Abstract
Stem bark of Oroxylum indicum (L) (SBOI) is used by ethnic communities of North East India as health tonic and in treating diseases of humans and animals. The objective of this research was to carry out a detailed investigation including total phenolic and flavonoid content, antioxidant, antimicrobial, cytotoxic and apoptotic activities of different solvent extracts of SBOI and to establish correlation between some parameters. Among petroleum ether (PE), dichloromethane and methanol (MeOH) extract of SBOI, MeOH extract contained the highest amount of total phenolic (320.7 ± 34.6 mg Gallic acid equivalent/g extract) and flavonoid (346.6 ± 15.2 mg Quercetin equivalent/g extract) content. In vitro antioxidant activity (IC50 22.7 μg/ml) was highest in MeOH extract (p > 0.05) and also a significant inverse correlation was observed between phenolic (r = 0.886)/flavonoid (r = 0.764) content and corresponding DPPH IC50. Only MeOH extract inhibited both bacteria and fungi. Although, individual extract showed cytotoxicity on HeLa cells with characteristic features of apoptosis, PE extract caused maximum cytotoxicity (IC50 of 112.3 μg/ml, p < 0.05) and apoptotic activity (33.2 % sub-G0/G1 population) on HeLa cells. But, there was a significant non-inverse correlation of the MTT IC50 with total phenolic (r = 0.812, p < 0.05)/flavonoid (r = 0.998, p < 0.05) content in the three solvent extracts. TLC analysis showed three unique compounds in PE extract which may have a role in apoptosis mediated cytotoxicity. These results called for futher chemical characterisation of MeOH and PE extract of SBOI for specific bioactivity.
Keywords: Oroxylum indicum, Antioxidant, Antimicrobial, Cytotoxicity, Apoptosis
Introduction
The plant kingdom has long been and continues to be one of the sources investigated to develop new phytochemical drugs. The medicinal properties of plants are generally ascribed to the presence of metabolites especially secondary metabolites. Among the secondary metabolites, phenolic compounds possess multiple biological activities (Middleton et al. 2000; Nichols et al. 2010; Carvalho et al. 2010). Oroxylum indicum (L.) Benth. ex Kurz (Bignoniaceae), commonly known as Midnight horror, is a deciduous tree well known among ethnic communities of South Asia including India for its medicinal property. The tree was distributed throughout the greater part of India but now the existence of Oroxylum indicum in natural population is highly threatened and has been categorized as vulnerable (Gokhale and Bansal 2006). A good stand of this plant is found to occur in the mountains of North East India. Different ethnic communities of the region use this plant for the treatment of various ailments and as food supplement. For example, Mao (2002) reported that the bark is taken for curing gastric ulcer and a paste made of the bark powder is used for treating mouth cancer, scabies and other diseases. The paste of the bark powder has also been found to be effective when applied to wounds to kill maggots, and decoction of fresh bark fed for de-worming of animals. Scientific research has progressively accumulated knowledge on biological potential of extracts of SBOI as antioxidant (Mishra et al. 2010), antimicrobial (Islam et al. 2010; Das and Choudhury 2010) and in exhibiting cytotoxic activity against B-16 (murine melanoma), HCT-8 (human colon carcinoma), CEM and HL-60 (leukemia) tumor cell lines (Costa-Lotufo et al. 2005) and HeLa cells (Siriwatanametanon et al. 2010). These previous studies reported separately only one or two of different biological activities of SBOI, but detailed reports on the entire spectrum of phytochemical potential of this plant are not available. Furthermore, very little is known about the possible mechanisms of cytotoxicity induced by SBOI. One earlier study (Costa-Lotufo et al. 2005) showed that the cytotoxic effect of extract of SBOI on mouse erythrocytes was not related to lytic activity or membrane instability. We carried out a detailed investigation on several aspects of three solvent extracts of SBOI including total phenolic and flavonoid content, antioxidant, antimicrobial, cytotoxic and apoptotic activities, and correlation study.
Materials and methods
Chemicals
Folin-Ciocalteu (FC) reagent, gallic acid, 2, 2-diphenyl-2-picryl hydrazyl-hydrate (DPPH), nitro blue tetrazolium (NBT), 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-tetrazolium bromide (MTT), acridine orange (AO), propidium iodide (PI) and cell culture chemicals were purchased from Sigma-Aldrich Chemicals Pvt. Ltd. (Mumbai, India). Ascorbic acid, curcumin, neomycin sulphate and riboflavin were purchased from HiMedia Laboratories Pvt. Limited (Mumbai, India). Proteinase-K, RNase and ethidium bromide (EB) were purchased from Bangalore Genei (Bangalore, India). Quercetin was purchased from Ozone International (Mumbai, India). All other chemicals and solvents used were of analytical grade.
Plant material
The SBOI was collected from Sorapat Hill, Phayeng, Manipur, India (N24°50′1.2″ E093°47′57.5″) at an elevation of 2,688 feet above sea level. The specimen was identified by Dr. Biseshori Thongam, Plant Bioresources Division, Institute of Bioresources and Sustainable Development (IBSD), Manipur, India and by Dr. S.K. Verma, National Bureau of Plant Genetics Resources, Meghalaya, India and voucher specimen (IBSD/C/101) has been deposited to IBSD herbarium.
Preparation of SBOI extraction
The plant materials were air dried at room temperature and powdered. The powdered bark was then exhaustively extracted serially by soaking in petroleum ether (PE), dichloromethane (DCM) and methanol (MeOH) to prevent the loss of biological activities of some heat sensitive ingredients. After filtration, the filtrate was concentrated using a vacuum rotary evaporator (EYELA, Japan) and finally freeze dried. The dried extracts were kept at 4 °C until further analysis.
Determination of total phenolic content (TPC)
The TPC was determined using the Folin-Ciocalteu (FC) reagent, following the procedure devised by Singleton and Rossi (1965), with some modification. Briefly, 20 μl extract (500 μg/ml) was mixed with 1.58 ml of distilled water. To the above mixture, 100 μl of FC reagent (1:2 dilutions) was added, followed by the addition of 300 μl of sodium carbonate (0.2 g/ml) and incubated for 2 h at room temperature in dark condition. The absorbance was then read at 765 nm using UV–visible spectrophotometer (UV-1700 Pharmaspec, SHIMZU). The result was expressed as gallic acid equivalence (GAE).
Determination of total flavonoid content (TFC)
The TFC of SBCG extracts were determined by the method described by Zhishen et al. (1999). Briefly, 250 μl of each sample was mixed with 1 ml of distilled water and subsequently with 75 µl sodium nitrite solution (150 g/l) nitrite solution. After 6 min, 75 μl aluminium chloride (100 g/l) was added and allowed to stand for a further 5 min before 1 ml sodium hydroxide solution (40 g/l) was added. The mixture was immediately made up to 2.5 ml with distilled water and mixed well. The absorbance was then measured at 510 nm. Total flavonoid content was expressed as Quercetin equivalence (QE).
Scavenging activity of DPPH radical
The free radical scavenging activity of the crude extracts was evaluated as described earlier by Mensor et al. (2001). Different concentrations of test samples were mixed with 1 ml DPPH (0.3 mM) in ethanol solution. After 30 min incubation at room temperature in the dark, the absorbance values were measured at 517 nm in a UV–visible spectrophotometer. Ascorbic acid was used as positive control. The inhibition ratio (%) was calculated as follows:
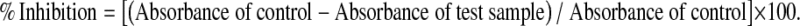 |
IC50 value was obtained by extrapolating from linear regression analysis of experimental data.
Reducing power
The reducing power was carried out as described by Oyaizu (1986). Various concentrations of test samples were mixed with 2.5 ml phosphate buffer (0.2 M; pH = 6.6), 2.5 ml potassium ferricyanide (1 %). After the mixture was incubated at 50 °C for 20 min, 2.5 ml of trichloroacetic acid (10 %) were added and the mixture was centrifuged at 3,000 rpm for 10 min. Supernatant (2.5 ml) was mixed with distilled water (2.5 ml) and 0.5 ml of ferric chloride (0.1 %). The absorbance was then read at 700 nm. Ascorbic acid was used as positive control.
Superoxide radical scavenging activity
Superoxide radical scavenging was carried out as described by Duan et al. (2007), with a slight modification. Various concentrations of test samples were mixed with 200 μl EDTA (40.2 mg/ml), 100 μl riboflavin (0.2 mg/ml), 200 μl ethanol and 100 μl of NBT (1 mg/ml) and were diluted to 3 ml with phosphate buffer (pH-7.6). The absorbance was then read at 560 nm after illumination for 15 min. Ascorbic acid was used as positive control. The inhibition ratio (%) was calculated as follows:
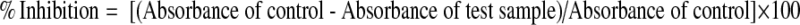 |
IC50 value was obtained by extrapolating from linear regression analysis of experimental data.
Microbial strains
The following bacterial and fungal strains were employed in the screening: Bacillus subtilis (2,451), Bacillus cereus (430), Pseudomonas aeruginosa (2,581), Aspergillus fumigatus (5,175), Aspergillus flavus (1,973) and Macrofomina phaseolina (472) were procured from the Microbial type culture collection (MTCC), Institute of Microbial Technology, Chandigarh, India. Escherichia coli, Klebsiella pneumoniae and Staphylococcus aureus were obtained from the Dehradun Medical College, Dehradun, India.
Preparation of disc
Whatmann filter paper (no. 1) discs (5 mm diameter) were impregnated with 20 μl of crude extracts to get a concentration of 1,000 μg/disc and were kept at 37 °C for 24 h. The reference antibiotic (neomycin) was prepared in appropriate concentration (4 μg/disc) as positive control and solvents PE, DCM and MeOH as negative control.
Agar disc diffusion method
Modified agar diffusion method was used to determine antibacterial activities and antifungal activities (Bauer et al. 1966). The bacterial cells suspension, 1 × 106 cfu/ml was mixed with sterile nutrient agar and poured into petridishes to give a solid plate. Similarly, fungi at 2 × 104 cfu/ml were inoculated to sterile potato dextrose agar for disc diffusion assay.
The discs were deposited on the surface of inoculated agar plates. The bacterial plates were then incubated for 24 h at 37 °C and fungi at 30 °C for 48 h. Inhibition zone diameters around each of the discs (diameter of inhibition zone plus diameter of the disc) were measured and recorded at the end of the incubation time. An average zone of inhibition was calculated for three replicates.
Minimal inhibitory concentrations (MICs)
MICs were determined by taking different concentrations with the highest concentration starting from 500 μg/disc and diluted one fold to obtain various concentration ranges and agar disc diffusion test was performed. The MIC is the lowest concentration of the test sample required to inhibit any visible growth.
Cell culture
The human cervical adenocarcinoma cell line (HeLa) was obtained from the National Centre for Cell science (Pune, India). The cells were grown as monolayer cultures in DMEM supplemented with 10 % (v/v) heat—inactivated fetal bovine serum (FBS) and 1 % antibiotic antimycotic solution (10,000 U/ml penicillin, 10 mg/ml streptomycin sulfate, 5 mg/ml gentamycin and 25 μg/ml amphotercin-B), and maintained at 37 °C in 5 % CO2/95 % air atmosphere with 90 % relative humidity.
MTT reduction assay on HeLa cells
Cytotoxicty was analysed using the MTT assay reported by Mosmann (1983). HeLa cells grown in T-25 culture flasks were harvested by trypsinization, plated at an approximate density of 1 × 105 cells/well in 96-well culture plates (Corning®), and were incubated for 24 h to achieve confluent growth. After 24 h the medium from each well was removed, and the cells were washed twice with Dulbecco’s Phosphate Buffered Saline (PBS). The cells were then exposed to increasing concentrations of extract. Each well contained 100 μl of serum free DMEM containing different concentrations of extracts. The cells were then incubated at 37 °C in 5 % CO2/95 % air atmosphere with 90 % relative humidity for 24 h. After incubation, the contents were replaced with equal amounts of MTT dissolved in serum free DMEM (0.5 mg/ml) after which the plates were further incubated for 3 h. The contents were then replaced with equal amounts of DMSO to solubilise the formazan grains formed by viable cells. Finally, the absorbance was read at 570 nm using a multi-well plate reader (TEKAN, infinite M200). The viability percentage was calculated by using the formula, as described below:
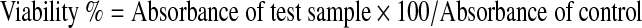 |
IC50 value was obtained by extrapolating from linear regression analysis of experimental data.
Fluorescence microscopy
Differential staining of extract treated (140 μg/ml) and untreated (24 h) HeLa cells was done using DNA-intercalate fluorescent dyes ethidium bromide (EB) and acridine orange (AO) and was analyzed under fluorescence microscope (Nikon, TS 100-F; Tokyo, Japan).
DNA fragmentation assay
For laddering experiments, cells were treated (140 μg/ml) as described above and incubated for 48 h in a CO2 incubator. Treated cells were then harvested, washed with ice cold Phosphate Buffered Saline (pH 7.2), and centrifuged at 3,000 rpm for 6 min at 4 °C. The resulting cell pellet was dispersed in 30 μl of lysis buffer (10 mM EDTA; 50 mM Tris HCl, pH-7.8; 1 % SDS) by gentle vortexing. About 4 μl of proteinase-K (10 μg/μl) was then added to the above mixture, followed by incubation at 45 °C for 1–2 h. Then, 2 μl of RNase (10 μg/μl) was added to the cell lysates, which were further incubated for 1 h at room temperature. After incubation cell lysates were mixed with 4 μl of 6X DNA sample dye and subjected to run at 2 % agarose gel electrophoresis. The gel was then stained with ethidium bromide (0.5 μg/ml) and visualized under a gel documentation system (BioRad, Hercules, CA, USA).
Flow cytometry
Apoptotic cells were detected using PI staining (Riccardi and Nicoletti, 2006) of HeLa cells followed by flow cytometry to detect the sub-G0/G1 cell population. Briefly, HeLa cells were treated with or without SBOI extract (140 μg/ml) for 24 h. After treatment, floating and adherent cells were harvested and fixed in ice-cold 70 % ethanol overnight at −20 °C. Fixed cells were then treated with 0.5 ml of DNA extraction buffer (192 ml of 0.2 M Na2HPO4 with 8 ml of 0.1 % Triton X-100 (v/v)) for 5 min at room temperature. DNA was stained with propidium iodide (20 μg/ml) and incubated for 1 h in the dark. Flow cytometric analysis was then performed using a flow cytometer (BD FACSCaliber). At least 10,000 cells were analyzed for each sample and data were analyzed and plotted by CellQuest software.
Thin layer chromatography
SBOI extracts were loaded on activated silica gel TLC plates. The plates were developed using petroleum ether:ethyl acetate (9:1). The spots were located by exposing the plates to UV light (254 nm) and iodine fumes.
Statistical analysis
The values were presented as mean ± SD of triplicate measurements. All statistical comparisons were made by unpaired t test and p-values less than 0.05 were considered statistically significant. Simple correlation analysis was carried out and significance was tested by using standard methods (Kwanchai and Arturo 1984).
Results
Total phenolic, flavonoid contents
Total phenolic and flavonoid content of SBOI extracts are shown in Table 1. The TPC in the three extracts were in a range of 134.07–320.7 mg GAE/g dried extract with highest content found in MeOH extract followed by DCM and PE extract. The TFC was found to be 346.6 ± 15.2, 160 ± 10 and 66.6 ± 5.7 mg QE/g dried MeOH, DCM and PE extract (p < 0.05), respectively.
Table 1.
Total phenolic and flavonoid contents of SBOI extracts
| Extract | Total phenolic content (mg/g)a | Total flavonoid content (mg/g)b |
|---|---|---|
| Petroleum ether | 134.07 ± 18.5z | 66.6 ± 5.7z |
| Dichloromethane | 287.4 ± 25.8y | 160 ± 10y |
| Methanol | 320.7 ± 34.6x | 346.6 ± 15.2x |
aData expressed as Gallic acid equivalents, Mean ± SD. Means marked with different letters, within each column, are significantly different (p < 0.05)
bData expressed as Quercetin equivalents, Mean ± SD. Means marked with different letters, within each column, are significantly different (p < 0.05)
Antioxidant activity
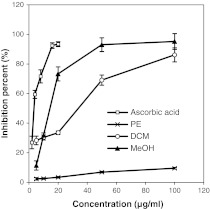
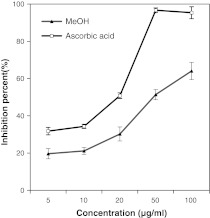
The antioxidant potency of the three extracts of SBOI tested on three test systems i.e. the DPPH free radical scavenging, reducing power and super oxide radical scavenging is presented in Figs. 1, 2, 3. Irrespective of the extracts, the DPPH free radical scavenging activity was found to be concentration dependent (Fig. 1) with MeOH extract showing highest scavenging activity whereas PE extract showed least scavenging activity (p < 0.05). The standard positive control i.e. ascorbic acid showed as high as 93.4 % of DPPH radical scavenging at just 20 μg/ml concentrations, whereas the highest scavenging activity exhibiting MeOH extract could show comparable scavenging activity of 93.07 % at 50 μg/ml. The IC50 value for the ascorbic acid and the three extracts is shown in Table 2. A lower IC50 indicates greater scavenging power. The IC50 value of ascorbic acid was the lowest at 4.1 μg/ml and IC50 of the extracts were in the order, MeOH (22.7 μg/ml) < DCM (37.8 μg/ml) < PE (607.5 μg/ml) (p < 0.05). The IC50 value based scavenging effect of MeOH extract was found to be 26.7 fold greater than PE extract. The result on reducing power study of PE extract treatment was not obtained because of precipitation. In reducing power, higher absorbance of the reaction mixture indicates increase in reducing power. Similar to DPPH scavenging activity, the reducing power activity was also dose dependent (Fig. 2). The absorbance of MeOH and DCM extract was 1.09 and 0.7 at 100 μg/ml concentration, respectively. Super oxide radical scavenging activity of MeOH extract was 64.04 % at 100 μg/ml and positive control, ascorbic acid was 64.04 % at 40.1 μg/ml. No result was obtained for PE and DCM extract due to precipitation. The IC50 of MeOH extract and ascorbic acid for super oxide radical scavenging activity was found to be 63.3 ± 4.6 μg/ml and 20.6 ± 1.2 μg/ml, respectively (p < 0.05).
Fig. 1.
Antioxidant activity of different solvent (PE, DCM and MeOH) extracts of SBOI and ascorbic acid (positive control) assessed by DPPH radical scavenging method. Each value represents the mean ± SD of three determinations
Fig. 2.
Antioxidant activity of different solvent (DCM and MeOH) extracts of SBOI and ascorbic acid (positive control) assessed by reducing power method. Each value represents the mean ± SD of three determinations
Fig. 3.
Antioxidant activity of MeOH extract of SBOI and ascorbic acid (positive control) assessed by superoxide oxide radical scavenging method. Each value represents the mean ± SD of three determinations
Table 2.
IC50 values of different antioxidant assays of SBOI extracts
| Extract | DPPH radical scavenging (μg/ml) | Superoxide radical scavenging (μg/ml) |
|---|---|---|
| Petroleum ether | 607.5 ± 32.7z | ND |
| Dichloromethane | 37.8 ± 3.6y | ND |
| Methanol | 22.7 ± 2.09x | 63.3 ± 4.6z |
| Ascorbic acid | 4.1 ± 1.4w | 20.6 ± 1.2y |
ND not determined. Data expressed as mean ± SD. Means marked with different letters, within each column, are significantly different (p < 0.05)
Antibacterial and Antifungal Activity
Inhibition zone diameter of six bacterial and three fungal pathogens due to the application of the three extracts at equivalent concentrations of 1,000 μg/disc and the reference antibiotic neomycin at 4 μg/disc are presented in Table 3. DCM and MeOH extracts inhibited all tested bacteria and MeOH extract inhibited only two out of the three fungi tested. PE extract inhibited only two bacterial stains. MIC values for DCM and MeOH extract ranged from 62.5 to 250 μg/disc (Table 4).
Table 3.
Antimicrobial activity of three solvent SBOI extracts
| Microorganisms | Inhibition zone diameter (mm) | |||
|---|---|---|---|---|
| PE (1,000 μg/disc) |
DCM (1,000 μg/disc) |
MeOH (1,000 μg/disc) |
Neomycin (4 μg/disc) |
|
| Bacteria | ||||
| Klebsiellapneumoniae | 0 | 7.8 ± 0.2 | 7.8 ± 0.2 | 14.5 ± 0.2 |
| Pseudomonas aeruginosa | 0 | 15 ± 1 | 14.1 ± 0.2 | 14.1 ± 0.2 |
| Escherichia coli | 7.8 ± 0.2 | 11.5 ± 0.5 | 14.5 ± 0.5 | 14.1 ± 0.5 |
| Bacillus subtilis | 0 | 8.3 ± 0.5 | 8 ± 0.5 | 9 ± 0.5 |
| Bacillus cereus | 0 | 11.3 ± 0.5 | 9.5 ± 0.5 | 7 ± 0.5 |
| Staphylococcus aureus | 7.8 ± 0.2 | 12.3 ± 0.5 | 14.1 ± 0.2 | 0 |
| Fungi | ||||
| Aspergillus flavus | 0 | 0 | 0 | ND |
| Aspergillus fumigatus | 0 | 0 | 6.5 ± 0.5 | ND |
| Macrofomina phaeolina | 0 | 0 | 6.5 ± 0.5 | ND |
ND not determined. Data expressed as mean ± SD
Table 4.
The minimum inhibitory concentration of different positive solvent extracts of SBOI
| Microorganisms | Minimum inhibitory concentration (μg/disc) | ||
|---|---|---|---|
| PE | DCM | MeOH | |
| Bacteria | |||
| Klebsiellapneumoniae | ND | 250 ± 0.0 | 125 ± 0.0 |
| Pseudomonas aeruginosa | ND | 62.5 ± 0.0 | 62.5 ± 0.0 |
| Escherichia coli | 1,000 ± 0.0 | 125 ± 0.0 | 125 ± 0.0 |
| Bacillus subtilis | ND | 125 ± 0.0 | 250 ± 0.0 |
| Bacillus cereus | ND | 250 ± 0.0 | 250 ± 0.0 |
| Staphylococcus aureus | 1,000 ± 0.0 | 62.5 ± 0.0 | 62.5 ± 0.0 |
| Fungi | |||
| Aspergillus flavus | ND | ND | ND |
| Aspergillus fumigatus | ND | ND | 250 ± 0.0 |
| Macrofomina phaeolina | ND | ND | 250 ± 0.0 |
ND not determined. Data expressed as mean ± SD
Effect of different extracts on the proliferation of HeLa cells
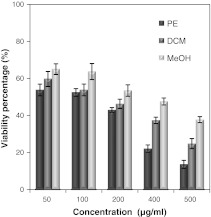
The percentage of viable HeLa cells due to treatment with the three extracts of SBOI are shown in Fig. 4. The relative number of surviving cells progressively decreased in a dose dependent manner. The number of surviving cells in PE extract treatment was lowest and in MeOH extract highest. PE extract at 50–500 μg/ml decreased the proliferation of HeLa cells by 53–13 %. On the other hand, 5–80 μg/ml of either the DCM or MeOH extract decreased the viable cells from 59 to 24 % and 65 to 37 %, respectively. Correspondingly, IC50 value of the PE extract (112.3 ± 4.4 μg/ml) was found to be lowest compared with the DCM (IC50—171.7 ± 7.4 μg/ml) and MeOH extract (IC50—315.7 ± 6.5 μg/ml) (p < 0.05).
Fig. 4.
Cytotoxic effect of different solvent (PE, DCM and MeOH) extracts of SBOI against HeLa cells. Cell survival was determined as the percentage of the control from three independent experiments. Each value represents the mean ± SD of three determinations
Apoptosis induced by different extracts on HeLa cells
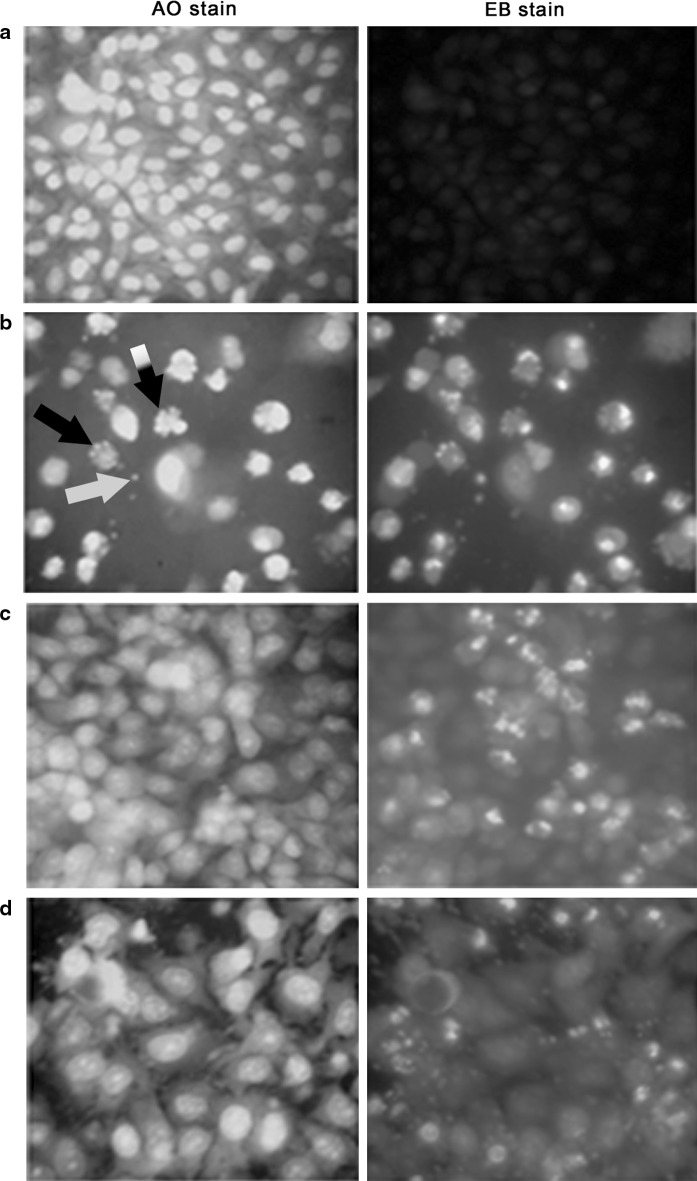
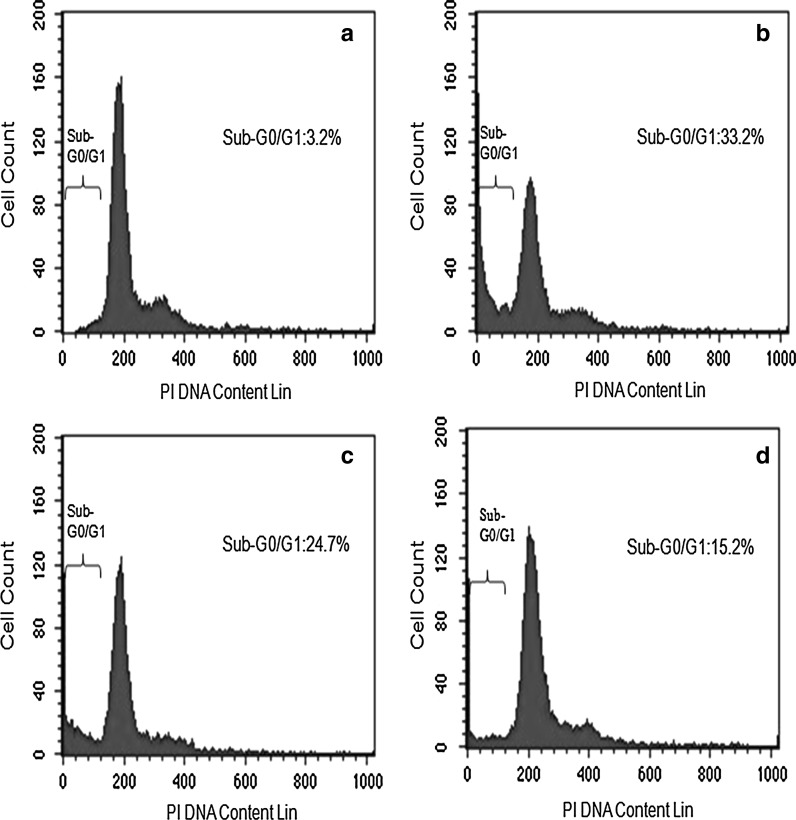
The density of HeLa cells emitting green fluorescence after treatment with PE extract was much lower compared to those in treatment with DCM and MeOH extract treated cells and considerably lower than extract untreated cell. Cytoplasmic membrane blebbing, nuclear fragmentation and apoptotic bodies which are the characteristic features of apoptotic cells were visible 24 h after treatment with the extract. These features are more prominent in PE extract treated cells (Fig. 5). The result of DNA fragmentation assay on HeLa cells is presented in Fig. 6. The DNA ladder of PE and DCM treated HeLa cells was comparable with that of the curcumin (positive control) treated cells. Due to technical reason, 100 bp ladder marker was used although a 1 kb ladder would have been ideal. The result of flow cytometry analysis is presented in Fig. 7. The sub-G0/G1 population (of HeLa cells) as a biochemical marker of apoptosis was considerably higher in all extracts compared to the untreated control with the highest population of 33.2 % in PE extract followed by DCM (24.73 %) and MeOH extract (15.2 %). The populations in other phases (G1, S and G2) of the cell cycle were lower irrespective of the extract.
Fig. 5.
HeLa cells stained with AO (green)/EB (orange or red) and viewed under fluorescence microscope (×400). a Untreated control cells; Cells after treatment with PE b DCM c and MeOH d SBOI (140 μg/ml) showing initiation of membrane blebbing (black and white striped arrow), nuclear fragmentation (black arrow) and apoptotic body (white arrow), a characteristic feature of apoptosis
Fig. 6.
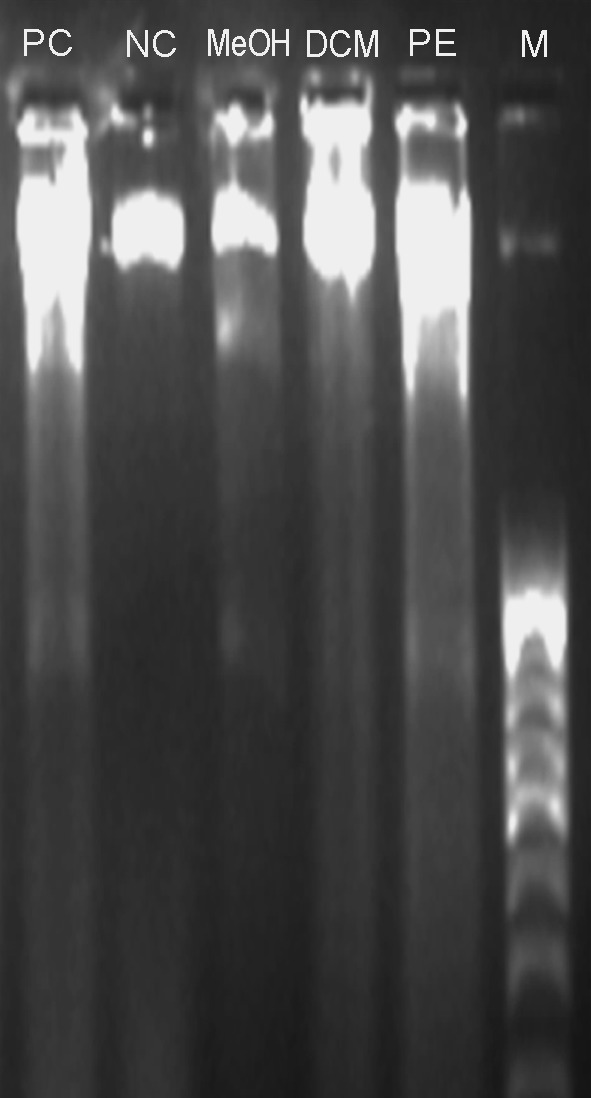
The gel electrophoresis image obtained after DNA fragmentation assay for apoptosis detection. From left lanes are: positive control/Curcumin treated (PC), negative control/no treatment (NC), MeOH, DCM and PE extracts of SBOI treated cells and marker/100 bp DNA ladder (M)
Fig. 7.
Effect of different SBOI on HeLa cells after 24 h treatment for apoptosis detection using flow cytometer. a Untreated. b–d Treated with 140 μg/ml of PE, DCM and MeOH extract, respectively
Correlation analysis
As shown in Table 5, the correlation between DPPH IC50 and total phenolic and flavonoid content is positive and that between MTT IC50 and total phenolic and flavonoid content is negative.
Table 5.
Simple correlations (r value) between total phenolic, total flavonoid in the three solvent extracts of SBOI and their DPPH IC50 and MTT IC50 values
| Correlations | r (p < 0.05) |
|---|---|
| Total phenolics and DPPH IC50 | 0.886i* |
| Total phenolics and MTT IC50 | 0.812n* |
| Total flavonoids and DPPH IC50 | 0.764i* |
| Total flavonoids and MTT IC50 | 0.998n* |
i inverse, n non-inverse
* Statistically significant at p < 0.05
TLC Analysis
The TLC profile of SBOI sequential extracts is shown in Fig. 8. The pattern of chromatogram of PE extract was distinctly different from that of the other two extracts. Three spots (two orange and one blue) found in case of PE extract were settled clearly at the Rf value 0.35, 0.8 and 0.98 and such spots were not visible in case of DCM and MeOH extract.
Fig. 8.
Thin layer chromatographic pattern of different SBOI extracts (PE, DCM and MeOH) along with Rf values. a TLC plate exposed to UV 254 nm. b TLC plate exposed to iodine fumes. PE extract showed unique features of spots as compare with the DCM and MeOH
Discussion
The result of this study has generated detailed information on content of polyphenols and flavonoids in successive extracts of stem bark of O. indicum (L.) Benth. ex Kurz. and their antioxidant, antimicrobial, cytotoxic and apoptotic activities. Various studies have revealed that high polyphenols and flavonoids present in plants have multiple biological effects (Middleton et al. 2000; Nichols et al. 2010; Carvalho et al. 2010). As a result, in our study, the TPC and TFC were determined. The total content of phenols was highest in MeOH followed by in DCM and PE extract. It was reported that solubility of the phenolic compounds increases with polarity of extracts (Siddhuraju and Becker 2003; Sultana et al. 2007) and polarity of MeOH is the highest among the three solvents used in this study. MeOH is a highly preferred solvent for the extraction of phenols (Yen et al. 1996). Similarly, total flavonoid content was also found to be highest in MeOH extract (p < 0.05) and an earlier report (Mohamed et al. 2010) showed increase in yield of flavonoid from Mirabilis jalapa tubers due to extraction using solvents of increasing polarity.
In our study, MeOH extract of SBOI exhibited highest level of antioxidant activity tested by the three models, suggesting that the MeOH extract of SBOI is a potential natural antioxidant. Further, this led us to hypothesize that polar antioxidants are stronger antioxidants than non polar antioxidants. Kalaivani and Mathew (2009) observed high antioxidant potential of ethanol extract of SBOI in β-carotene bleaching assay and chloroform extract in reducing power assay. They attributed variation among antioxidant potential to the phytochemical diversity in the extracts. Again, several authors have reported association of high total phenolic content and high antioxidant activity in numerous plants (Anesini et al. 2008; El Babili et al. 2010; Carvalho et al. 2010). In agreement with those reports, in our study a significant inverse correlation was obtained between phenolic content and DPPH IC50 (r = 0.886, p < 0.05) value and also between flavonoid content and DPPH IC50 (r = 0.764, p < 0.05) suggesting that the high antioxidant activity is due to the high polyphenol and flavonoid content in the SBOI. Antioxidant activity of plant extracts containing polyphenol components is due to their capacity to be donors of hydrogen atoms or electrons and to capture free radicals (Shon et al. 2003). Sankara and Nair (1972a, b) reported that stem bark and leaves of O. indicum contain flavonoids such as baicalein, chrysin and scutellarin, and baicalein is shown to possess antioxidant activity (Ng et al. 2000). Moreover, baicalein and chrysin of different plant extracts including that of O. indicum are also shown to possess antimicrobial properties (Kujumgier et al. 1999; Tahara et al. 1987). Das and Choudhury (2010) reported inhibitory effects of ethanol and methanol extracts of SBOI on Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa. Similarly, Islam et al. (2010) demonstrated antimicrobial activities from different fractions of methanol extract of SBOI of Chittagong, Bangladesh against gram positive and gram negative bacteria and fungi. By 1H NMR spectrometric analysis they confirmed that n-hexane fraction of methanolic crude extract contained flavonoid type compounds. We found that MeOH and DCM extract displayed a broad antibacterial spectrum against three gram negative (Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae) and gram positive (Bacillus subtilis, Bacillus cereus and Staphylococcus aureus) bacteria while PE extract showed antibacterial activity only against Escherichia coli and Staphylococcus aureus. MeOH extract inhibited two pathogenic fungi (Aspergillus fumigatus and Macrofomina phaeolina). It has been reported that plant extracts are not as effective against fungi as against bacteria (Heisey and Gorham 1992). Fungi are more resistant to plant extracts. Therefore, the result of MeOH extract of SBOI obtained in this study is of great importance as it has shown a wide spectrum of antimicrobial activity and contains higher amounts of polyphenols and flavonoids to which the observed broad spectrum antimicrobial effect can be attributed. Thus, this potential extract might find therapeutic use in place of antibiotics against which pathogens have developed resistance.
The other important aspect of this study is that we have observed cytotoxicity of three solvent extracts on HeLa cells and obtained evidence on whether the extracts induce apoptosis. Results of cytotoxicity test using MTT assay on HeLa cells showed that PE extract had the strongest cytotoxic effect with the lowest IC50 value 112.3 ± 4.4 μg/ml (p < 0.05). The other two extracts were not as effective. Recently, Siriwatanametanon et al. (2010) found the highest cytotoxic effect of ethyl acetate extract from SBOI of Thailand on HeLa cell lines (IC50—55.22 ± 0.58 μg/ml) followed by PE extract (IC50—96.1 ± 1.3 μg/ml) and MeOH extract (IC50—417.9 ± 1.7 μg/ml). In a previous study, Costa-Lotufo et al. (2005) showed ethanol extract of SBOI from Bangladesh to possess the highest cytotoxic activity in MTT assay using tumour cell lines such as B16 (murine carcinoma), HCT-8 (human colon carcinoma) and HL-60 (leukemia) and also reported that the cytotoxic activity was not related to the lytic properties or membrane instability induced by the extract, but rather the activity was related to inhibition of DNA or protein synthesis. To ascertain whether the cytotoxicity against HeLa cells was mediated through apoptosis, several studies including morphological, biochemical, and sub-G0/G1 population studies were carried out on treated HeLa cells. In morphological study, most of the dead cells showed characteristic features of apoptosis such as cytoplasmic membrane blebbing, nuclear fragmentation and apoptotic bodies (Fig. 5) on treatment with extracts. These features are very prominent in PE extract. Biochemically, apoptosis is characterized by activation of endogenous nucleases and DNA degradation into fragment multiples of 185 bp (Bortner et al. 1995) which has also been seen in DNA fragmentation assay of our study (Fig. 6). Furthermore, based on flow cytometry analysis of HeLa cells after treatment with SBOI extracts, it was found that the sub-G0/G1 population, a biochemical marker of apoptosis (Yu et al. 2007) with hypo-diploid DNA increased considerably in all extracts with highest increase of 33.2 % in PE extract followed by 24.7, 15.2 and 3.2 % in DCM, MeOH extract and the untreated control, respectively (Fig. 7). This accumulation directly relates to the decrease of the cell population in the other phases of the cell cycle, indicating cell death through interference on cell cycle programme. Thus, our study clearly shows that the cytotoxic activity of SBOI extract, in particular of PE extract was due to induction of apoptosis. There are 1,100 publications reporting anticancer activities of polyphenols in the peer-reviewed journals (Lamoral-Theys et al. 2010). This seems to indicate that polyphenols are the main phytochemicals of higher plants with antiproliferative properties. But in our study, a non—inversely significant correlation was observed between MTT IC50 value and total phenolic (r = −0.812, p < 0.05)/flavonoid (r = −0.998, p < 0.05) content of the three extracts suggesting that the cytotoxic activity of these extracts may be related to some chemical components other than the phenolic and flavonoid compounds or may be explained by the fact that polyphenolic compounds of these extracts were different between the extracts as far as they were extracted with solvents having different polarities. To further confirm the unique phytochemical present in the PE extract involved in cytotoxicity, TLC analysis was done among the extracts. As expected, some unique spots (three spots) were observed in the PE extract (Fig. 8) suggesting that the presence of these phytochemicals in this extract was responsible for the observed cytotoxic effect on HeLa cells. However, these results do not rule out the possible combined action of all different compounds present originally in the SBOI powder as DCM and MeOH extracts also caused HeLa cell death, although to a lower extent.
Our finding suggests that MeOH and DCM extracts of SBOI contain high levels of polyphenols and flavonoids and that these bioactive compounds may be predominantly responsible for high level of antioxidant, antibacterial and antifungal activity and a mild cytotoxic effect. The PE extract contains some unique classes of phytochemicals responsible for high level of apoptosis mediated cytotoxicity. Thus, MeOH and PE extract of SBOI contains specific phytochemicals with desirable bioactivity which are currently fractionated, based on bioactivity guided fractionation for isolation of specific bioactive compounds.
Acknowledgments
The senior author thanks Institute of Bioresources and Sustainable Development (IBSD), Imphal and Department of Biotechnology, Government of India for the Ph.D-JRF fellowship. The technical help of Mr. Lokesh Deb, Dr. Reena Langoljam and Miss Surbala Laishram of IBSD and Mr. Arghya Sett of IIT, Guwahati is duly acknowledged.
References
- Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agric Food Chem. 2008;5:9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;36:493–496. [PubMed] [Google Scholar]
- Bortner CD, Oldenburg NBE, Cidlowski JA. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995;5:21–77. doi: 10.1016/S0962-8924(00)88932-1. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jerónimo C, Silva BM. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol. 2010;48:441–447. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Costa-Lotufo LV, Khan MT, Ather A, Wilke DV, Jimenez PC, Pessoa C, de Moraes ME, de Moraes MO. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacol. 2005;99:21–30. doi: 10.1016/j.jep.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Das S, Choudhury MD. Antimicrobial activity of stem bark extracts from the plant Oroxylum indicum Vent. Assam Univ J Sci Technol Biol Environ Sci. 2010;5:95–99. [Google Scholar]
- Duan X, Wu G, Jiang Y. Evaluation of antioxidant properties of phenolics from lichi fruit in relation to pericarp browning prevention. Molecules. 2007;12:759–771. doi: 10.3390/12040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Babili F, Bouajila J, Fouraste I, Valentin A, Mauret S, Moulis C. Chemical study, antimalarial and antioxidant activities, and cytotoxicity to human breast cancer cells (MCF7) of Argania spinosa. Phytomedicine. 2010;17:157–160. doi: 10.1016/j.phymed.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Gokhale M, Bansal YK. Avowal of importance of endangered tree Oroxylum indicum (Linn.) Vent. IJNPR. 2006;5:112–114. [Google Scholar]
- Heisey RM, Gorham BK. Antimicrobial effects of plant extracts on Streptococcus mutans, Candida albican, Trichophyton rubrum and other microorganisms. Lett Appl Microbiol. 1992;14:136–139. doi: 10.1111/j.1472-765X.1992.tb00668.x. [DOI] [Google Scholar]
- Islam MK, Eti IZ, Chowdhury JA. Phytochemical and antimicrobial analysis on the extract of Oroxylum indicum Linn. Stem-Bark IJPT. 2010;9:25–28. [Google Scholar]
- Kalaivani T, Mathew L. Phytochemistry and free radical scavenging activities of Oroxylum indicum. Environ We Int J Sci Tech. 2009;4:45–52. [Google Scholar]
- Kujumgier A, Tsvetkoova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64:235–240. doi: 10.1016/S0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- Kwanchai AG, Arturo AG. Statistical procedures for agricultural research. New York: Wiley; 1984. [Google Scholar]
- Lamoral-Theys D, Pottier L, Dufrasne F, Nève J, Dubois J, Kornienko A, Kiss R, Ingrassia L. Natural polyphenols that display anticancer properties through inhibition of kinase activity. Curr Med Chem. 2010;17:812–825. doi: 10.2174/092986710790712183. [DOI] [PubMed] [Google Scholar]
- Mao AA. Oroxylum indicum Vent. A potential anticancer medicinal plant. IJTK. 2002;1:17–21. [Google Scholar]
- Mensor LL, Menezes FS, Leitao GG, Reis AS, Santos TC, Coube CS, Leitao SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Middleton- E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Mishra SL, Sinhamahapatra PK, Nayak A, Das R, Sannigrahi S. In vitro antioxidant potential of different parts of Oroxylum indicum: a comparative study. Indian J Pharm Sci. 2010;72:267–269. doi: 10.4103/0250-474X.65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed H, Raoudha J, Imen L, Ons M, Damak M, Moncef N. GC/MS and LC/MS analysis, and antioxidant and antimicrobial activities of various solvent extract from Mirabilis jalapa tubers. Process Biochem. 2010;45:1486–1493. doi: 10.1016/j.procbio.2010.05.027. [DOI] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ng TB, Liu F, Wang ZT. Antioxidant activity of natural products from plants. Life Sci. 2000;68:709–723. doi: 10.1016/S0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of the browning reaction. Antioxidative activities of browning reaction products prepared from glucosamine. Jpn J Nutr. 1986;44:307–314. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Sankara S, Nair AGR. Flavonoids of the stem bark of Oroxylum indicum. Curr Sci. 1972;41:62–63. [Google Scholar]
- Sankara S, Nair AGR. Flavonoids from the leaves of Oroxylum indicum and Pajanelia longifolia. Phytochem. 1972;11:439–440. doi: 10.1016/S0031-9422(00)90042-6. [DOI] [Google Scholar]
- Shon MY, Kim TH, Sung NJ. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetoceae) extracts. Food Chem. 2003;82:593–597. doi: 10.1016/S0308-8146(03)00015-3. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorometry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Siriwatanametanon N, Fiebich BL, Efferth T, Prieto JM, Heinrich M. Traditionally used Thai medicinal plants: invitro anti inflammatory, anticancer and antioxidant activities. J Ethnopharmacol. 2010;130:196–207. doi: 10.1016/j.jep.2010.04.036. [DOI] [PubMed] [Google Scholar]
- Sultana B, Anwar F, Przybylski R. Antioxidant activity of phenolic components present in barks of barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007;104:1106–1114. doi: 10.1016/j.foodchem.2007.01.019. [DOI] [Google Scholar]
- Tahara S, Hashihidoka Y, Mizutani J. Flavonoids as medicines. Agri Biol Chem. 1987;51:1039–1045. doi: 10.1271/bbb1961.51.1039. [DOI] [Google Scholar]
- Yen G, Wu S, Duh P. Extraction and identification of antioxidant components from the leaves of mulberry (Morus alba L.) J Agric Food Chem. 1996;44:1687–1690. doi: 10.1021/jf9503725. [DOI] [Google Scholar]
- Yu JQ, Liu HB, Lei JC, Tan WJ, Hu XM, Zou GL. Antitumor activity of chloroform fraction of Scutellaria barbata and its active constituents. Phytother Res. 2007;21:817–822. doi: 10.1002/ptr.2062. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and there scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]