Abstract
Subgroup J avian leukosis virus (ALV-J) is an avian retrovirus that causes severe economic losses in the poultry industry. The early identification and removal of virus-shedding birds are important to reduce the spread of congenital and contact infections. In this study, a TaqMan-based real-time PCR method for the rapid detection and quantification of ALV-J with proviral DNA was developed. This method exhibited a high specificity for ALV-J. Moreover, the detection limit was as low as 10 viral DNA copies. The coefficients of variation (CVs) of both interassay and intra-assay reproducibility were less than 1%. The growth curves of ALV-J in DF-1 cells were measured by real-time PCR, yielding a trend line similar to those determined by 50% tissue culture infective dose (TCID50) and p27 antigen detection. Tissue samples suspected of ALV infection were evaluated using real-time PCR, virus isolation, and routine PCR, and the positivity rates were 60.1%, 41.6% and 44.5%, respectively. Our data indicated that the real-time PCR method provides a sensitive, specific, and reproducible diagnostic tool for the identification and quantification of ALV-J for clinical diagnosis and in laboratory research.
INTRODUCTION
Avian leukosis viruses (ALVs) are members of the Alpharetrovirus genus of the family Retroviridae that are associated with a variety of neoplasms, including lymphoid and myeloid leukoses. ALVs are prevalent in poultry flocks worldwide and cause serious economic losses due to decreased egg production and quality, tumor mortality, and low growth rate. ALV isolates from chickens are classified into subgroups A, B, C, D, and E and the recently identified subgroup J according to host range, viral envelope glycoprotein properties, and cross-neutralization patterns (1). Subgroup A and B ALVs occur as common pathogenic exogenous viruses, and subgroup C and D ALVs have been rarely reported in the field. Subgroup E viruses are endogenous leukosis viruses with low pathogenicity. Subgroup J ALV (ALV-J) is an exogenous virus that causes myeloid leukosis (ML) in chickens. ALV-J was first isolated from meat-type chickens and was designated subgroup J due to its distinct envelope characteristics (2). The env gene sequence of the ALV-J prototype (HPRS-103 strain) is considerably different from those of other subgroups. However, it shares high identities (75 to 97%) with env-like sequences from the endogenous avian retrovirus (EAV) family members, suggesting that it evolved by recombination with the env-like sequences of this family (3, 4).
ALV-J was first isolated from broiler chickens with myeloid leukosis in the United Kingdom. Since its first report, ALV-J infection has been widely detected in multiple countries in the Americas, Asia, and Oceania (5–7). Although the virus has a broad host range and all chicken lines tested have been susceptible to infection (2, 8), no field cases of ALV-J infection and tumors in layer chickens were reported until 2004 (9). In recent years, cases of ALV-J infection and tumors have been widely reported for commercial layer chickens and breeders of layer chickens in the People's Republic of China (7, 9, 10). Many factors, such as the type of virus, the dosage and route of inoculation, the chicken's age, and the type of chicken, determine the infection rate, morbidity, timing of tumor formation, and mortality of ALV-J. It has been reported that the infection rate was up to 87% in breeders of broiler chickens, as detected by virological and serological methods. ALV-J cases can appear at any time after 14 weeks, but the peak incidence usually occurs before or after sexual maturity. The minimum age of a broiler infected with ALV-J presenting myeloid sarcoma in the field was only 4 weeks (1). Experimental results have shown that ALV-J can be detected in the blood and cloaca within 1 week after infection. ALV-J spreads by both vertical and horizontal transmission, and no commercial vaccine is available. Therefore, to reduce the spread of congenital and contact infection, the control of ALV infections depends mainly on the early identification and removal of virus-shedding birds.
Many methods for ALV-J detection have been established, and virus isolation in cell culture is often used as the “gold standard.” However, this method is time-consuming, because a minimum of 7 days is required to obtain the results (11). Recently, several (PCR) methods have been developed and applied to ALV-J detection; these methods are more sensitive and efficient than virus isolation in cell culture (6, 11–15). Loop-mediated isothermal amplification (LAMP) has also been established, with a detection limit of five DNA copies (16). However, this method is not quantitative. Although quantitative competitive reverse transcription-PCR (QC-RT-PCR) has been developed and modified for quantification of ALV-J, this method is less convenient and accurate than real-time PCR (17, 18). Moreover, Kim et al. developed a real-time RT-PCR method for ALV-J detection using the H5/H7 primer pair. This method is highly reproducible, and the results are positively correlated with those of QC-RT-PCR and 50% tissue culture infective dose (TCID50) as determined by routine methods. However, the sensitivity of this method is much lower than was expected (10 to 100 copies per sample) (17).
In the present study, a sensitive and specific real-time PCR method based on a TaqMan probe for ALV-J DNA quantification was developed. ALV-J replication curves were then established by routine methods and compared to the results of this real-time PCR method. Moreover, the applications of this real-time PCR method for lab experiments and for clinical detection are described.
MATERIALS AND METHODS
Virus and clinical sample treatment.
ALV-A (RAV-1), ALV-B (RAV-2), ALV-C (RAV-49), ALV-E (RAV-0), ALV-J (HPRS-103), chicken infectious anemia virus (CAV; M9905 strain), and avian reticuloendotheliosis virus (REV; HLJR0901 strain) were maintained in our laboratory. Fourteen ALV-J strains (LN08SY10, JL08CH3-1, LN08SY31, SD09DP04, HLJ09SH01, HLJ09SH02, HuB09JY03, HuB09HW02, HuB09HW03, HLJ10SH03, HLJ10SH04, JL10HW01, JL10HW02, and HLJ08MDJ01) were identified and isolated previously by our laboratory (19). Infectious laryngotracheitis virus (ILTV) and Marek's disease virus (MDV) were obtained from the corresponding subject groups of the Harbin Veterinary Research Institute. A total of 173 liver and spleen samples suspected of ALV infection were obtained from flocks in China (20). Tissue samples were homogenized in phosphate-buffered saline (PBS) containing 1,000 units/ml of penicillin and streptomycin and subsequently centrifuged at 6,000 × g for 5 min at 4°C. An aliquot of the supernatant was subjected to proviral DNA extraction as a template for real-time PCR and routine PCR assays. The remaining portions of the supernatants were passed through 0.22-μm filters and inoculated onto a DF-1 cell monolayer cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). After 2 h, the cells were overlaid with DMEM supplemented with 2% FBS and incubated at 37°C in a 5% CO2 atmosphere for 7 days. The DF-1 cell cultures were then harvested for p27 antigen detection.
Proviral DNA extraction.
The proviral DNA of cell culture and tissue samples was extracted as previously described (19). Briefly, the supernatants were lysed in tissue lysis buffer (4 M guanidine hydrochloride, 25 mM sodium citrate, and 1% Triton X-100) and extracted twice with phenol-chloroform-isoamyl alcohol (25:24:1). The DNA was precipitated with absolute isopropanol, washed with 70% ethanol, and dried at room temperature. Subsequently, the DNA was resuspended in nuclease-free water and stored at −80°C.
Primer design.
To detect and distinguish ALV-J from other ALV subgroups (ALV-A, ALV-B, ALV-C, ALV-D, and ALV-E) and the EAV family, a pair of primers was designed by aligning previously published ALV sequences. The forward primer (ALV-JNF, nucleotide positions 5296 to 5315 [5′-TTGCAGGCATTTCTGACTGG-3′]) was designed against the 3′ region of the pol gene, which is conserved among several subgroups but not in the EAV family, according to the ALV-J sequence (HPRS-103; GenBank accession number Z46390.1). The reverse primer (ALV-JNR, nucleotide positions 5490 to 5509 [5′-ACACGTTTCCTGGTTGTTGC-3′]) was designed from a well-conserved region of the gp85 gene of ALV-J, which could be distinguished from other subgroups. The TaqMan probe (nucleotide positions 5376 to 5400 [5′-CCTGGGAAGGTGAGCAAGAAGGA-3′]) was a 23-bp oligonucleotide located downstream of ALV-JNF. The 5′ end of the probe was labeled with FAM (6-carboxyfluorescein), and the 3′ end was labeled with BHQ1 (Black Hole Quencher 1).
Real-time PCR.
Real-time PCRs were performed with a LightCycler 480 real-time thermocycler (Roche Instrument Center, Switzerland). To obtain optimal specific fluorescent signals, the annealing temperature and primer, probe, and Mg2+ concentration were optimized. The reaction was performed in a 25-μl system containing 1 μl of cDNA, 2.5 μl of 10× Ex Taq buffer, 2 μl of deoxynucleoside triphosphate (dNTP) (2.5 mM), 1 μl of MgCl2 (75 mM), 1 μl of ALV-JNF (10 pM), 1 μl of ALV-JNR (10 pM), 0.5 μl of probe (10 pM), 1 unit of Ex Taq HS (TaKaRa, China), and the appropriate volume of double-distilled water (ddH2O). The real-time PCR was carried out with a predenaturation step at 95°C for 5 min and amplification for 40 cycles at a melting temperature of 95°C for 10 s and an annealing/elongation temperature of 65°C for 40 s. Fluorescent signals were collected during the elongation step.
Plasmid standard preparation.
A 214-bp fragment of ALV-J was amplified with the primer pair ALV-JNF/ALV-JNR and then cloned into the pMD-18T vector (TaKaRa) to obtain the recombinant plasmid pMD-JNS. The concentration of the plasmid was determined with a UV spectrophotometer, and the plasmid copy number was calculated using the following formula: number of copies = (concentration in ng × 6.022 × 1023)/(genome length × 109 × 650) (21). Serial dilutions from 1 × 101 to 1 × 1010 copies/μl of the plasmid standard DNA were used to produce a standard curve.
Cell infection.
DF-1 cells were infected with ALV-J (the HLJ08MDJ01 strain, previously isolated and identified in our lab) at a multiplicity of infection (MOI) of 0.1 and incubated at 37°C in DMEM supplemented with 2% FBS. At 1, 2, 3, 4, 5, 6, and 7 days postinfection, infected cell cultures were harvested, and the supernatant fluid was gathered after three rounds of freeze-thawing. A portion of the supernatant was subjected to proviral DNA extraction. The remaining portion was used for p27 antigen detection using an avian leukosis virus antigen test kit.
Animal infection.
Twenty-five specific-pathogen-free (SPF) chickens were infected by intra-abdominal injection of 1 × 105 TCID50s of HLJ08MDJ01 at 1 day old. Twenty-four uninfected control birds were injected with DMEM. Infected and control birds were reared separately in negative-pressure isolators. At 1, 2, 3, 4, 8, 12, 16, and 24 weeks postinfection, three chickens from each group were randomly chosen to be bled. The anticoagulant blood samples were collected for proviral DNA extraction.
Routine PCR.
The routine PCR amplification of ALV-J was performed with the subgroup-specific primers described by Smith et al. (14). The primers used were H5, 5′-GGATGAGGTGACTAAGAAAG-3′, and H7, 5′-CGAACCAAAGGTAACACACG-3′. A 545-bp band was amplified by these primers, as observed via gel electrophoresis. The reaction was performed in a 25-μl mixture containing 1 μl of cDNA, 2.5 μl of 10× Ex Taq buffer, 2 μl/liter of dNTP (2.5 mM), 1 μl of H5 primer (10 pM), 1 μl of H7 primer (10 pM), 1 U of Ex Taq HS (TaKaRa), and the appropriate volume of ddH2O. The PCR procedure was as follows: 95°C for 5 min; 35 cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 10 min. PCR products were evaluated by 1.0% agarose gel electrophoresis.
p27 antigen detection by ELISA.
ALV p27 antigen was detected with the avian leukosis virus antigen test kit (IDEXX, Inc., Westbrook, MA) by following the procedure recommended by the manufacturer. In brief, 100 μl of diluted sample was dispensed into appropriate wells, and the plates were covered and incubated for 60 min at room temperature. The wells were then washed 5 times with distilled water. Then, 100 μl of (rabbit) anti-p27-horseradish peroxidase (anti-p27-HRPO) conjugate was added to each well, and the plate was incubated for 60 min at room temperature. The wells were then washed 5 times with distilled water, after which 100 μl of TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution was added to each well, and the plate was incubated for 15 min at room temperature. Finally, 100 μl of stop solution was dispensed into each well to stop the reaction. Absorbance values were measured and recorded at 650 nm.
RESULTS
Standard curve for real-time PCR.
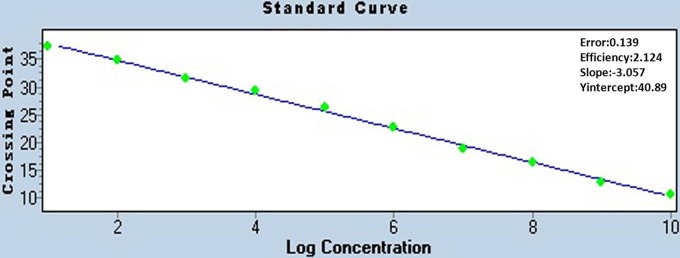
To generate a standard curve, the plasmid pMD-JNS was serially diluted from 1 × 1010 copies/μl to 1 × 101 copies/μl, and the nucleotide concentrations were detected by real-time PCR assay. Threshold cycle (CT) values were plotted against the known copy numbers of the standard controls. Figure 1 demonstrates the good correlation between copy number and CT value (error = 0.139, efficiency = 2.214).
Fig 1.
Standard curve of real-time PCR for the detection of ALV-J. The copy number of the plasmid pMD-JNS was determined spectrophotometrically. The plasmid was serially diluted from 1 × 1010 copies/μl to 1 × 101 copies/μl for use as standard controls. The concentration refers to the template copy number per reaction.
Real-time PCR specificity for ALV-J.
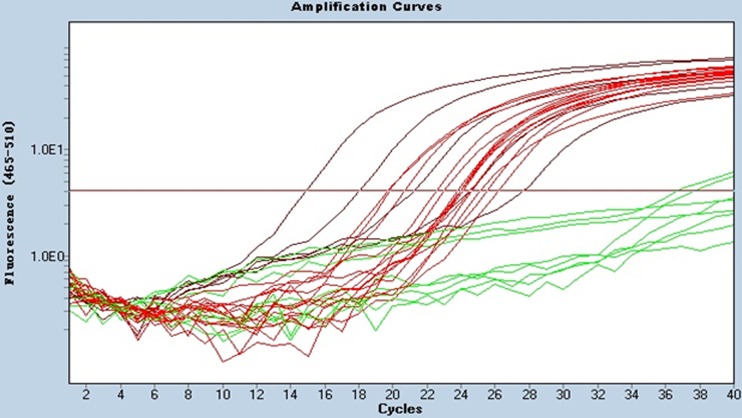
Viral DNA from 15 ALV-J isolates, other ALV subgroups (A, B, C, and E), and some other DNA viruses and RNA viruses, including REV, CAV, MDV, and ILTV, were subjected to real-time PCR. The results showed that the proviral DNA of all 15 ALV-J viruses (HPRS-103, LN08SY10, JL08CH3-1, LN08SY31, SD09DP04, HLJ09SH01, HLJ09SH02, HuB09JY03, HuB09HW02, HuB09HW03, HLJ10SH03, HLJ10SH04, JL10HW01, JL10HW02, and HLJ08MDJ01) could be detected (Fig. 2). In contrast, we did not detect fluorescent signals from the other virus samples, suggesting the specificity of the real-time PCR for ALV-J.
Fig 2.
Specificity of the real-time PCR method for the detection of ALV-J. Twenty-three virus strains, including 15 ALV-J strains, were used to test the specificity of the assay. Red curves (HPRS-103, LN08SY10, JL08CH3-1, LN08SY31, SD09DP04, HLJ09SH01, HLJ09SH02, HuB09JY03, HuB09HW02, HuB09HW03, HLJ10SH03, HLJ10SH04, JL10HW01, JL10HW02, and HLJ08MDJ01) were considered positive, and green curves (ALV-A [RAV-1], ALV-B [RAV-2], ALV-C [RAV-49], ALV-E [RAV-0], CAV [M9905 strain], and REV [HLJR0901 strain]) were considered negative.
Real-time PCR sensitivity and reproducibility.
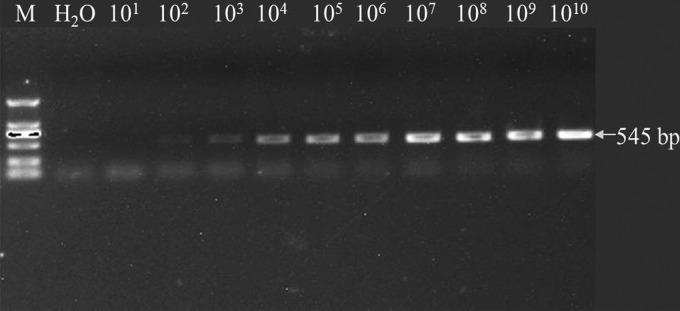
Next, the sensitivity of the real-time PCR was compared with that of routine PCR. The recombinant plasmid pMD-H57, containing a 545-bp fragment that could be amplified with primers H5 and H7, was used as a template. The data showed that the detection limit of the real-time PCR was fewer than 10 copies (data not shown), whereas the limit for routine PCR with the H5/H7 primer pair was 100 copies (Fig. 3). Therefore, the sensitivity of our real-time PCR assay was at least 10 times higher than that of the routine PCR assay.
Fig 3.
Routine PCR with H5 and H7 primers for the detection of ALV-J. PCR revealing a 545-bp amplicon specific to ALV-J. The detection limit was 100 copies. Lane M, DNA marker DL-2000 (TaKaRa, Japan).
The interassay and intra-assay coefficients of variation (CVs) in the CT values for input copy number were determined with the plasmid standard DNA (1 × 105, 1 × 106, and 1 × 107 plasmid copies/μl). All tests were repeated three times. Table 1 shows that all CVs were less than 1% (the interassay CV was 0.438 to 0.651%, while the intra-assay CV was 0.070 to 0.571%).
Table 1.
Reproducibility of real-time PCRa
| Reproducibility | No. of DNA copies | CT (mean ± SD) | CV (%) |
|---|---|---|---|
| Intra-assay | 105 | 23.17 ± 0.016 | 0.070 |
| 106 | 19.84 ± 0.069 | 0.350 | |
| 107 | 16.49 ± 0.094 | 0.571 | |
| Interassay | 105 | 23.30 ± 0.102 | 0.438 |
| 106 | 19.91 ± 0.130 | 0.651 | |
| 107 | 16.59 ± 0.076 | 0.457 |
The interassay and intra-assay coefficients of variation (CV) in the CT values for input copy number were determined with the DNA plasmid standard (1 × 105, 1 × 106, and 1 × 107 plasmid copies/μl). All tests were repeated three times.
Virus growth curve in cell culture.
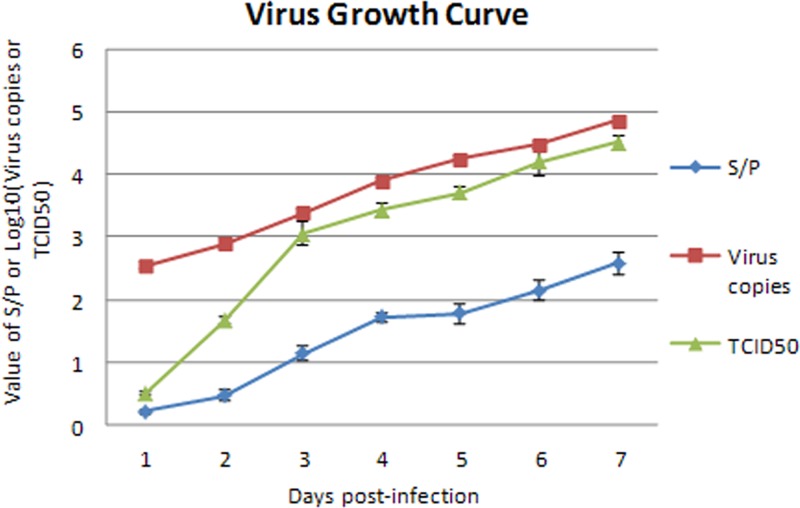
Growth curves of ALV-J in DF-1 cells were determined based on virus copies, the value of the ratio of the sample to the positive control (S/P) for p27 antigen detection, and the TCID50 of the supernatant collected daily. The curves established by virus copies and S/P values were very similar, though the numerical values of the virus copy curve were higher than those of the S/P value curve (Fig. 4). Both curves increased constantly for the first 7 days postinfection. The curve of TCID50 also demonstrated a consistently increased trend line, with a lower level, 0.5 log10 TCID50/100 μl, at the 1st day postinfection and a much higher level, 4.5 log10 TCID50s/100 μl, at the 7th day postinfection. This result indicated that the real-time PCR method could be used to measure the viral growth of ALV-J.
Fig 4.
Growth curves of ALV-J established by S/P values, virus copies, and TCID50s. DF-1 cells were infected with ALV-J at an MOI of 0.1. At 1, 2, 3, 4, 5, 6, and 7 days postinfection, the supernatants were gathered after three rounds of freeze-thawing. Growth curves of the ALV-J in DF-1 cells were determined by virus copies, value of the ratio of the sample to the positive control (S/P) for p27 antigen detection, and TCID50 of the supernatant collected daily.
Detection of ALV-J in the sera of experimental animals.
Routine PCR and real-time PCR methods were used to measure ALV-J in blood samples taken from infected and control chickens. ALV-J infection was detected from 1 week postinfection in the infected group and lasted for the entire experimental period (24 weeks postinfection). No ALV-J was detected in the control group. The positivity ratios of detection in the infected group by routine PCR and real-time PCR methods were 80.0% and 88.0%, respectively (Table 2).
Table 2.
Analysis of experimental animal serum samples
| Group | Test | No. positive/no. of samples |
Total no. positive/total no. tested (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 wk | 2 wks | 3 wks | 4 wks | 8 wks | 12 wks | 16 wks | 24 wks | |||
| ALV-J infected | PCR (H5/H7) | 3/3 | 2/3 | 1/3 | 3/3 | 3/3 | 3/3 | 2/3 | 3/4 | 20/25 (80.0) |
| Real-time PCR | 3/3 | 3/3 | 2/3 | 3/3 | 3/3 | 3/3 | 2/3 | 3/4 | 22/25 (88.0) | |
| Mock infected | PCR (H5/H7) | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/24 (0) |
| Real-time PCR | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/24 (0) | |
Detection of ALV-J from clinical samples.
A total of 173 tissue samples suspected of ALV infection were evaluated for ALV-J proviral DNA using the real-time PCR method. The positivity rate was 60.1% (104/173) (Table 3). Routine PCR and virus isolation were also performed on the same samples. However, the positivity rates of ALV-J infection determined using routine PCR and virus isolation with p27 detection were 44.5% (77/173) and 41.6% (72/173), respectively (Table 3). Among those that were positive by real-time PCR (104 samples), 45 samples were also positive by both routine PCR and virus isolation with p27 detection. Another 59 samples were scored as negative by either routine PCR or virus isolation with p27 detection or both. These results showed that the real-time PCR screen was more sensitive than routine PCR and virus isolation with p27 detection.
Table 3.
Analysis of clinical samplesa
| Real-time PCR | Routine PCR (H5/H7) | Virus isolation (p27 detection) | No. of samples | Positivity rate (%) |
|---|---|---|---|---|
| P | P | P | 45 | 26.0 |
| P | P | N | 32 | 18.5 |
| P | N | P | 8 | 4.6 |
| P | N | N | 19 | 10.9 |
| N | P | P | 0 | 0 |
| N | N | P | 19 | 0.9 |
| N | P | N | 0 | 0 |
| N | N | N | 50 | 28.9 |
P, positive; N, negative.
DISCUSSION
Since 2004, cases of ALV-J infection and tumors have been widely reported for commercial layer hens and breeders of layer chickens in the People's Republic of China (7, 9, 10). ALV-J spreads by both vertical and horizontal transmission, and no commercial vaccine is available. Therefore, the control of ALV infections depends mainly on the early identification and removal of virus-shedding birds to minimize the spread of congenital and contact infection. Virus isolation is a standard method for diagnosis, but it requires complex cell culture procedures and requires more than a week to obtain results (14). Obviously, this is not a satisfactory method for the identification and removal of virus-shedding birds. Recently, several molecular methods, including PCR and LAMP, have been developed and applied (6, 11–16). In this study, a specific, sensitive, and quantitative real-time PCR method was developed for ALV-J detection. ALV-J is the member of the family Retroviridae, which shares the same basic genomic structure and mode of replication as other retroviruses. The RNA genome in the viral particles is reverse transcribed into a DNA form, the provirus, in the infected cells. Several PCR methods have been established based on either the RNA or DNA form of the genome (13, 14, 17, 18, 22). Because DNA is more stable and can be manipulated more conveniently than RNA, the DNA genome was selected as the PCR template in this study.
The env gene of ALV-J differs greatly from those of ALV subgroups A through E but has high identity to env-like sequences of the EAV family of endogenous avian retroviruses (3). The existence of EAV-HP elements in several lines of chickens could interfere with the specific amplification of the env gene sequences of ALV-J; thus, it was necessary to choose primers that selectively amplify the region specific to the exogenous virus. Smith et al. developed a PCR with the H5/H7 primers that was successfully used to detect ALV-J (14). The forward primer (H5) was designed based on the 3′ region of the pol gene and was conserved across several ALV subgroups. The reverse primer (H7) was designed based on a well-conserved region of the env gene of ALV-J, which could distinguish this virus from ALVs of other subgroups. In this study, we designed a set of real-time PCR primers (JNF/JNR) similar to the H5/H7 primer pair; the JNF primer is located within the 3′ region of the pol gene, and JNR is in the 5′ region of the env gene of ALV-J. To evaluate the specificity of this real-time method, we prepared 23 virus DNA samples extracted from virus-infected cell cultures, including samples from ALV subgroups A, B, C, and E; 15 ALV-J isolates; and some other types of viruses (REV, CAV, MDV, and ILTV). As expected, the 15 ALV-J samples were detected by this method, while ALV subgroups A, B, C, and E and other viruses were not detected. This indicated that this real-time PCR method has good specificity for ALV-J.
Kim et al. developed a real-time RT-PCR method for ALV-J detection using primers H5 and H7 (17). This assay is highly reproducible, and the results are positively correlated with the QC-RT-PCR and TCID50s determined by the routine methods. However, the sensitivity of this method is much lower than was expected (10 to 100 copies per sample), possibly because the PCR product size is longer than typically recommended for RT-PCR (17). In this study, we aligned large numbers of ALV sequences available in GenBank and designed a pair of primers with a much shorter product (214 bp). As a result, as few as 10 copies of DNA could be detected, making this assay at least 10 times more sensitive than routine PCR (16). Clinical samples and virus supernatants from infected cell cultures were diluted, and the proviral DNA was extracted. These proviral DNA samples were then screened by real-time PCR and routine PCR. The results showed that the limit for the routine PCR was 100 copies; however, the limit for the real-time PCR was less than 10 copies. This finding also indicated that the sensitivity of our real-time PCR was at least 10 times higher than that of the routine PCR method for detection in clinical samples.
Because there is no observable cytopathic effect (CPE) of ALV-J infection, TCID50 could not be calculated by observing CPE. TCID50 has been determined previously by inoculating chicken embryo fibroblast (CEF) cells from the C/E chicken line with serial dilutions of a viral sample. At 7 to 9 days postinoculation, viral growth in the cell culture can be detected using an antigen capture enzyme-linked immunosorbent assay (ELISA) for a group-specific p27 antigen or an immunofluorescence assay (IFA) with anti-gp85 monoclonal antibodies (MAbs). However, this technique is very time-consuming, laborious, expensive, and difficult to perform in some laboratories due to a lack of C/E cells or MAbs (23). Because real-time PCR is able to quantitatively determine nucleotide (viral DNA or viral RNA) content, this method can be used to track viral growth curves (17, 24, 25). A comparison of ALV-J growth curves determined according to copy numbers of the viral genome, p27 viral antigen detection in the culture supernatants, and conventional TCID50 was performed. The curves established according to copies of the viral genome or S/P values were very similar, although the values in the curve of virus copies were higher than those in the S/P ratio curve. Both curves constantly increased over 7 days postinfection. The TCID50 curve also demonstrated a consistently increasing trend line. However, the three curves established for virus copies, the value of the S/P ratio for p27 antigen, and TCID50 were all slightly different. These differences may be because the virus copies and p27 antigen results reflect absolute virus numbers, including noninfectious virus, while TCID50 describes the replication curve of infectious viruses only. However, any of the three methods could be used to evaluate ALV-J growth kinetics. The real-time PCR method could be used to measure the viral growth of ALV-J, providing a fast, easy, and convenient approach for monitoring ALV-J replication.
A total of 173 tissue samples suspected of ALV infection was collected from commercial layer and breeder flocks that had dramatically reduced egg production and hemorrhages in the skin and feather follicles. Some birds had gray-white nodules on the liver, spleen, or kidneys, and the liver and spleen were enlarged up to several times their normal size. These samples were subjected to real-time PCR, routine PCR (with the H5/H7 primer pair), and virus isolation with p27 detection. The virus positivity rates were 60.1% (104/173), 44.5% (77/173), and 41.6% (72/173) as detected by real-time PCR, routine PCR, and virus isolation with p27 detection, respectively. Although virus isolation is considered the “gold standard,” it has limitations, one of which is that it can identify only samples containing live virus. However, routine PCR and real-time PCR can detect live viruses as well as defective nonreplicating viruses. Therefore, it is believed that routine PCR is more sensitive than virus isolation. However, the positivity rates for routine PCR and virus isolation were very similar. This finding may be because p27 is a group antigen and is conserved in all ALVs, such as ALV-A and ALV-B. In fact, some of these clinical samples (about 49.72% according to LAMP detection) were infected with ALV-A, as determined by our lab (20). Among those screening as positive by real-time PCR (104 samples), 45 samples also screened positive by both routine PCR and virus isolation with p27 detection. Another 59 samples were negative when screened by routine PCR or virus isolation with p27 detection or both. In addition, an animal infection experiment was carried out in which ALV-J was detected from 1 week postinfection throughout the experimental period (24 weeks postinfection) by both PCR methods. No ALV-J was detected in the control group. The rates of infection detected by routine PCR and real-time PCR were 80.0% and 88.0%, respectively. Both the animal infection experiment and clinical sample detection results indicate that our real-time PCR method is more sensitive than routine PCR and more specific than virus isolation with p27 antigen detection.
Although real-time PCR has been widely used in the detection of many viruses and other pathogenic microorganisms, it has limitations. For example, a matching primer pair and probe as well as special, expensive equipment are needed to run real-time PCR. In addition, false-positive results are inevitable due to its high sensitivity, typically requiring no more than 10 copies of RNA or DNA for detection. In general, to avoid false-positive results, suspicious samples and samples detected after 35 cycles must be repeated at least three times. Our final results were determined by performing at least three separate experiments. In conclusion, this report describes a TaqMan-based real-time quantitative PCR method for the detection of ALV-J. This method is specific, sensitive, and convenient. It can detect ALV-J in clinical and experimental infection samples and thus might be used for the early detection and eradication of ALV-J in breeder flocks. Moreover, this method can be used to quantitatively analyze ALV-J and track viral growth curves, taking the place of time-consuming and laborious routine TCID50 calculations. This newly developed real-time PCR method will be highly valuable in the detection and eradication of ALV-J in breeder flocks as well as for basic ALV-J laboratory research.
ACKNOWLEDGMENTS
The study was supported by the Natural Science Foundation of China (no. 31072146), Modern Agro-Industry Technology Research System (no. nycytx-42-G3-01), and Harbin Programs for Science and Technology Development (no. 2010AA6AN034).
Footnotes
Published ahead of print 24 October 2012
REFERENCES
- 1. Fadly AM, Payne LN. 2003. Leukosis/sarcoma group, p 465–516 In Saif YM, Barnes HJ, Fadly AM, Glisson JR, McDougald LR, Swayne DE. (ed), Diseases of poultry, 11th ed. Iowa State University Press, Ames, IA [Google Scholar]
- 2. Payne LN, Brown SR, Bumstead N, Howes K, Frazier JA, Thouless ME. 1991. A novel subgroup of exogenous avian leukosis virus in chickens. J. Gen. Virol. 72: 801– 807 [DOI] [PubMed] [Google Scholar]
- 3. Bagust TJ, Fenton SP, Reddy MR. 2004. Detection of subgroup J avian leucosis virus infection in Australian meat-type chickens. Aust. Vet. J. 82: 701– 706 [DOI] [PubMed] [Google Scholar]
- 4. Venugopal K, Smith LM, Howes K, Payne LN. 1998. Antigenic variants of J subgroup avian leucosis virus: sequence analysis reveals multiple changes in the env gene. J. Gen. Virol. 79: 757– 766 [DOI] [PubMed] [Google Scholar]
- 5. Bai J, Payne LN, Skinner MA. 1995. HPRS-103 (exogenous avian leukosis virus, subgroup J.) has an env gene related to those of endogenous elements EAV-0 and E51 and an E element found previously only in sarcoma virus. J. Virol. 69: 779– 784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silva RF, Fadly AM, Hunt HD. 2000. Hypervariability in the envelope genes of subgroup J avian leukosis viruses obtained from different farms in the United States. Virology 272: 106– 111 [DOI] [PubMed] [Google Scholar]
- 7. Sun S, Cui Z. 2007. Epidemiological and pathological studies of subgroup J avian leukosis virus infections in Chinese local “yellow” chickens. Avian Pathol. 36: 221– 226 [DOI] [PubMed] [Google Scholar]
- 8. Payne LN, Howes K, Gillespie AM, Smith LM. 1992. Host range of Rous sarcoma virus pseudotype RSV(HPRS-103) in 12 avian species: support for a new avian retrovirus envelope subgroup, designated J. J. Gen. Virol. 73: 2995– 2997 [DOI] [PubMed] [Google Scholar]
- 9. Xu B, Dong W, Yu C, He Z, Lv Y, Sun Y, Feng X, Li N, Lee LF, Li M. 2004. Occurrence of avian leukosis virus subgroup J in commercial layer flocks in China. Avian Pathol. 33: 13– 17 [DOI] [PubMed] [Google Scholar]
- 10. Gao YL, Qin LT, Pan W, Wang YQ, Qi XL, Gao HL, Wang XM. 2010. Avian leukosis virus subgroup J in layer chickens, China. Emerg. Infect. Dis. 16: 1637– 1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. García M, EI-Attrache J, Riblet SM, Lunge VR, Fonseca AS, Villegas P, Lkuta N. 2003. Development and application of reverse transcriptase nested polymerase chain reaction test for the detection of exogenous avian leukosis virus. Avian. Dis. 47: 41– 53 [DOI] [PubMed] [Google Scholar]
- 12. Silva RF, Fadly AM, Taylor SP. 2007. Development of a polymerase chain reaction to differentiate avian leukosis virus (ALV) subgroups: detection of an ALV contaminant in commercial Marek's disease vaccines. Avian Dis. 251: 663– 667 [DOI] [PubMed] [Google Scholar]
- 13. Smith EJ, Williams SM, Fadly AM. 1998. Detection of avian leukosis virus subgroup J using the polymerase chain reaction. Avian Dis. 42: 375– 380 [PubMed] [Google Scholar]
- 14. Smith LM, Brown SR, Howes K, McLeod S, Arshad SS, Barron GS, Venugopal K, Mckay JC, Payne LN. 1998. Development and application of polymerase chain reaction (PCR) tests for the detection of subgroup J avian leukosis virus. Virus Res. 54: 87– 98 [DOI] [PubMed] [Google Scholar]
- 15. Zavala G, Jackwood MW, Hilt DA. 2002. Polymerase chain reaction for detection of avian leukosis virus subgroup J in feather pulp. Avian Dis. 46: 971– 978 [DOI] [PubMed] [Google Scholar]
- 16. Zhang X, Liao M, Jiao P, Luo K, Zhang H, Ren T, Zhang G, Xin C, Cao W. 2010. Development of a loop-mediated isothermal amplification assay for rapid detection of subgroup J avian leukosis virus. J. Clin. Microbiol. 48: 2116– 2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim Y, Gharaibeh SM, Stedman NL, Brown TP. 2002. Comparison and verification of quantitative competitive reverse transcription polymerase chain reaction (QC-RT-PCR) and real time RT-PCR for avian leukosis virus subgroup J. J. Virol. Methods 102: 1– 8 [DOI] [PubMed] [Google Scholar]
- 18. Kim Y, Brown TP. 2004. Development of quantitative competitive-reverse transcriptase-polymerase chain reaction for detection and quantitation of avian leukosis virus subgroup J. J. Vet. Diagn. Invest. 16: 191– 196 [DOI] [PubMed] [Google Scholar]
- 19. Gao Y, Yun B, Qin L, Pan W, Qu Y, Liu Z, Wang Y, Qi X, Gao H, Wang X. 2012. Molecular epidemiology of avian leukosis virus subgroup J in layer flocks in China. J. Clin. Microbiol. 50: 953– 960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Kang Z, Gao Y, Qin L, Chen L, Wang Q, Li J, Gao H, Qi X, Lin H, Wang X. 2011. Development of loop-mediated isothermal amplification for rapid detection of avian leukosis virus subgroup A. J. Virol. Methods 173: 31– 36 [DOI] [PubMed] [Google Scholar]
- 21. Lamien CE, Lelenta M, Goger W, Silber R, Tuppurainen E, Matijevic M, Luckins AG, Diallo A. 2011. Real time PCR method for simultaneous detection, quantitation and differentiation of capripoxviruses. J. Virol. Methods 171: 134– 140 [DOI] [PubMed] [Google Scholar]
- 22. van Woensel PA, van Blaaderen A, Moorman RJ, de Boer GF. 1992. Detection of proviral DNA and viral RNA in various tissues early after avian leukosis virus infection. Leukemia 6(Suppl 3):135S–137S [PubMed] [Google Scholar]
- 23. Fadly AM, Witter RL. 1998. Oncornaviruses: leukosis/sarcomas and reticuloendotheliosis, p 185–196 In Swayne DE, Glisson JR, Jackwood MW, Pearson JE, Reed WM. (ed), A laboratory manual for the isolation and identification of avian pathogens, 4th ed. AAAP Press, Jacksonville, FL [Google Scholar]
- 24. Sachs L, Schnurr D, Yagi S, Lachowicz-Scroggins ME, Widdicombe JH. 2011. Quantitative real-time PCR for rhinovirus, and its use in determining the relationship between TCID50 and the number of viral particles. J. Virol. Methods 171: 212– 218 [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Qi X, Gao H, Gao Y, Lin H, Song X, Pei L, Wang X. 2009. Comparative study of the replication of infectious bursal disease virus in DF-1 cell line and chicken embryo fibroblasts evaluated by a new real-time RT-PCR. J. Virol. Methods 157: 205– 210 [DOI] [PubMed] [Google Scholar]