87.32°C, whereas 40 strains had a melting temperature range of 86.8 to 86.95°C, indicating the presence of the blaKPC-3-like gene. There was no amplification in the 73 known KPC-negative isolates by PCR (CT > 29), and no peaks were observed in melting curve analysis (<1 on the plot of fluorescence and time derivatives [dF/dT] against temperature).
Of 27 unknown isolates that demonstrated positive or weak positive results with the MHT, 13 were KPC positive by PCR, 6 had blaKPC-2-like Tms, and 7 had blaKPC-3-like Tms. Although the other 14 unknown isolates also showed positive or weakly positive MHT results, these isolates were negative for KPC using this assay and other previously validated endpoint KPC-specific PCRs available in our laboratory (14). The mechanisms of resistance in 11 of these isolates remain unresolved. However, three E. coli isolates were positive for the blaCMY-2-like gene (data not shown), which when combined with a loss of the porin OmpF has been shown to result in decreased susceptibility to ertapenem (15).
Differentiation of blaKPC-2-like and blaKPC-3-like gene variants by HRM genotyping analysis.
In order to fully utilize the Rotor-Gene Q's HRM capabilities, we also differentiated the presence of blaKPC-2-like and blaKPC-3-like genes by genotyping. The resulting difference plots for the blaKPC-2-like or blaKPC-3-like genes are shown in Fig. 2b and c, respectively. PS28, which carried the blaKPC-5 gene, was correctly identified as being positive for a blaKPC-2-like gene with a Tm of 87.2. There was one isolate (Kp324) that was positive for the presence of blaKPC and had a Tm of 86.88, which suggested that it encoded the blaKPC-3 gene. This sample was unable to be genotyped initially as blaKPC-3-like because its curve on the HRM difference plot was outside the 80% CI cutoff we established for this assay. Upon repeat testing, this was resolved, and Kp324 was correctly genotyped as encoding the blaKPC-3-like gene.
Quantitative genotyping using ScreenClust HRM software.
To successfully implement an assay into the clinical microbiology laboratory, the results must be easy to interpret. Current software for both traditional melting curve and HRM analyses are not able to quantify differences between genotypes using statistical analysis. To overcome this limitation, we chose to use the Rotor-Gene ScreenClust HRM software, which statistically genotyped our samples into one of three clusters (KPC negative, blaKPC-2-like, and blaKPC-3-like). The results are displayed in both a spreadsheet (Table 4) and cluster plot (Fig. 2d). The spreadsheet identifies the cluster for each sample sorted, the typicality (i.e., how well the sample sorted into that cluster), and the probabilities of that sample being sorted into one of the three clusters correctly. The cluster plot shows each sample as a distinct colored square with a color-coded ellipse that designates to which cluster the samples belong. ScreenClust correctly identified all 87 of the KPC-negative samples into the KPC-negative cluster. ScreenClust also correctly clustered 32 and 47 isolates previously identified as being in the blaKPC-2-like and blaKPC-3-like clusters, respectively.
Table 4.
ScreenClust HRM analysis cluster, typicality, and probability results
| ID | Isolate | Clustera | Typicalityb | Posterior probabilityc |
||
|---|---|---|---|---|---|---|
| KPC-2-like | KPC-3-like | KPC negative | ||||
| 15 | Entb247 | KPC negative | 0.40697278 | 0.0000 | 0.0000 | 1.0000 |
| 16 | Kleb225 | KPC negative | 0.58822841 | 0.0000 | 0.0000 | 1.0000 |
| 17 | UMM3 | KPC-2-like | 0.62458202 | 1.0000 | 0.0000 | 0.0000 |
| 18 | 233 | KPC-3-like | 0.64076957 | 0.0000 | 1.0000 | 0.0000 |
| 19 | Kleb352 | KPC-2-like | 0.41996172 | 1.0000 | 0.0000 | 0.0000 |
| 20 | Ec351 | KPC-2-like | 0.91755836 | 1.0000 | 0.0000 | 0.0000 |
| 21 | 236 | KPC-3-like | 0.29703921 | 0.0000 | 1.0000 | 0.0000 |
The genotype result for a sample.
The typicality measures how well a sample falls within the cluster for which it has been classified.
The probability of each sample fitting into a particular cluster is given as a value from 0 to 1. The sum of all probability values for a single sample is 1. Each sample is called into the cluster with the highest probability. Samples with a probability of less than 0.7 of belonging to a particular cluster should be treated with caution.
Verification of blaKPC-2-like and blaKPC-3-like Tms and ScreenClust results by direct sequencing.
Of the 66 previously characterized KPC-producing Gram-negative organisms that were tested in this assay, direct sequencing of the blaKPC gene was performed on 10 isolates (8). Using ScreenClust analysis, five samples were identified as blaKPC-2-like; four isolates carried blaKPC-2, and 1 isolate carried blaKPC-5. The five samples identified as blaKPC-3-like all carried blaKPC-3. To further validate both the HRM and ScreenClust results, the blaKPC gene was sequenced from an additional 21 isolates (Table 3). Nine isolates had an average Tm of 87.2°C ± 0.03°C; these were shown to encode KPC-2. Another 12 isolates had an average Tm of 86.8°C ± 0.03°C, and sequence analysis verified that these isolates carried blaKPC-3.
DISCUSSION
Combination therapy is warranted when patients are infected with KPC-producing organisms (16). Therefore, rapid and accurate detection of KPC-producing Gram-negative organisms in the clinical laboratory is paramount in directing appropriate therapy and preventing dissemination. Conventional phenotypic assays to screen and identify these organisms are time-consuming and can be difficult to interpret. Therefore, we developed a rapid assay to detect the presence of blaKPC, as well as differentiate between the two most prevalent blaKPC gene variants on one analyzer without downstream sample manipulation.
This KPC HRM assay demonstrated 100% specificity and sensitivity for the detection of blaKPC since we were able to detect blaKPC in all of our previously characterized KPC-positive isolates while avoiding any false positives from the 73 previously characterized KPC-negative isolates. For the differentiation of the two most prevalent blaKPC gene variants, we compared the melting-curve Tms, HRM genotyping, and ScreenClust genotypes to direct sequencing results. The differentiation of blaKPC-2 and blaKPC-3 genes in 31 isolates by HRM using all three analyses had 100% sensitivity and specificity compared to direct sequencing of the blaKPC gene. However, utilization of the ScreenClust software gave the most easily interpreted results, supported by quantitative measurements (i.e., cluster typicalities and probabilities for each sample). For this reason, we consider its incorporation to be the optimal analytical tool regarding this assay.
A common concern when introducing a molecular assay into the clinical laboratory is the expense. This assay takes advantage of the Rotor-Gene Q's ability to perform both the PCR and HRM analysis on a single analyzer without additional equipment. In addition, the Type-It HRM master mix has also been optimized for use on the LightCycler 480 analyzer. The total cost of supplies required for this assay from template preparation to PCR and HRM analysis is ∼$5 per sample.
The turnaround time from the isolation of individual colonies to PCR and HRM results in the present study was 24 h when inoculated broth medium grown overnight for template preparation was used. This turnaround time was decreased to <3 h with the preparation of template made directly from isolated colonies in RNase-free, DNase-free sterile water. The desired template preparation method should be determined for each individual laboratory and standardized using control strains for implementation of this assay.
In conclusion, we have demonstrated that this assay reliably detects the presence of blaKPC and can differentiate between the two most prevalent blaKPC gene variants. This assay is cost-effective, is simple to set up, and provides rapid results that require little interpretation. These characteristics allow for easy incorporation into the workflow of a clinical microbiology or reference laboratory to aid in the identification of KPC-producing Gram-negative organisms.
ACKNOWLEDGMENTS
We thank Kenneth Thomson and Stephen Cavalieri for the clinical isolates used in this study. We also thank Qiagen for providing the Rotor-Gene Q analyzer, the Rotor-Gene Q software, and the ScreenClust HRM software for laboratory use.
Footnotes
Published ahead of print 17 October 2012
REFERENCES
- 1. Cuzon G, Naas T, Truong H, Villegas MV, Wisell KT, Carmeli Y, Gales AC, Venezia SN, Quinn JP, Nordmann P. 2010. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 16:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies TA, Marie Queenan A, Morrow BJ, Shang W, Amsler K, He W, Lynch AS, Pillar C, Flamm RK. 2011. Longitudinal survey of carbapenem resistance and resistance mechanisms in Enterobacteriaceae and non-fermenters from the U.S.A. in 2007–09. J. Antimicrob. Chemother. 66:2298–2307 [DOI] [PubMed] [Google Scholar]
- 3. Mangold KA, Santiano K, Broekman R, Krafft CA, Voss B, Wang V, Hacek DM, Usacheva EA, Thomson Richard B, Jr, Kaul KL, Peterson LR. 2011. Real-time detection of blaKPC in clinical samples and surveillance specimens. J. Clin. Microbiol. 49:3338–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodford N, Tierno Philip M, Jr, Young K, Tysall L, Palepou MI, Ward E, Painter RE, Suber DF, Shungu D, Silver LL, Inglima K, Kornblum J, Livermore DM. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical Center. Antimicrob. Agents Chemother. 48:4793–4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, Yao JDC, Wengenack NL, Rosenblatt JE, Cockerill FR, III, Smith TR. 2006. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin. Microbiol. Rev. 19. 1:165–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen L, Chavda KD, Mediavilla JR, Zhao Y, Fraimow HS, Jenkins SG, Levi MH, Hong T, Rojtman AD, Ginocchio CC, Bonomo RA, Kreiswirth BN. 2012. Multiplex real-time PCR for detection of an epidemic KPC-producing Klebsiella pneumoniae ST258 Clone. Antimicrob. Agents Chemother. 56:3444–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole JM, Schuetz AN, Hill CE, Nolte FS. 2009. Development and evaluation of a real-time PCR assay for detection of Klebsiella pneumoniae carbapenemase genes. J. Clin. Microbiol. 47:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hindiyeh M, Smollen G, Grossman Z, Ram D, Davidson Y, Mileguir F, Vax M, Ben David D, Tal I, Rahav G, Shamiss A, Mendelson E, Keller N. 2008. Rapid detection of blaKPC carbapenemase genes by real-time PCR. J. Clin. Microbiol. 46:2879–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chromá M, Hricová K, Koláø M, Sauer P, Koukalová D. 2011. Using newly developed multiplex polymerase chain reaction and melting curve analysis for detection and discrimination of β-lactamases in Escherichia coli isolates from intensive care patients. Diagn. Microbiol. Infect. Dis. 71:181–191 [DOI] [PubMed] [Google Scholar]
- 10. Mendes RE, Kiyota KA, Monteiro J, Castanheira M, Andrade SS, Gales AC, Pignatari ACC, Tufik S. 2007. Rapid detection and identification of metallo-beta-lactamase-encoding genes by multiplex real-time PCR assay and melt curve analysis. J. Clin. Microbiol. 45:544–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang S, Ramachandran P, Rothman R, Hsieh Y, Hardick A, Won H, Kecojevic A, Jackman J, Gaydos C. 2009. Rapid identification of biothreat and other clinically relevant bacterial species by use of universal PCR coupled with high-resolution melting analysis. J. Clin. Microbiol. 47:2252–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reja V, Kwok A, Stone G, Yang L, Missel A, Menzel C, Bassam B. 2010. ScreenClust: advanced statistical software for supervised and unsupervised high resolution melting (HRM) analysis. Methods 50:S10–S14 [DOI] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed Approved standard M7-A6, vol 28 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14. Roth AL, Kurpiel PM, Lister PD, Hanson ND. 2011. blaKPC RNA expression correlates with two transcriptional start sites but not always with gene copy number in four genera of Gram-negative pathogens. Antimicrob. Agents Chemother. 55:3936–3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan JJ, Wu JJ, Lee CC, Ko WC, Yang FC. 2010. Prevalence and characteristics of ertapenem-nonsusceptible Escherichia coli in a Taiwanese university hospital, 1999 to 2007. Eur. J. Clin. Microbiol. Infect. Dis. 29:1417–1425 [DOI] [PubMed] [Google Scholar]
- 16. Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin. Microbiol. Infect. 17:1798–1803 [DOI] [PubMed] [Google Scholar]
- 17. Won SY, Munoz-Price L, Lolans K, Hota B, Weinstein RA, Hayden MK. 2011. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin. Infect. Dis. 53:532–540 [DOI] [PubMed] [Google Scholar]