Abstract
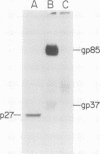
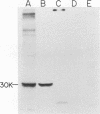
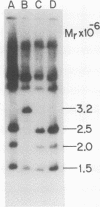
We have studied the pattern of glycoprotein synthesis in two nonconditional mutants of Rous sarcoma virus. One mutant, SE33, produces no viral particles but synthesizes Pr92env, which is cleaved intracellularly to mature glycoproteins. The second mutant, SE521, encodes a gPr92env which is not cleaved to gp85 or gp37 and therefore produces virions with the phenotype of Bryan RSV(-) or NY8. Neither of these mutants have detectable genomic deletions. The study of these mutants has led to the following conclusions. (i) In the absence of particle production or p15 synthesis, gPr92env can be cleaved to the mature glycoprotein which is found on the cell surface. (ii) Noncleaved gPr92env is not packaged into virions but is found on the cell surface. (iii) gPr92env alone can account for subgroup specific viral interference. (iv) gPr92env is probably transported to the cell surface before additional glycosylation or cleavage to mature virion glycoprotein. The nonprocessed precursor of SE521 appears to be glycosylated normally, and thus far we have been unable to determine the basis for the defect in this mutant.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolognesi D. P. Structural components of rna tumor viruses. Adv Virus Res. 1974;19:315–359. doi: 10.1016/s0065-3527(08)60663-6. [DOI] [PubMed] [Google Scholar]
- Buchhagen D. L., Hanafusa H. Intracellular precursors to the major glycoprotein of avian oncoviruses in chicken embryo fibroblasts. J Virol. 1978 Mar;25(3):845–851. doi: 10.1128/jvi.25.3.845-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggelmann H. Biosynthesis of an unglycosylated envelope glycoprotein of Rous sarcoma virus in the presence of tunicamycin. J Virol. 1979 Jun;30(3):799–804. doi: 10.1128/jvi.30.3.799-804.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Kawai S., Wang L. H., Vogt P. K., Murphy H. M., Hanafusa H. RNA of replication-defective strains of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1569–1573. doi: 10.1073/pnas.72.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R., Shaikh R., Mason W. S. Identification of an avian oncovirus polyprotein in uninfected chick cells. Cell. 1978 May;14(1):89–104. doi: 10.1016/0092-8674(78)90304-5. [DOI] [PubMed] [Google Scholar]
- England J. M., Bolognesi D. P., Dietzschold B., Halpern M. S. Evidence that a precursor glycoprotein is cleaved to yield the major glycoprotein of avian tumor virus. J Virol. 1977 Feb;21(2):810–814. doi: 10.1128/jvi.21.2.810-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Lewandowski L. J. Isolation of the major viral glycoprotein and a putative precursor from cells transformed by avian sarcoma viruses. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2342–2346. doi: 10.1073/pnas.71.6.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H. Interaction among avian tumor viruses giving enhanced infectivity. Proc Natl Acad Sci U S A. 1967 Sep;58(3):818–825. doi: 10.1073/pnas.58.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. Synthesis and processing of avian sarcoma virus glycoproteins. Virology. 1978 Apr;85(2):475–486. doi: 10.1016/0042-6822(78)90454-3. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. doi: 10.1128/jvi.15.6.1367-1377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. Extracellular cleavage of the glycoprotein precursor of Rous sarcoma virus. J Virol. 1979 Jan;29(1):285–292. doi: 10.1128/jvi.29.1.285-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. The generation of the two envelope glycoproteins of Rous sarcoma virus from a common precursor polypeptide. Virology. 1978 Mar;85(1):63–74. doi: 10.1016/0042-6822(78)90411-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Halpern M. S. Subunit structure of the glycoprotein complex of avian tumor virus. J Virol. 1976 Jun;18(3):956–968. doi: 10.1128/jvi.18.3.956-968.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Varmus H. E., Bishop J. M. Virus-specific messenger RNAs in permissive cells infected by avian sarcoma virus. J Biol Chem. 1979 Aug 25;254(16):8015–8022. [PubMed] [Google Scholar]
- Linial M., Brown S., Neiman P. A nonconditional mutant of Rous sarcoma virus containing defective polymerase. Virology. 1978 Jun 1;87(1):130–141. doi: 10.1016/0042-6822(78)90165-4. [DOI] [PubMed] [Google Scholar]
- Linial M., Neiman P. E. Infection of chick cells by subgroup E viruses. Virology. 1976 Sep;73(2):508–520. doi: 10.1016/0042-6822(76)90412-8. [DOI] [PubMed] [Google Scholar]
- Moelling K., Hayami M. Analysis of precursors to the envelope glycoproteins of avian RNA tumor viruses in chicken and quail cells. J Virol. 1977 Jun;22(3):598–607. doi: 10.1128/jvi.22.3.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Scott A., Dittmar K. E., Owada M. Effect of p15-associated protease from an avian RNA tumor virus on avian virus-specific polyprotein precursors. J Virol. 1980 Feb;33(2):680–688. doi: 10.1128/jvi.33.2.680-688.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Pawson T., Mellon P., Duesberg P. H., Martin G. S. env Gene of Rous sarcoma virus: identification of the gene product by cell-free translation. J Virol. 1980 Mar;33(3):993–1003. doi: 10.1128/jvi.33.3.993-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R. Immunofluorescence on avian sarcoma virus-transformed cells: localization of the src gene product. Cell. 1979 Jan;16(1):11–24. doi: 10.1016/0092-8674(79)90183-1. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Wassarman P. M. [3H]Dansyl chloride: a useful reagent for the quantitation and molecular weight determination of nanogram amounts of protein. Anal Biochem. 1977 Jan;77(1):25–32. doi: 10.1016/0003-2697(77)90286-x. [DOI] [PubMed] [Google Scholar]
- Shaikh R., Linial M., Brown S., Sen A., Eisenman R. Recombinant avian oncoviruses. II. Alterations in the gag proteins and evidence for intragenic recombination. Virology. 1979 Jan 30;92(2):463–481. doi: 10.1016/0042-6822(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Shealy D. J., Rueckert R. R. Proteins of Rous-associated virus 61, an avian retrovirus: common precursor for glycoproteins gp85 and gp35 and use of pactamycin to map translational order of proteins in the gag, pol, and env genes. J Virol. 1978 May;26(2):380–388. doi: 10.1128/jvi.26.2.380-388.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Varmus H. E., Bishop J. M. Nucleotide sequences related to the transforming gene of avian sarcoma virus are present in DNA of uninfected vertebrates. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4102–4106. doi: 10.1073/pnas.75.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology. 1966 Aug;29(4):642–653. doi: 10.1016/0042-6822(66)90288-1. [DOI] [PubMed] [Google Scholar]
- Stohrer R., Hunter E. Inhibition of Rous sarcoma virus replication by 2-deoxyglucose and tunicamycin: identification of an unglycosylated env gene product. J Virol. 1979 Nov;32(2):412–419. doi: 10.1128/jvi.32.2.412-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. Separation of morphological conversion and virus production in Rous sarcoma virus infection. Cold Spring Harb Symp Quant Biol. 1962;27:407–414. doi: 10.1101/sqb.1962.027.001.038. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966 Nov;30(3):368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]